- Accueil
- volume 12 (2008)
- Nutritional and environmental consequences of dietary fibre in pig nutrition: a review
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Nutritional and environmental consequences of dietary fibre in pig nutrition: a review

Notes de la rédaction
Received on September 6, 2007, accepted on October 2, 2007
Résumé
Conséquences nutritionnelles et environnementales des fibres alimentaires dans la nutrition du porc : une revue. Etant donné leurs fonctionnalités multiples, une attention croissante a été portée aux fibres alimentaires en nutrition porcine durant la dernière décennie, en dépit de leur impact négatif sur les performances dues à une digestibilité de l'énergie et des protéines plus faible. Cette revue examine l'influence des fibres alimentaires sur les processus digestifs et leurs conséquences sur la nutrition protéique et énergétique du porc, sur les questions de santé et sur les problèmes environnementaux. Les fibres alimentaires sont définies comme les polysaccharides d'origine végétale qui résistent aux sécrétions digestives et sont potentiellement disponibles pour les fermentations bactériennes dans les intestins des monogastriques. L'amidon résistant est également considéré comme une fibre alimentaire. Les acides gras volatils produits par les bactéries contribuent à la satisfaction des besoins en énergie du porc et régulent à la fois la composition de la flore et la croissance des cellules épithéliales, particulièrement dans le cas du butyrate. La croissance bactérienne induite par les fermentations provoque un transfert de l'excrétion de N de l'urine vers les fèces. A côté de la fermentescibilité, les propriétés physiques des fibres alimentaires telles que la capacité de rétention en eau, la viscosité et la solubilité influencent la digestion, la satiété et le temps de transit. Les opportunités et les risques liés à l'incorporation de fibres alimentaires dans les rations des porcs élevés en conditions intensives ou dans des systèmes tropicaux plus extensifs sont discutés en rapport avec les mécanismes d'interaction des fibres alimentaires avec les processus digestifs. Les fibres alimentaires sont en effet un moyen possible pour réduire les pertes en N des unités de production et pour améliorer la santé intestinale du porc et le bien-être animal. Enfin, le rôle potentiel des méthodes de fermentation in vitro pour étudier le devenir des fibres alimentaires dans l'appareil digestif est discuté.
Abstract
Despite its negative impact on performances because of lower protein and energy digestibility, increasing attention has been paid in the past decade to dietary fibre in swine nutrition due to its multiple functionalities. The present review examines the influence of dietary fibre on the digestive processes and the consequences on pig protein and energy nutrition, health concerns and environmental issues. Dietary fibre is defined as the plant polysaccharides that are resistant to digestive secretions and are potentially available for bacterial fermentation in the intestines of single-stomached animals. Resistant starch is also considered as a dietary fibre. The short-chain fatty acids released by bacteria contribute to the host energy supply and both regulate the composition of the flora and the growth of epithelial cells, especially in the case of butyrate. The bacterial growth supported by the fermentation induces a shift of N excretion from urine to faeces. Beside the fermentability, the physical properties of dietary fibre such as the water-holding capacity, the viscosity and the solubility influence the digestion, the satiety and the transit time. In relationship with the mechanisms of dietary fibre interaction with the digestive processes exposed in this review, the opportunities and treats of dietary fibre inclusion in swine rations for intensified and for more extensive tropical production systems are discussed. Dietary fibre is indeed a possible means to reduce nitrogen losses of production units and to improve pig intestinal health and animal welfare. Finally, the potential role of in vitro fermentation methods to investigate the fate of dietary fibre in the digestive system is discussed.
Table des matières
1. Introduction
1The second half of the XXth century has seen a sharp increase of the world pork (Sus scrofa) production, which reaches nowadays a herd of 964 million pigs (FAO, 2006). Intensification of the rearing techniques, breeding programs and genetic progresses have resulted in lower production costs. However, intensive production systems have caused nitrate leaching and phosphorus accumulation in the soils receiving pig manure. These systems also induced animal welfare concerns such as stereotypies in gestating sows and human health problems such as the development of a gut microflora resistant to antibiotics (Manero et al., 2006). All these issues seriously question the social and environmental sustainability of intensive pig production (Basset-Mens et al., 2005).
2During the last 15 years, different solutions have been proposed to cope with these problems. Efforts have been spent to formulate diets that better meet the pig's requirements or contribute to reduce odour and pollutant excretion. In particular, attention is paid to dietary fibre (DF), for its capacity to reduce ammonia emission (Nahm, 2003; Aarnink et al., 2007) and to improve gut health (Williams et al., 2001; Montagne et al., 2003) and pig welfare (Meunier-Salaun, 1999; Courboulay et al., 2001).
3Increasing fibrous ingredients provided by the food industry are now incorporated in rations for pigs, despite the negative impact of DF on performances due to lower digestibility of dietary energy and protein (Noblet et al., 2001) and fatter carcasses. The use of forages, rich in DF, is also envisaged in more extensive systems such as herbage in outdoor production systems (Rivera Ferre et al., 2001; Blair, 2007) or in tropical countries where alternative feeding systems are studied, developed and extended (Pérez, 1997; Leterme et al., 2007) because grains are needed for human consumption.
4The present review examines the influence of DF fermentation in the pig intestines on the digestive processes. Their consequences on pig protein and energy nutrition, health concerns and environmental issues in intensified and in more extensive tropical production systems are also discussed.
2. Dietary fibre fermentation
2.1. Dietary fibre definition and chemical structure
5DF is commonly defined as all plants polysaccharides and lignin that are resistant to hydrolysis by human digestive secretions (Trowell, 1976). This definition is also commonly used for all non-ruminant animal species, including the pig. DF covers a wide range of carbohydrates known as non-starch polysaccharides (NSP) that include pectins, cellulose, hemicelluloses, β-glucans and fructans. Oligosaccharides and resistant starch are also considered in the DF fraction. As shown in table 1, the hydrolysis of these carbohydrates invariably produces the same pentoses, hexoses, deoxyhexoses and uronic acids (Chesson, 1995).
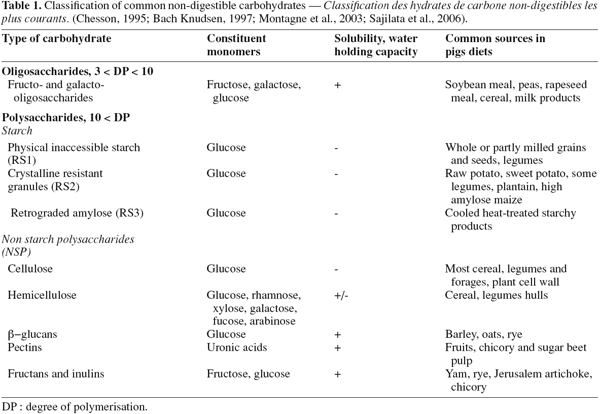
6The physiological properties of NSP and their fermentability are poorly predictable from the monomeric composition and are more related to their solubility, viscosity, physical structure and water-holding capacity (Asp, 1996).
7Starch is susceptible to hydrolysis by salivary and pancreatic enzymes. However, the hydrolysis is not always complete (Sajilata et al., 2006). A part of the starch, termed “ resistant starch ” (RS), escapes digestion in the small intestine and reaches the large intestine because of physical inaccessibility (RS1 according to Cummings et al., 1995)), crystalline structure (RS2) or amylose retrogradation after cooking (RS3). Resistant starch is also considered as a DF (Chesson, 1995).
2.2. Gut microbial population and animal health
8DF that escapes digestion in the upper part of the gastro-intestinal tract, is potentially available for bacterial fermentation in the large intestine. The anaerobic bacteria concentration in the pig gastro-intestinal tract passes thus from log 7-8 CFU.g-1 in the stomach and the small intestine to log 10-11 CFU.g-1 in the large intestine (Jensen et al., 1994).
9Approximately 90% of the cultivable bacteria in the pig colon are Gram-positive, strict anaerobes belonging to the Streptococcus, Lactobacillus, Eubacterium, Clostridium and Peptostreptococcus genus. The Gram-negative represent about 10% of the total flora and belong to the Bacteroides and Prevotella groups (Russell, 1979; Robinson et al., 1984; Leser et al., 2002).
10The gut microflora of healthy animals is subject to modifications in terms of predominant species according to the diet. The presence of DF seems to play an important role (Moore et al., 1987; Awati et al., 2005). The potential prebiotic influence of DF sources has been investigated in humans and monogastric animals. For example, Mc William et al. (2007) observed different bacterial communities in the primary colonizers of three insoluble colonic substrates (wheat bran, high amylose starch and mucin). Oligofructose, galacto-oligosaccharides and lactulose were clearly shown to increase Bifidobacteria and Lactobacilli in the large intestine of humans (Macfarlane et al., 2006). The addition of guar gum or cellulose to a standard diet was also shown to increase ileal Bifidobacteria and Enterobacteria populations in growing pigs (Owusu-Asiedu et al., 2006). On the contrary, diets high in fermentable NSP and resistant starch have been associated with increased incidence of clinical swine dysentry in grower pigs and diarrhoea in weaning piglets (Pluske et al., 1998; Pluske et al., 2003).
11The stability of the flora depends on numerous bacterial antagonisms between endogenous and exogenous species, including the resistance to colonisation by pathogens (Bourlioux, 1997). The bacteria species and the mechanisms involved are still poorly documented, but they are both of bacterial and animal origin (Williams et al., 2001). The resistance to Clostridium perfringens, for example, seems to be linked to the synthesis by Ruminococcus species of an antimicrobial substance, which is activated in the presence of trypsin. Conversely, the resistance to Clostridium difficile is probably due to an interaction between mucin and the microflora (Bourlioux, 1997).
12Furthermore, in acidic environment, SCFA produced by DF fermentation, as presented below, are capable of inhibiting the growth of some intestinal pathogens such as Escherichia coli, Salmonella spp. and Clostridium spp. (Montagne et al., 2003). Butyrate, in particular, seems to play a selective antimicrobial role, since studies in pigs indicate that Lactobacillus sp. and Streptococcus bovis are less sensitive to n-butyrate, compared to Escherichia coli, Salmonella spp., Clostridium acetobutylicum, Streptococcus cremoris, Lactococcus lactis and Lactococcus cremoris (Williams et al., 2001).
13In summary, the presence of DF significantly modifies the microbial equilibrium in the intestines with a positive or detrimental impact on animal health according the DF source and the physiological status of the pig.
2.3. Fermentation pathways and products
14The intestinal bacteria hydrolyse the polysaccharides composing the DF and metabolise their constituent sugars through a series of anaerobic energy-yielding reactions leading to the production of ATP which is used for bacteria basal and growth metabolism (Figure 1) (Macfarlane et al., 1995). Except for Bifidobacteria, the majority of the anaerobes of the large intestine use the Embden-Meyerhof-Parnas pathway, also known as glycolysis, that degrades glucose to pyruvate via glucose-6-phosphate (Prescott et al., 1996), to ferment the carbohydrates (Miller et al., 1996). Polysaccharides made of pentoses and pectins are first metabolised by the Pentose phosphate pathway (Macfarlane et al., 2003) starting from the pentose to fructose-6-phosphate and glyceraldehyde-3-phosphate via xylulose-5-phosphate (Prescott et al., 1996).
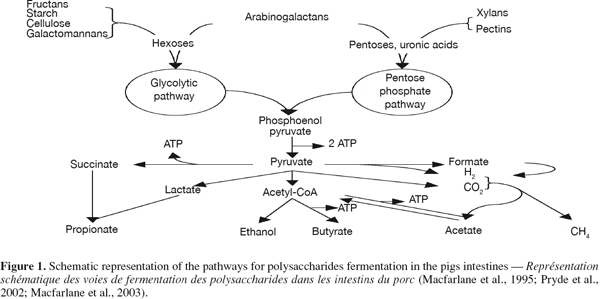
15As shown on figure 1, later steps include methanogenesis from H2 and CO2 or formic acid, reductive acetogenesis, butyrogenesis and acetogenesis from acetyl-CoA and propionogenesis via the acrylate pathway or the succinate decarboxylation (Pryde et al., 2002).
16Short-chain fatty acids (acetate, propionate and n-butyrate) and gases (CO2, H2 and CH4) are the main end-products of intestinal fermentation. Other metabolites such as lactate, ethanol and succinate are also formed by different types of bacteria (Drochner et al., 2004). With the possible exceptions of ethanol, these products do not accumulate in a healthy gut, because they serve as substrate and electron donors for cross-feeding bacteria and are further converted into SCFA (Macfarlane et al., 1995).
17The general stoichiometry follows the general equation (Williams et al., 2001):
1857.5 C6H12O6 + 45 H2O → 65 acetate + 20 propionate + 15 n-butyrate + 140 H2 + 95 CO2 + 288 ATP
19Despite this general equation, the amounts and the relative molar proportion of SCFA vary widely. Low SCFA yields recorded with some substrates like wheat bran may equate with incomplete fermentation or may mean that other intermediates are being formed. As presented in the general equation, acetate is the major anion produced during DF fermentation. However, the fermentation of pectin yields 80:12:8 (acetate:propionate:butyrate), other NSP yield 63:22:8 and starch 62:15:23 (Cummings, 1997; Drochner et al., 2004). Resistant starch is indeed known as a butyrogenic substrate of special interest in terms of intestinal health as discussed below (Sajilata et al., 2006). Beside the DF source, the quantity of substrate available also influences the way of its utilisation. Using pure cultures in chemostats, Macfarlane et al. (1995) reported that Bacteriodes ovatus and Clostridium perfringens produced more acetate at the expense of propionate and lactate, respectively when fermenting in a carbon-limited instead of a carbon-excess environment. In the same study, the bacterial growth rate was also shown to influence the SCFA molar ratio.
3. Feeding value of diet enriched in DF
3.1. Energy loss and metabolic utilisation of SCFA
20Increased DF level is associated with a reduced metabolisable energy content of the feed (Noblet et al., 2001). The overall energy cost in terms of heat production associated with the ingestion and excretion of indigestible fibrous ingredients is minimal and cannot be considered as significant (De Lange et al., 2006). Nevertheless, beside the unfermented DF, the main loss of energy due to DF is ascribed to the gases of fermentation (CH4, H2 and CO2), the heat of fermentation and the heat due to metabolic utilisation of SCFA. A significant part is also lost as bacterial biomass in the faeces. Even though it has not been clearly quantified yet, this loss was estimated to 0.2 of the neutral detergent fibre (NDF) energy content or 0.5 of the energy content of digestible NDF (Noblet et al., 2001).
21Average energy loss as methane ranges from 0.001 to 0.012 of the gross energy, the highest values being obtained with diets rich in highly digestible DF sources (soybean hulls or sugar beet pulp) (Noblet, 2001). Sows loose a higher proportion of digestible energy (DE) as methane than growing pigs at the same dietary level of fibre (Jørgensen, 2007). This is a consequence of their greater capacity for fermentation due to a higher intestinal transit time (Le Goff et al., 2003).
22Energy produced from hindgut fermentation varies from 0.07 to 0.17 of the total available energy, depending on the fermentable carbohydrates content of the diet (Anguita et al., 2006). Fermentation products contribute to the host maintenance energy supply from 0.15 for growing-finishing pigs (Dierick et al., 1989) to 0.3 for gestating sows (Varel et al., 1997). This contribution of DF to energy supply is conditioned by the absorption and metabolic utilisation by the host's cells of the SCFA. Propionate is a very effective glucogenic substrate and acetate is less easily taken up by the liver where it stimulates lipogenesis. Unlike propionate and acetate, butyrate does not pass in blood but it is directly metabolised by the colonocytes (Rémésy et al., 1995). Breves et al. (1997) observed substantial differences between hindgut mucosal uptake of SCFA and serosal release using the Ussing Chamber technique. They also noticed that the proportion of acetate increased at the expense of butyrate, confirming the higher metabolism of butyrate in the colonocytes, as compared to the other SCFA. Butyrate has been shown to regulate epithelial cell growth, to induce differentiation and apoptosis in the small intestine, to increase intestinal cell proliferation in piglets (Kien et al., 2007) and to improve digestive and absorptive capacities of the small intestine in pigs (Claus et al., 2007). Feeding diets rich in pectin to rats, Pirman et al. (2007) also noticed that the protein synthesis rates increased significantly in all parts of the intestines, consequently to the fermentation of the DF.
23In humans, approximately 90% of the SCFA produced in the large intestine are absorbed (Macfarlane et al., 1995). In growing pigs, Jørgensen et al. (1997) observed that less than 1% of SCFA infused intracaecally was excreted in the faeces. The efficiency of SCFA energy utilisation reached 0.82, 12% for the building of protein and 88% for fat production. This is approximately 5 to 10% lower than starch digested and absorbed in the small intestine (Jørgensen et al., 1997). Combined to the higher loss of energy through methane production, the lower efficiency in utilisation of SCFA energy from DF fermentation compared to glucose explains why the efficiency of DE utilisation for maintenance and growth reaches respectively 0.43 and 0.54 for NDF, as compared to 0.81 and 0.82 for starch (Noblet, 2001).
24In brief, among the DF fermentation products, only the SCFA contribute to the energy supply of the animal especially for fat production, but with a lower efficiency compared to glucose. Butyrate however, as major energy source for epithelial cells, improves the health of the intestines.
3.2. Digestibility
25The digestibility of DF varies from 0.40 to 0.60, as compared to the other nutrients (protein, fat, sugars or starch) which are above 0.80 (Noblet et al., 2001). DF fermentability is also more variable due to high diversity in physical structure and in chemical bounds between monomers. The reduction of energy digestibility will thus vary according to the DF source and the amount of total DF in a diet is an inadequate criterion for predicting energy digestibility (Noblet, 2001). Pastuszewska et al. (2000) observed that potato starch and pectins were more extensively fermented than cellulose in the caecum of rats (Rattus norvegicus). In growing pigs, NSP digestibility was shown to vary from 0.163 for wheat straw, 0.435 for wheat bran, 0.695 for sugar beet pulp to 0.791 for soybean hulls (Chabeauti et al., 1991). The presence of lignin explains the poor digestibility of wheat straw. Hemicellulose and cellulose composing wheat bran NSP are also less fermentable than the highly-digestible pectic substances of sugar beet pulp and soybean hulls (Karr-Lilienthal et al., 2005). Comparing the influence of soluble and insoluble DF, Owusu-Asiedu et al. (2006) observed that the replacement of 7% maize starch by guar gum (galactomannan) and cellulose in a maize-soybean meal diet for grower pigs decreased the energy digestibility from 0.878 to 0.866 and 0.849 respectively, while a combination of 7% guar gum and 7% cellulose led to an energy digestibility of 0.758. Conversely, the addition of resistant starch in a pig diet has no influence on the organic matter (OM) digestibility. The faecal digestibility of resistant starch is almost complete as shown by Martinez-Puig et al. (2003) who compared the digestibilty of starch in diets containing 250 g of raw potato starch or 250 g of corn starch (0.994 vs. 0.999).
26The adaptation of pigs to DF digestion is also a long process that requires 5 weeks (Martinez-Puig et al., 2003). Noblet (2001) calculated that during the 30 to 100 kg period, energy digestibility increases by 0.003 to 0.0045 per 10 kg of live weight for diets containing 4 to 6% of crude fibre. The largest effect is observed between growing pigs and sows. Two different DE values should therefore been provided for fibrous ingredients (Noblet et al., 2003). Energy digestibility is always higher with sows, because of their higher transit time consecutive to their higher gastrointestinal tract volume combined to lower feed intake per live weight (Le Goff et al., 2002).
27Thus, beside the lower efficiency in the utilisation of SCFA energy compared to glucose, the low digestibility of some DF sources contribute to their negative impact on the energy content of the diet.
3.3. Voluntary intake and performances
28The bulking capacity of DF reduces the transit time in the entire gastro-intestinal tract and the digestibility of the other nutrients of the diet. An increase in fibre content decreases the mean retention time in the small and the large intestines (Wilfart et al., 2007), reducing the time of exposure of the diet to the host's digestive enzymes (Low, 1982). The amount of digesta flow at the terminal ileum is greater in pigs fed diets with high levels of DF than in pigs fed low-fibre diets (Varel et al., 1997). Unlike that in the intestines, the retention time in the stomach can increase in presence of DF, causing earlier satiety due to elongation of the stomach wall (Wenk, 2001). Early satiety is important for the welfare of gestating sows (Meunier-Salaun, 1999), but detrimental in grower pigs where a maximum energy intake is desired. When green forages are fed, intake reduction is even more important, probably due to a poor palatability and a tilling of the fibrous ingredients increases voluntary intake as shown with tree leaves in sows by Leterme et al. (2005).
29The slower emptying of the stomach is a consequence of the water-holding capacity of the DF source (Table 1). Soluble DF are therefore efficient to prolong satiety, while insoluble DF have a lower impact (Wenk, 2001). The reduction in digestibility varies also according to the level and type of fibre since the rate of diffusion, towards the mucosal surface, of the host enzymes to the diet and the diffusion of the solubilised components, such as sugars and peptides, are slowed down by the viscosity of the intestinal content, depending on the water-holding capacity of DF (Table 1) (Asp, 1996; Wenk, 2001; Hopwood et al., 2004).
30Despite the negative impact on digestibility, farmers in the tropics use extensively fibrous crop by-products and forages as alternative ingredients to prohibitive cereals in pig diets. For example, in Vietnam, the incorporation of 15 % spinach or sweet-potato leaves in a diet for Mon Cai × Large White grower pigs increased the crude fibre content of the diets, but also the crude protein (0.172 to 0.182 g CP.kg-1DM) and the α-linolenic acid (0.14 g ALA.MJ-1ME) contents. This eventually stimulated the growth performances of the pigs (Nguyen et al., 2004). In Nigeria, similar performances were observed in grower pigs fed a diet containing 75% maize and 3% full fat soybean and a diet containing 27% maize, 38% cassava peels, 9% soybean and 5% palm oil. The inclusion of cassava peels doubled the crude fibre content, but the inclusion of palm oil counterbalanced the decrease in DE content and, finally, the cost saving per kg of weight gain using cassava peels reached 24% (Balogun et al., 1997). Leterme et al. (2006a) also showed that the inclusion of 30% of tropical tree leaves in sow diets did not affect the digestive processes. In their study, tree leaves provided from 8.53 to 12.0 MJ DE per kg DM despite a decrease in energy digestibility. DE of the leaves alone was calculated to vary from 0.54 to 0.69, while the NDF content ranged from 468 to 310 g.kg-1DM.
31The use of fibrous ingredients in pig diet as an alternative to cereals may not always be efficient in terms of animal performances but the economical asset of the operation is mostly at the advantage of a substantial substitution (Ogle, 2006). Furthermore, despite their low energy density, tropical unconventional fibrous ingredients were also shown to provide other valuable nutrients to the animals such as well-balanced protein (Balogun et al., 1997; Leterme et al., 2005; 2006a) and minerals, especially calcium, iron and manganese (Leterme et al., 2005; 2006b).
32In summary, the lower energy content of high-fibre diets and their influence on satiety reduce growth performances of the animals. This practise may however be interesting for sows or to reduce the feeding costs of growing pigs.
4. Influence of DF on protein nutrition and nitrogen excretion and emission
4.1. Protein digestibility
33As indicated by the following examples, tropical dicotyledons used to feed pigs in the tropics have high CP contents: Manihot esculenta, 324 g.kg-1DM; Ipomea batatas, 244; Desmodium intortum, 272; Amaranthus hybridus, 261; Psophocarpus scandens, 297; Arachis hypogaea, 223; Trichanthera gigantea, 216; Morus alba, 190; Xanthosoma sagittifolium, 240 (Leterme et al., 2005; Phuc, 2006; Bindelle et al., 2007). However, an average of 31% of this nitrogen is bound to the NDF and is not available for the animal (Bindelle et al., 2005). Moreover, green forages are often associated with anti-nutritional factors that interfere with the digestive processes (Phuc, 2006). As discussed above, the reduction in digestibility varies according to the level and type of fibre and its water-holding capacity (Wenk, 2001). High-fibre diets are also known to increase endogenous nitrogen losses (Leterme et al., 1996; Souffrant, 2001) and erosion of the intestinal wall (Varel et al., 1997). The source and nature of the DF as well as their physico-chemical properties seem to influence the ileal digestibility of protein. However, controversial results are found in the literature on this topic (Souffrant, 2001).
34As a consequence of microbial growth, DF intestinal fermentation reduces protein apparent faecal digestibility and increases faecal N excretion (Mroz et al., 1993) since the bacterial biomass that accumulates in faeces is composed of approximately 625 g.kg-1DM of crude protein (Russell et al., 1992). Leek et al. (2007), for example, recorded a CP digestibility of 0.82 with barley-based diets, as compared to 0.85 and 0.88 with maize- and wheat-based diets. Simultaneously, barley induced higher N retention than the two other cereals (0.54 vs. 0.47 and 0.41 respectively). Zervas et al. (2002) also observed that adding fermentable DF sources (soybean hulls or sugar beet pulp) to a low-fibre, high-protein diet reduced the CP digestibility from 0.85 to 0.80 for soybean hulls and 0.74 for sugar beet pulp. In the case of a low protein diet, the reduction was less important but still significant since it passed from 0.82 to 0.76 and 0.74, respectively. This decrease in N digestibility did not affect N retention. In ruminant studies, the nitrogen incorporation per g OM fermented by the flora was shown to vary according to the substrate, with rapid fermentable DF yielding higher bacterial growth than slow fermentable DF (Hall et al., 2001).
35The studies by Leek et al. (2007) and Zervas et al. (2002) obviously show that reduced faecal CP digestibility consecutive to intestinal DF fermentation is not necessarily related to lower protein value of the diet. They are consistent with Canh et al. (1997) who recorded a N faecal digestibility of 0.85 and a N retention of 0.30 with a grain-based diet, whereas with sugar beet pulp the digestibility was reduced to 0.75 but N retention increased to 0.44.
36Bacterial carbohydrate fermentation in pigs mainly takes place in the caecum and the colon, but also in a certain proportion before the end of the ileum (Rowan et al., 1992). As an example, Böhmer et al. (2005) found that more than 55% of dietary inulin, a highly fermentable and soluble fructan, was digested in the small intestine. In piglets, the capability of a bacterial inoculum to produce SCFA from fructo-oligosaccharides passes 2 mmol.h-1.kg-1digesta when bacteria are harvested in the stomach to 16.4 in the distal small intestine, 43.6 in the caecum and 65.1 in the colon (Mikkelsen et al., 2004). This indicates that the population of active bacteria is very low in the stomach, but becomes significant in the distal small intestine. As a consequence, the bacterial biomass accumulation occurring before the intestinal content reaches the large intestine contributes in a small but significant extent to the amino acid requirements of pigs (Torrallardona et al., 2003).
37It can be concluded that the presence of DF in the diets lowers the apparent faecal digestibility of the crude protein and possibly the ileal digestibility, but not necessarily the efficiency of nitrogen retention by the animal.
4.2. Nitrogen excretion pathways
38As the intestinal content passes through the caecum and the colon, it becomes depleted in fermentable carbohydrates. The energy source for the flora evolves from rapidly fermentable to slowly fermentable DF and, finally, to dietary resistant and endogenous proteins. Bacterial proteolysis induces the production of branched-chain fatty acids (mainly valerate, i-valerate, i-butyrate), malodorous compounds such as skatole, which contributes to unpleasant smell and taste of boars meat (Jensen et al., 1995), and amines and ammonia, originating from the deamination of amino acids.
39However, the combination of different sources of DF affects both the SCFA patterns and the site of fermentation (Henningsson et al., 2002). Substrates with lower rates of fermentation, like wheat bran used in combination with more fermentable NSP or RS, maintain the microbial activity throughout the entire large intestine and decrease proteolysis occurring in the distal colon (Govers et al., 1999). The undigested dietary proteins and the endogenous proteins are in this state of figure used for the building up of bacterial proteins and the intense bacterial growth in the intestine enhances the urea transfer from the blood to the large intestine (Younes et al., 1996; Pastuszewska et al., 2000). As a consequence, urinary N excretion is decreased. Zervas et al. (2002) showed in their study that the inclusion of fermentable DF from sugar beet pulp and soybean hulls in low protein wheat- and barley-based diets increased faecal N output from 5.1 to 7.7 g.d-1 and lowered urinary:faecal N excretion ratio, decreasing from 2 for the control diet to 1.3 with soybean hulls and 1 for sugar beet pulp. Total N excretion remained unaffected. Kreuzer et al. (1993) also found that the addition of 100 to 220 g NSP.kg-1 in pig's diet reduced urinary N excretion from 20 to 28%. The same observation was made by Canh et al. (1997). With a grain-based diet, they recorded a urinary:faecal N ratio of 3.83 vs. 1.21 with a diet containing 250 g.kg-1 of sugar beet pulp.
4.3. N emission through manure
40The N-excretion shift from urea in urine to bacterial protein in faeces, as exposed in the previous section, is a potential means for reducing the environmental load of pig facilities (Nahm, 2003). The breakdown of protein in manure is a slow process taking weeks and even months depending on the temperature. Conversely, the degradation of urea to ammonia and CO2 covers only several hours (Aarnink et al., 2007). The ammonia production during 10 d of manure from pigs fed a barley-based diet is limited to 0.066 of the N intake, as compared to 0.113 for maize and 0.121 for wheat. When NSP enzymes are added to the diets, the emission from barley-based diets increases while the emission from maize- and wheat-based diets decreases, confirming the role of the NSP in the reduction of ammonia emission (Leek et al., 2007). Sutton et al. (1999) observed that during a 7 d-storage, the manure of pigs fed a grain-based diet lost 0.24 of the initial N as NH3, as compared to 0.14 in the case of sugar beet pulp. Kreuzer et al. (1998) showed that feeds with high contents in pectin and hemicellulose, like citrus pulp and sugar beet pulp, were the most effective DF sources to reduce N loss in manure, as compared to cellulose from rye bran and RS from cassava.
41Bach Knudsen et al. (1991) observed that SFCA concentration increases from less than 20 mmol.l-1 in the small intestine to 100-140 mmol.l-1 in the caecum. As a consequence the pH drops from 6.6-7.2 to 5.7-6.8 (Jensen et al., 1994). The intestinal pH and the content of total SCFA in the digesta are linearly related (Högberg et al., 2004). The pH was shown to raise again when passing from the caecum to the proximate and latter the distal colon, but this increase was higher with low-fibre diets, as compared to high fibre diets (Bach Knudsen et al., 1991; Jensen et al., 1994), as a consequence of NH3 production. The lower pH of faeces and manure of pigs fed diets with high fermentable DF content is also an efficient means for reducing ammonia emission since it is soluble under its protonated form (NH4+) (Aarnink et al., 2007). Canh et al. (1998) found that for each increase of 100 g NSP in pig diets, the slurry pH decreased by 0.12 units and ammonia emission was reduced by 5.4%.
42In tropical extensive production systems, decreasing urinary-N emission is also of great interest. Indeed, tropical soils are generally poor in OM and nutrient recycling is critical to maintain productivity since mineral fertilizers are prohibitive to the small farmers. N is the most limiting nutrient in agricultural systems because it can be easily be lost through gaseous losses, leaching or runoff, while large amounts are essential for non-leguminous plant growth (Campbell et al., 1995; Smil, 1999). In mixed livestock-cropping systems, urinary-N is particularly susceptible to loss despite improved manure collection and storage practises. A reduction in N loss requires to maximise faecal-N excretion (Rufino et al., 2006) as influenced in pig production by the DF content of the diet. However, manure from highly-fermentable fibre diets contains lower proportions of nitrogen directly utilizable by the plants since protein degradation is a slow process (Kreuzer et al., 1993; Aarnink et al., 2007).
43Finally, the accumulation of bacterial protein in manure consecutive to high-fibre diets is also valorised in integrated fish and pig production systems which are very common in Southeast Asia (Payne et al., 1999). However, these practises require further investigation because they are controversial (Kumar et al., 2004).
5. Conclusion and perspectives
44This review of the literature pointed out the influence of the DF source on the microbial flora equilibrium, on energy and protein digestibility and on the N excretion pathways in pigs. The potentialities offered by fibrous ingredients for extensive production systems in the tropics were also illustrated.
45Further studies devoted to the relationship between DF fermentability and its functionalities are necessary in order to identify appropriate DF sources that reduce ammonia emission, promote intestinal health and still allow fair pig performances. The influence of crude protein bound to NDF on protein availability, bacterial growth and N excretion pathways requires also further investigation. Finally, the loss of energy as bacterial biomass in the faeces should be properly quantified in the future.
46In vivo models for describing the fate of DF in the digestive system are difficult and expensive, especially if a wide range of ingredients must be evaluated. There is thus an important potential role for in vitro techniques, providing an adequate in vivo validation of the method (Coles et al., 2005). Enzymatic methods based on substrates disappearance during incubation, e.g. the protocol of Boisen et al. (1997), succeed in the prediction of in vivo OM and energy digestibility, but limit the understanding of fermentation in the large intestine. The use of a living bacterial inoculum as in the gas production technique, used in ruminant nutrition (Menke et al., 1988), instead of an enzymatic complex such as viscozyme, allows to record the substrates disappearance during fermentation and also bacterial accumulation, SCFA production and gas release, which are of first importance when the functionalities of DF are considered. As showed by Awati et al. (2005), in vitro fermentation methods can also highlight the effect of the DF source on the microbial composition. Nevertheless, there is still a lack of proper in vivo validation of such studies results.
47Finally, numerous studies highlighted the potential of fermentable DF to reduce the environmental load of pig facilities. However, a combined economical and environmental evaluation of this practise is necessary for intensified as well as for extensive production systems.
48List of the abbreviations
49CP: crude protein
50DE: digestible energy
51DF: dietary fibre
52NDF: neutral detergent fibre
53NSP: non-starch polysaccharides
54OM: organic matter
55RS: resistant starch
56SCFA: short-chain fatty acids
57Acknowledgements
58The authors gratefully acknowledge the National Fund for Scientific Research (FNRS, Brussels, Belgium) for the financial support of the author's mobility.
Bibliographie
Aarnink A.J.A. & Verstegen M.W.A., 2007. Nutrition, key factor to reduce environmental load from pig production. Livest. Sci., 109, 194-203.
Anguita M., Canibe N., Pérez J.F. & Jensen B.B., 2006. Influence of the amount of dietary fiber on the available energy from hindgut fermentation in growing pigs: use of cannulated pigs and in vitro fermentation. J. Anim. Sci., 84, 2766-2778.
Asp N.G., 1996. Dietary carbohydrates: classification by chemistry and physiology. Food Chem., 57, 9-14.
Awati A. et al., 2005. Effect of substrate adaptation on the microbial fermentation and microbial composition of faecal microbiota of weaning piglets studied in vitro. J. Sci. Food Agric., 85, 1765- 1772.
Bach Knudsen K.E., 1997. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol., 67, 319-338.
Bach Knudsen K.E., Jensen B.B., Andersen J.O. & Hansen I., 1991. Gastrointestinal implications in pigs of wheat and oat fractions. 2. Microbial activity in the gastrointestinal tract. Brit. J. Nutr., 65, 233-248.
Balogun T.F. & Bawa G.S., 1997. Cassava peels in the diet of young pigs in Nigeria. Trop. Anim. Health Prod., 4, 209-215.
Basset-Mens C. & van der Werf H.M.G., 2005. Scenario-based environmental assessment of farming systems: the case of pig production in France. Agric. Ecosystems Environ., 105, 127-144.
Bindelle J. et al., 2005. A rapid estimation of nitrogen bound to neutral detergent fibre in forages by near infrared reflectance spectroscopy. In: O'Mara F.P. et al. Proceedings of the XXth International Grassland Congress. June 26th - July 1st, 2005. Dublin: University College Dublin, 259.
Bindelle J. et al., 2007. Voluntary intake, chemical composition and in vitro digestibility of fresh forages fed to Guinea pigs in periurban rearing systems of Kinshasa (Democratic Republic of Congo). Trop. Anim. Health Prod., 39, 419-426.
Blair R., 2007. Nutrition and feeding of organic pigs. Wallingford, UK: CABI.
Böhmer B.M., Branner G.R. & Roth-Maier D.A., 2005. Precaecal and faecal digestibility of inulin (DP 10-12) or an inulin/Enterococcus faecium mix and effects on nutrient digestibility and microbial gut flora. J. Anim. Physiol. Anim. Nutr., 89, 388-396.
Boisen S. & Fernández J.A., 1997. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol., 68, 277-286.
Bourlioux P., 1997. What is currently known about the molecular mechanisms of colonisation resistance. Anaerobe, 3, 179-184.
Breves G. & Krumscheid R., 1997. In vitro studies on transport and metabolism of short-chain fatty acids in pig hindgut. Comp. Biochem. Physiol. Part A: Physiol., 118, 399-401.
Campbell C.A., Myers R.J.K. & Curtin D., 1995. Managing nitrogen for sustainable crop production. Nutrient Cycling in Agroecosystems, 42, 277-296.
Canh T.T., Verstegen M.W.A., Aarnink A.J.A. & Schrama J.W., 1997. Influence of dietary factors on nitrogen partitioning and composition of urine and feces of fattening pigs. J. Anim. Sci., 75, 700-706.
Canh T.T. et al., 1998. Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. J. Anim. Sci., 76, 1887-1895.
Chabeauti E., Noblet J. & Carré B., 1991. Digestion of plant cell walls from four different sources in growing pigs. Anim. Feed Sci. Technol., 32, 207-213.
Chesson A., 1995. Dietary fiber. In: Stephen A.M. Food polysaccharides and their applications. New York: Marcel Dekker, 547-576.
Claus R., Günthner D. & Letzguß H., 2007. Effects of feeding fat-coated butyrate on mucosal morphology and function in the small intestine of the pig. J. Anim. Physiol. Anim. Nutr., 91, 312-318.
Coles L.T., Moughan P.J. & Darragh A.J., 2005. In vitro digestion and fermentation methods, including gas production techniques, as applied to nutritive evaluation of foods in the hindgut of humans and other simple-stomached animals. Anim. Feed Sci. Technol., 123-124, 421-444.
Courboulay V., Dubois A. & Meunier-Salaün M.C., 2001. La distribution d'aliments riches en fibres affecte l'activité alimentaire des truies gestantes logées en groupe. 33es Journées de la Recherche Porcine, Paris, 30 janvier-1 février 2001. Paris : ITP, 307-312.
Cummings J.H., 1997. The large intestine in nutrition and disease. Brussels: Institut Danone.
Cummings J.H. & Englyst H.N., 1995. Gastrointestinal effects of food carbohydrate. Am. J. Clin. Nutr., 61, 928S-945S.
De Lange C., Van Milgen J., Dubois S. & Noblet J., 2006. Energy cost of ingesting and excreting indigestible material in growing pigs is minimal. Anim. Res., 55, 551-562.
Dierick N.A., Vervaeke I.J., Demeyer D.I. & Decuypere J.A., 1989. Approach to the energetic importance of fibre digestion in pigs. I. Importance of fermentation in the overall energy supply. Anim. Feed Sci. Technol., 23, 141-167.
Drochner W., Kerler A. & Zacharias B., 2004. Pectin in pig nutrition, a comparative review. J. Anim. Physiol. Anim. Nutr., 88, 367-380.
FAO, 2006. Statistical Yearbook 2005-2006. Rome: FAO.
Govers M.J. et al., 1999. Wheat bran affects the site of fermentation of resistant starch and luminal indexes related to colon cancer risk: a study in pigs. Gut, 45, 840-847.
Hall M.B. & Herejk C., 2001. Differences in yields of microbial crude protein from in vitro fermentation of carbohydrates. J. Dairy Sci., 84, 2486-2493.
Henningsson Å.M., Björck I.M.E. & Nyman E.M.G.L., 2002. Combinations of indigestible carbohydrates affect short-chain fatty acid formation in the hindgut of rats. J. Nutr., 132, 3098-3104.
Högberg A. & Lindberg J.E., 2004. Influence of cereal non-starch polysaccharides on digestion site and gut environment in growing pigs. Livest. Prod. Sci., 87, 121-130.
Hopwood D.E., Pethick D.W., Pluske J.R. & Hampson D.J., 2004. Addition of pearl barley to a rice-based diet for newly weaned piglets increases the viscosity of the intestinal contents, reduces starch digestibility and exacerbates post-weaning colibacillosis. Br. J. Nutr., 92, 419-427.
Jensen B.B. & Jørgensen H., 1994. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl. Environ. Microbiol., 60, 1897-1904.
Jensen B.B. & Jensen M.T., 1995. Effect of diet composition on microbial production of skatole in the hindgut of pigs and its relation to skatole in backfat. In: Nunes A.F., Portugal A.V., Costa J.P. & Ribeiro J.R. Proceedings of the 7th International Symposium on Protein Metabolism and Nutrition, May 24-27th, 1995. Vale de Santarém, Portugal: EZN, 489-494.
Jørgensen H., 2007. Methane emission by growing pigs and adult sows as influenced by fermentation. Livest. Sci., 109, 216-219.
Jørgensen H., Larsen T., Zhao X.Q. & Eggum B.O., 1997. The energy value of short-chain fatty acids infused into the caecum of pigs. Br. J. Nutr., 77, 745-756.
Karr-Lilienthal L.K., Kadzere C.T., Grieshop C.M. & Fahey J.G.C., 2005. Chemical and nutritional properties of soybean carbohydrates as related to non-ruminants: a review. Livest. Prod. Sci., 97, 1-12.
Kien C.L., 2007. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J. Nutr., 137, 916-922.
Kreuzer M. & Machmüller A., 1993. Reduction of gaseous nitrogen emission from pig manure by increasing the level of bacterially fermentable substrates in the ration. In: Verstegen M.W.A., den Hartog L.A., Van Kempen G.J.M. & Metz J.H.M. Proceedings of the 1st International Symposium on nitrogen flow in pig production and environmental consequences, June 8-11th, 1993. Wageningen, The Netherlands: Pudoc Scientific Publishers, 151-156.
Kreuzer M. et al., 1998. Reduction of gaseous nitrogen loss from pig manure using feeds rich in easily-fermentable non-starch polysaccharides. Anim. Feed Sci. Technol., 73, 1-19.
Kumar M.S., Burgess S.N. & Luu L.T., 2004. Review of nutrient management in freshwater polyculture. J. Appl. Aquacult., 16, 17-44.
Leek A.B.G. et al., 2007. Apparent component digestibility and manure ammonia emission in finishing pigs fed diets based on barley, maize or wheat prepared without or with exogenous non-starch polysaccharides enzymes. Anim. Feed Sci. Technol., 135, 86-99.
Le Goff G., van Milgen J. & Noblet J., 2002. Influence of dietary fibre on digestive utilization and rate of passage in growing pigs, finishing pigs, and adult sows. Anim. Sci., 74, 503-515.
Le Goff G., Noblet J. & Cherbut C., 2003. Intrinsic ability of the faecal microbial flora to ferment dietary fibre at different growth stages of pigs. Livest. Prod. Sci., 81, 75-87.
Leser T.D. et al., 2002. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol., 68, 673-690.
Leterme P. et al., 1996. Chemical composition of pea fibre isolates and their effect on the endogenous amino acid flow at the ileum of the pig. J. Sci. Food Agric., 72, 127-134.
Leterme P. et al., 2005. Chemical composition, nutritive value and voluntary intake of tropical tree foliage and cocoyam in pigs. J. Sci. Food Agric., 85, 1725-1732.
Leterme P. et al., 2006a. Nutritive value of tropical tree leaf meals in adult sows. Anim. Sci., 82, 175-182.
Leterme P., Buldgen A., Estrada F. & Londoño A.M., 2006b. Mineral content of tropical fruits and unconventionnal foods of the Andes and the rain forest of Colombia. Food Chem., 95, 644-652.
Leterme P., Buldgen A., Murgueitio E.R. & Cuartas C., 2007. Fodder banks for sustainable pig production systems. Cali, Colombia: CIPAV Foundation.
Low A.G., 1982. Digestibility and availability of amino acids from feedstuffs for pigs: a review. Livest. Prod. Sci., 9, 511-520.
Macfarlane G.T. & Gibson G.D., 1995. Microbiological aspects of the production of short-chain fatty acids in the large bowel. In: Cumming J.H., Rombeau J.L., Sakota T. Physiological and clinical aspects of short-chain fatty acids. Cambridge, UK: Cambridge University Press, 87-105.
Macfarlane S. & Macfarlane G.T., 2003. Regulation of short-chain fatty acid production. Proc. Nutr. Soc., 62, 67-72.
Macfarlane S., Macfarlane G.T. & Cummings J.H., 2006. Review article: prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther., 24, 701-714.
Manero A., Vilanova X., Cerdà-Cuéllar M. & Blanch A.R., 2006. Vancomycin- and erythromycin-resistant enterococci in a pig farm and its environment. Environ. Microbiol., 8, 667-674.
Martinez-Puig D. et al., 2003. Consumption of raw potato starch increases colon length and fecal excretion of purine bases in growing pigs. J. Nutr., 133, 134-139.
Mc William L.E.C. et al., 2007. Selective colonization of insoluble substrates by human faecal bacteria. Environ. Microbiol., 9, 667-679.
Menke K.H. & Steingass H., 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Develop., 28, 7-55.
Meunier-Salaun M.C., 1999. Fibre in diets of sows. In: Garnsworthy P.C. & Wiseman J. Recent advances in animal nutrition. Nottingham, UK: Nottingham University Press, 57-273.
Mikkelsen L.L., Bach Knudsen K.E. & Jensen B.B., 2004. In vitro fermentation of fructo-oligosaccharides and transgalacto-oligosaccharides by adapted and unadapted bacterial populations from the gastrointestinal tract of piglets. Anim. Feed Sci. Technol., 116, 225-238.
Miller T.L. & Wolin M.J., 1996. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol., 62, 1589-1592.
Montagne L., Pluske J.R. & Hampson D.J., 2003. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol., 108, 95-117.
Moore W.E.C. et al., 1987. Effect of high-fiber and high-oil diets on the fecal flora of swine. Appl. Environ. Microbiol., 53, 1638-1644.
Mroz Z. et al., 1993. Preliminary studies on excretory patterns of nitrogen and anaerobic deterioration of faecal protein from pigs fed various carbohydrates. In: Verstegen M.W.A., de Hartog L.A., van Kempen G.J.M. & Metz J.H.M. Proceedings of the 1st International Symposium on nitrogen flow in pig production and environmental consequences, June 8-11th, 1993. Wageningen, The Netherlands: EAAP, 247-252.
Nahm K.H., 2003. Influence of fermentable carbohydrates on shifting nitrogen excretion and reducing ammonia emission of pigs. Crit. Rev. Environ. Sci. Technol., 30, 165-186.
Nguyen L.Q., Everts H., Hue H.T. & Beynen A.C., 2004. Feeding of spinach or sweet potato leaves and growth performance of growing pigs kept on smallholder farms in Central Vietnam. Trop. Anim. Health Prod., 36, 815-822.
Noblet J., 2001. Digestive and metabolic utilization of dietary energy in pig feeds: comparison of energy systems. In: Garnsworthy P.C. & Wiseman J. Recent Developments in Pig Nutrition 3. Nottingham, UK: Nottingham University Press, 161-184.
Noblet J. & Le Goff G., 2001. Effect of dietary fibre on the energy value of feeds for pigs. Anim. Feed Sci. Technol., 90, 35-52.
Noblet J., Bontems V. & Tran G., 2003. Estimation de la valeur énergétique des aliments pour le porc. Prod. Anim., 13, 197-210.
Ogle B., 2006. Forages for pigs: nutritional, physiological and practical implications. In: Preston T.R. & Ogle B. Proceedings of the workshop-seminar on forages for pigs and rabbits, August 21-24th, 2006. Phnom Penh, Cambodia: MEKARN-CelAgrid, http://www.mekarn.org/proprf/ogle.htm, (19/07/07).
Owusu-Asiedu A. et al., 2006. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci., 84, 843-852.
Pastuszewska B., Kowalczyk J. & Ochtabińska A., 2000. Dietary carbohydrates affect caecal fermentation and modify nitrogen excretion patterns in rats I. Studies with protein free diets. Arch Anim. Nutr., 53, 207-225.
Payne W.J.A. & Wilson R.T., 1999. An introduction to animal husbandry in the tropics. 5e ed. Oxford, UK: Blackwell Science.
Pérez R., 1997. Feeding pigs in the tropics. Rome: FAO.
Phuc B.H.N., 2006. Review of the nutritive value and effects of inclusion of forages in diets for pigs. In: Preston T.R. & Ogle B. Proceedings of the workshop-seminar on forages for pigs and rabbits, August 21-24th, 2006. Phnom Penh, Cambodia: MEKARN-CelAgrid, http://www.mekarn.org/proprf/phuc.htm, (19/07/07).
Pirman T. et al., 2007. Dietary pectin stimulates protein metabolism in the digestive tract. Nutrition, 23, 69-75.
Pluske J.R. et al., 1998. Confirmation of the role of rapidly fermentable carbohydrates in the expression of swine dysentery in pigs after experimental infection. J. Nutr., 128, 1737-1744.
Pluske J.R. et al., 2003. Effects of different sources and levels of dietary fibre in diets on performance, digesta characteristics and antibiotic treatment of pigs after weaning. Anim. Feed Sci. Technol., 107, 129-142.
Prescott L.M., Harley J.P. & Klein D., 1996. Microbiology. 3d ed. Boston, MA, USA: WCB/McGraw-Hill.
Pryde S.E. et al., 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett., 217, 133-139.
Rémésy C., Demigné C. & Morand C., 1995. Metabolism of short-chain fatty acids in the liver. In: Cummings J.H., Rombeau J.L. & Sakata T. Physiological and clinical aspects of short-chain fatty acids. Cambridge, UK: Cambridge University Press, 171-190.
Rivera Ferre M.G. et al., 2001. The effect of season and level of concentrate on the voluntary intake and digestibility of herbage by outdoor sows. Anim. Sci., 72, 501-510.
Robinson I.M., Whipp S.C., Bucklin J.A. & Allison M.J., 1984. Characterization of predominant bacteria from the colons of normal and dysentric pigs. Appl. Environ. Microbiol., 48, 964-969.
Rowan A.M., Moughan P.J. & Wilson M.N., 1992. The flows of deoxyribonucleic acid and diaminopimelic acid and the digestibility of dietary fibre components at the terminal ileum, as indicators of microbial activity in the upper digestive tract of ileostomised pigs. Anim. Feed Sci. Technol., 36, 129-141.
Rufino M.C., Rowe E.C., Delve R.J. & Giller K.E., 2006. Nitrogen cycling efficiencies through resource-poor African crop-livestock systems. Agric. Ecosystems Environ., 112, 261-282.
Russell E.G., 1979. Types and distribution of anaerobic bacteria in the large intestine of pigs. Appl. Environ. Microbiol., 37, 187-193.
Russell J.B. et al., 1992. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci., 70, 3551-3561.
Sajilata M.G., Singhal R.S. & Kulkarni P.R., 2006. Resistant starch - a review. Compr. Rev. Food Sci. Food Saf., 5, 1-17.
Smil V., 1999. Nitrogen in crop production: an account of global flows. Global Biogeochem. Cycles, 13, 647-662.
Souffrant W., 2001. Effect of dietary fibre on ileal digestibility and endogenous nitrogen losses in the pig. Anim. Feed Sci. Technol., 90, 93-102.
Sutton A.L. et al., 1999. Potential for reduction of odorous compounds in swine manure through diet modification. J. Anim. Sci., 77, 430-439.
Torrallardona D., Harris C.I. & Fuller M.F., 2003. Pigs'gastrointestinal microflora provide them with essential amino acids. J. Nutr., 133, 1127-1131.
Trowell H., 1976. Definition of dietary fibre and hypothesis that it is a protective factor in certain diseases. Am. J. Clin. Nutr., 29, 417-427.
Varel V.H. & Yen J.T., 1997. Microbial perspective on fiber utilization by swine. J. Anim. Sci., 75, 2715-2722.
Wenk C., 2001. The role of dietary fibre in the digestive physiology of the pig. Anim. Feed Sci. Technol., 90, 21-33.
Wilfart A. et al., 2007. Effect of fibre content in the diet on the mean retention time in different segments of the digestive tract in growing pigs. Livest. Sci., 109, 27-29.
Williams B.A., Verstegen M.W.A. & Tamminga S., 2001. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev., 14, 207-227.
Younes H. et al., 1996. A blend of dietary fibers increases urea disposal in the large intestine and lowers urinary nitrogen excretion in rats fed a low protein diet. Nutr. Biochem., 7, 474-480.
Zervas S. & Zijlstra R.T., 2002. Effects of dietary protein and fermentable fiber on nitrogen excretion patterns and plasma urea in grower pigs. J. Anim. Sci., 80, 3247-3256.
Pour citer cet article
A propos de : Jérôme Bindelle
Gembloux Agricultural University – FUSAGx. Department of Animal Husbandry, Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: bindelle.j@fsagx.ac.be
A propos de : André Buldgen
Gembloux Agricultural University – FUSAGx. Department of Animal Husbandry, Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Pascal Leterme
Prairie Swine Centre Inc. Box 21057. 2105 8th Street East, Saskatoon. Saskatchewan S7H 5N9. Canada.






