- Portada
- volume 12 (2008)
- numéro 4
- Modelling the effect of temperature, water activity and solute on the in vitro growth of the biocontrol yeast Pichia anomala strain K
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Modelling the effect of temperature, water activity and solute on the in vitro growth of the biocontrol yeast Pichia anomala strain K

Notes de la rédaction
Received on July 23, 2007, accepted on March 11, 2008
Résumé
Modélisation de l'effet de la température, l'activité de l'eau et des solutés sur la croissance in vitro de l'agent du biocontrôle Pichia anomala (souche K). L'objectif de ce travail était d'évaluer et de modéliser l'effet combiné de la température (5-25° C) et de l’activité de l’eau (aw, 0.890-0.980) sur la croissance et le temps de latence de Pichia anomala souche K. L'analyse de la variance a montré un effet hautement significatif d'aw, du soluté et de la température sur la croissance de la souche K. La diminution d'aw et de la température réduit et retarde significativement la croissance, l'effet étant plus accentué avec le NaCl en comparaison aux autres solutés testés. Les modèles de surface de réponse reliant le logarithme népérien de la croissance avec aw et la température ont été développés pour chaque soluté. La croissance optimum a été obtenue à des valeurs d’aw comprises entre 0,980 et 0,995 à 25 °C. Pour tous les modèles, il y avait un bon ajustement entre les valeurs observées et prédites. Cependant, seuls les modèles basés sur les substrats modifiés au sorbitol et au glucose ont précisément décrit les données de croissance expérimentales. La mise en œuvre de ces résultats, en combinaison avec ceux que nous avons publiés sur les quatre principaux agents pathogènes de post-récolte, devrait aboutir à une stratégie de lutte biologique plus efficace pour limiter les pertes de fruits en conservation.
Abstract
The objective of this work was to evaluate and model the combined effect of temperature (5-25°C) and water activity (aw, 0.890-0.980) on Pichia anomala strain K growth and lag phase. Variance analysis showed a highly significant effect of aw, solute and temperature on strain K growth. The decrease of aw and temperature significantly reduced and delayed growth, the effect being more drastic with NaCl compared to the other solutes. Response surface models relating the natural logarithm of growth with aw and temperature were developed for each solute. Optimum growth was obtained at aw values ranging from 0.980 to 0.995 at 25°C. For all models, there was good agreement between observed and predicted values. However, only models based on sorbitol and glucose accurately described experimental growth data. Implementation of the present results in combination with our previous findings on four main post-harvest pathogens should result in a more effective biocontrol strategy to limit fruit losses
Tabla de contenidos
1. Introduction
1The yeast Pichia anomala strain K was isolated from the surface of Golden Delicious apples and selected for its high and reliable biocontrol activity against Botrytis cinerea and Penicillium expansum, two worldwide serious pathogens of stored apples (Jijakli et al., 1993). The underlying mechanisms responsible of its biocontrol activity have been unravelled considering B. cinerea/apple as a model and using microbiological, biochemical, genetic and molecular approaches. Competition for nutrients and mycoparasitism seem to be the main modes of action of strain K (Jijakli et al., 1993; Jijakli et al., 1998; Massart et al., 2006; Friel et al., 2007). In order to specifically track the population dynamics of this strain after its application on apples, different monitoring systems have been developed (De Clercq et al., 2003; Pujol et al., 2003). Strain K was also found to be very effective in the biocontrol of Penicillium digitatum and Penicillium italicum, two devastating post-harvest pathogens of citrus (Lahlali et al., 2004). Its biocontrol activity has always been proven very efficient for post-harvest applications where environmental conditions are generally well controlled. For pre-harvest applications, however, strain K population density and efficacy during a two-year trial were largely influenced by meteorological conditions (Jijakli et al., 2002). In pre-harvest applications, the biocontrol agent will face large changes in temperature, relative humidity, light intensity, etc. To be successful in such application, the optimal and limits of environmental conditions in which strain K might develop must be determined. Water availability and temperature are among the main factors able to alter strain K growth and establishment.
2Response surface methodology (RSM) is a collection of statistical tools for designing experiments, evaluating the effects of factors, and searching for optimal conditions of factors for desirable responses (Myers et al., 2002; Liew et al., 2005). In our previous works, RSM has been successfully applied to study the combined effect of water activity (aw) and temperature on the in vitro growth of B. cinerea (Lahlali et al., 2007), P. expansum (Lahlali et al., 2005), P. digitatum and P. italicum (Lahlali et al., 2006). These studies have enabled us to determine and compare the ecological niches of these economically important pathogens. Regarding the biocontrol agent strain K, however, the range of aw and temperature that allows its proliferation are still unknown. Whether or not strain K occupies the same niches as its pathogen targets is not known either. With this in mind, an in vitro study has been undertaken in order to:
3– evaluate the effect of temperature, aw and solute on strain K growth and lag phase,
4– construct models predicting growth response to these factors.
2. Material and methods
2.1. Yeast isolate
5Pichia anomala strain K was isolated from the surface of " Golden Delicious " apples at the Plant Pathology Unit (Gembloux Agricultural University, Belgium) and identified by the Industrial Fungi & Yeast’s Collection (BCCMTM/MUCL, Belgium). Stock cultures were stored at 4°C on potato dextrose agar (PDA) plates (Merck, Darmstadt, Germany). Prior to each test, strain K was cultured at 25°C for three successive generations on PDA medium with an interval of 24 hours.
2.2. Media preparation
6The basic medium used for the present study was PDA with a aw of 0.995. The water activity was modified by the addition of increasing amounts of glycerol, sorbitol, glucose or NaCl to obtain levels of 0.980, 0.930, and 0.890 at 25, 15 and 5°C (Lahlali et al., 2005; 2007). The aw of all media was measured by a CX 3 Aqua Lab device (Decagon Devices, Inc.).
2.3. Growth and lag phase assessment
7A yeast concentration of 1 x 104 colony-forming units (cfu) (cfu.ml-1) was prepared. The final concentration of yeast was adjusted using D.O measurement as previously described (Jijakli et al., 1998). An aliquot of 100 µl was plated on each PDA medium. Prepared Petri dishes were sealed with the parafilm and kept in a growth room at tested temperatures (25, 15 and 5°C). Petri dishes were daily examined and the number of visible colonies was counted. Then, the effect of solute, aw and temperature on strain K growth (cfu.ml-1) and lag phase (time required for growth) was evaluated.
2.4. Experimental design and model validation
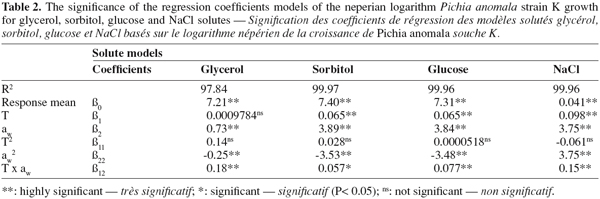
8Growth (cfu.ml-1) data were statistically analyzed with a general linear model (GLM) available as SAS software (SAS Institute, version 8.2, Cary, NC, USA). Statistical significance was judged at the P < 0.05 level. Before modelling, growth data were subjected to box-cox transformation (Box et al., 1987) to correct the homogeneity of variance. The natural logarithm of growth was modelled by means of three full factorial design including three levels of temperature (25, 15, and 5°C) and aw (0.980, 0.930 and 0.890) (Lahlali et al., 2005; 2006). This design was applied in triplicate combinations with a single block. A second-order polynomial equation was used to fit the natural logarithm (ln) of strain K growth for each solute:
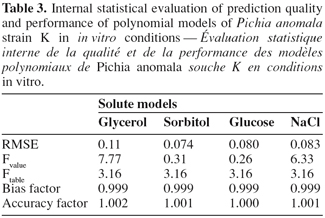
9ln (cfu.ml-1) = β0 + β1T + β2 aw + β11 T2 + β22 aw2 + β12 T aw
10with six coefficients:
11– β0: intercept
12– β1, β2: linear coefficients
13– β12: interaction coefficient
14– β11, β22: squared coefficients
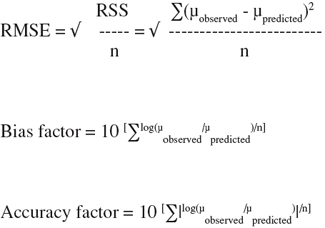
15by means of the statistical software package DESIGN-EXPERT® version 6.0. (StatEase, Inc., Minneapolis, USA). To evaluate the fitting and prediction accuracy of response surface model, the following evaluation criteria were employed (Ross, 1996; Samapundo et al., 2005; Lahlali et al., 2007):
16– Root-Mean-Square Error (RMSE)
17– F-value, regression coefficient (R2)
18– Bias factor
19– Accuracy factor
20F-values were calculated and compared with tabulated F-values.
21The RMSE and the bias and accuracy factors were calculated as follows:
3. Results
3.1. Effect of temperature, aw and solute on strain K growth
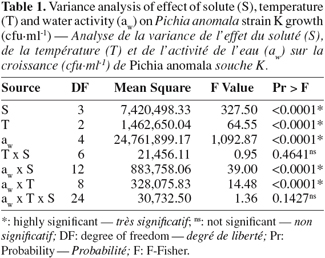
22Statistical analysis showed a highly significant effect of temperature, aw, solute, as well as of water activity-temperature and aw-solute interactions (Table 1).
23At 25°C, the growth of strain K decreased with decreasing aw of the medium, the effect being dependent on the solute (Figure 1A). In fact, strain K growth was inhibited at 0.930 in the case of NaCl and at 0.890 for sorbitol and glucose. Regarding glycerol, however, strain K was still able to grow even at the lowest aw (0.890).
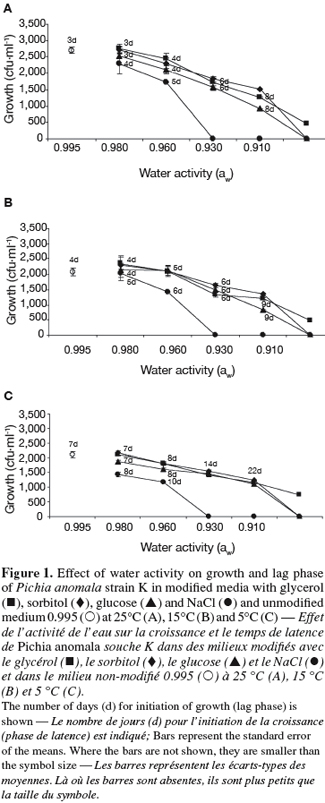
24The lag phase (time prior growth) of strain K growth was also influenced by the aw of the medium as well as by the type of the solute (Figure 1A). At 0.980 for example, strain K growth required 4 days of incubation at 25°C in media supplemented with NaCl and only 3 days for media amended with glycerol, sorbitol or glucose. As aw decreased, the lag phase increases and reached 10 days at the lowest aw (Figure 1A).
25At the two other temperatures tested (15 and 5°C), the same tendencies were observed regarding the effect of aw and solute on the growth and lag phase of strain K (Figures 1B and 1C). The reduction of incubation temperature accentuated the above-described effects observed at 25°C.
3.2. Modelling growth
26The response surface curves representing the predicted effect of aw and temperature on natural logarithm (ln) of strain K growth (cfu.ml-1) showed that the optimal growth was observed at aw ranging between 0.960 and 0.980 and at 25°C (Figure 2). Model curves based on sorbitol and glucose illustrate well a net curvature for aw > 0.96, which suggests a significant effect of interaction between the temperature of incubation and the aw of the medium. An almost nonexistent growth of the strain K was observed at the lowest aw (0.890) whatever the incubation temperature and the solute model (data not shown).
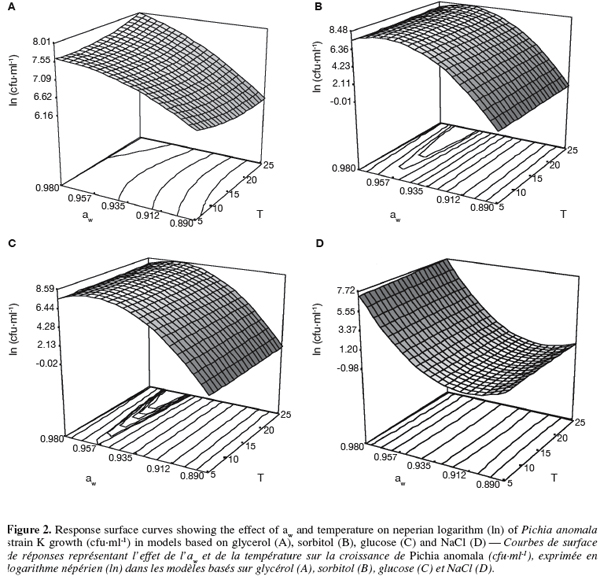
27The two factors, temperature and aw, explained more than 97% of variation in the different models whatever the solute (R2, Table 2). The estimated regression coefficients given by the multiple regression analysis are listed in table 2. It can be seen that all coefficients models have a significant effect, except effect of temperature (β1) for the model based on glycerol and the quadratic effect of temperature (β11), regardless of the solute model. Whatever the quadratic model, temperature (β1) and aw (β2) have a positive effect on strain K growth and aw has more highly influenced strain K growth than incubation temperature. The negative quadratic effect of temperature (β11) was only observed for the model based on NaCl. However, the quadratic effect of aw (β22) was revealed negative only for non-ionic models such as sorbitol, glycerol and glucose. In all constructed models, the interaction coefficient (β12) between aw and temperature seemed to be significant.
3.3. Models fit
28All quadratic polynomial equations of built solute models for strain K growth were subjected to internal statistical validation (Table 3) using the following mathematical and statistical indices: the RMSE; the lack of fit test, bias and accuracy factors (Lahlali et al., 2007). Models based on sorbitol and glucose have small values of RMSE with respectively 0.074 and 0.08, followed by models based on NaCl and glycerol. Sorbitol and glucose models were revealed not significant to lack of fit test at P < 0.05, suggesting that both models adequately describe strain K growth in the limits of experiments. Therefore, they are considered as the best predictors of in vitro strain K growth with respect to the two studied factors, temperature and aw. These selected models possess a bias factor of 0.999 and an accuracy factor equal to unit, indicating a slight difference between observed and predicted values.
4. Discussion
29The efficacy of biocontrol agents in pre-harvest application is generally variable and much lower as compared to their efficacy when applied post-harvest. This could be explained, at least partly, by the fluctuation of field environmental conditions, such as temperature, rainfall and water availability (Teixidõ et al., 1999; Jijakli et al., 2002). Accordingly, the objective of our work was to assess the influence of temperature, aw and solute on the in vitro growth of P. anomala strain K, an efficient biocontrol agent of post-harvest diseases of apple and citrus fruits (Jijakli et al., 1993; Lahlali et al., 2004).
30Our results show that in vitro growth of strain K was significantly affected by incubation temperature, aw and by the nature of the solute used to adjust the aw of the medium. Similar findings were also reported for other biocontrol agents such as Candida sake (Teixidõ et al., 1998a) and Pantoea agglomerans (Costa et al., 2002). Strain K growth was found to decrease with decreasing temperature and aw. Regarding solutes, the most detrimental effect of these parameters was observed in media supplemented with NaCl where no growth was recorded at 0.930 aw. In the presence of glycerol, however, strain K was still able to grow even at 0.890 aw but the lag phase was lengthened with the decrease in incubation temperature. A differential response to ionic (e.g. NaCl) and nonionic solutes (e.g. glycerol) has been reported for other microorganisms, including yeasts (Van Eck et al., 1993; Teixidõ et al., 1998a; Fredlund et al., 2002) and fungi (Lahlali et al., 2005; 2006; 2007). When compared to two other strains of the same species CSIR Y207 (Van Eck et al., 1993) and J121 (Fredlund et al., 2002), strain K showed an intermediate tolerance to NaCl-adjusted aw. Two major explanations may be advanced to explain the differential growth of microorganisms observed in media with the same aw but adjusted with either non-ionic (especially glycerol) or ionic (NaCl) solutes. The first one is the fact that NaCl exerts a double effect compared to glycerol. Glycerol may affect the microorganism through an osmotic component that compromises water uptake whereas NaCl had, in addition to an osmotic effect, an ionic effect linked to the accumulation of toxic Na and Cl ions and to the impairment of mineral nutrition. The second explanation may be the possible active uptake of glycerol by microorganisms and its accumulation as a compatible solute at low glycerol-adjusted aw values compared to NaCl-adjusted ones.
31In the present work, we used the response surface methodology (RSM) to model the combined effect of temperature, aw, and solute on strain K growth because it remains the approach largely applied for its precise yielded results and its lower cost (Myers et al., 2002). The optimal growth of strain K was observed at aw values ranging from 0.960 to 0.980 and at 25°C regardless the solute. Also, strain K growth appears to be highly sensitive to the decrease of aw of the medium and of incubation temperature. These results are in agreement with those reported by Teixidõ et al. (1998a; 1998b) on C. sake. All solute models have a coefficient R2 close to 1.00, which suggests a higher part of variation explained by the studied factors (temperature and aw) involved in models conception (Box et al., 1987). In order to select the best predictor solute model among the designed ones, some statistical and mathematical criteria such as RMSE, lack of fit and bias and accuracy factors were evaluated. These parameters were largely used in predictive microbiology for internal statistical validation of the models (Ratkowsky, 2003; Panagou et al., 2003; Dantigny et al., 2005; Samapundo et al., 2005; Lahlali et al., 2007). Quadratic models based on sorbitol and glucose yielded small values of RMSE and possessed a no significant lack of fit test at P < 0.05. Also, both models had a bias and accuracy factors close to unit. Consequently, they are considered the best predictors models of the in vitro growth of strain K within the limits of experiments. Moreover, a good adjustment between observed and predicted values was observed with both selected models. The modelling part of our work confirmed previous studies on food spoilage microorganism indicating that aw has a greater effect than that of temperature (Samapundo et al., 2005; Lahlali et al., 2007). In the present work, we also found that the logarithm transformation, usually used to fit the maximum specific growth of bacteria, appears to be very adequate to describe strain K growth. For moulds, however, radial growth rate was adequately fitted by the square root transformation (Dantigny et al., 2005; Lahlali et al., 2006).
32Recently, we performed similar studies on the main pathogenic targets of our antagonistic strain K: B. cinerea (Lahlali et al., 2007), P. expansum (Lahlali et al., 2005), P. digitatum and P. italicum (Lahlali et al., 2006). Until now, little attention has been paid to the impact of environmental factors like temperature and aw on the growth of both the antagonist and its pathogenic targets in order to compare their ecological fitness. According to this work and to our previous ones, it seems that strain K had roughly the same ecological niche as the above-mentioned wound pathogens of apple and citrus fruits. The optimal range of temperature and aw may be respectively 20-25°C and 0.960-0.980, except for B. cinerea (0.981-0.987). The minimal temperature for growth may be 1°C for strain K and 0°C for the pathogens. The minimal aw allowing growth depends on the nature of solutes: 0.930 (NaCl) and 0.890 (non-ionic solutes), except for P. italicum, 0.960 (NaCl) and for P. digitatum and B. cinerea, 0.910 (non-ionic solutes). The main difference between strain K and its targets was the time required to start growth, i.e. lag phase. This phase was higher for strain K compared to wound pathogens, especially Penicillium sp. at low aw and low temperatures. Such difference supports the necessity to apply strain K as soon as possible after harvest in order to pre-colonize wounded fruits before the arrival of pathogen conidia.
5. Conclusion
33The present study has determined the range of temperature and aw within which strain K may proliferate in vitro. We have shown that this yeast may tolerate a broad range of these environmental factors and may occupy the same ecological niche as its pathogenic targets. We have to emphasize however that these conclusions are drawn from data obtained in in vitro conditions and any extrapolation to in vivo or natural conditions may be hazardous because of the involvement of other factors not considered here.
34Acknowledgements
35This research work received a grant from the " Agence Universitaire de la Francophonie " and from the Plant Pathology Unit of Gembloux Agricultural University (Belgium) for which the authors are grateful.
Bibliographie
Box G.E.P. & Draper N.R., 1987. Least squares for response surface work. In: Empirical model building and response surfaces. New York, USA: John Wiley, 34-103.
Costa E., Teixidõ N., Delgado J. & Vinas I., 2002. Water activity, temperature, and pH effects on growth of the biocontrol agent Pantoea agglomerans CPA-2. Can. J. Microbiol., 48, 1082-1088.
Dantigny P. et al., 2005. Modelling the effect of ethanol on growth rate of food spoilage moulds. Int. J. Food Microbiol., 98, 261-269.
De Clercq D. et al., 2003. Development of a SCAR marker and a semi-selective medium for specific quantification of Pichia anomala strain K on apple fruit surfaces. Postharvest Biol. Technol., 29, 237-247.
Fredlund E. et al., 2002. Physiological characteristics of biocontrol yeast Pichia anomala J121. FEMS Yeast Res., 2, 395-402.
Friel D., Gomez Pessoa N.M., Vandenbol M. & Jijakli H.M., 2007. Separate and combined disruptions of two exo-β-1,3-glucanase genes decrease the efficiency of Pichia anomala (strain K) biocontrol against Botrytis cinerea on apples. Mol. Plant-Microbe Interactions, 20(4), 371-379.
Jijakli M.H. & Lepoivre P., 1993. Biological control of post-harvest Botrytis cinerea and Penicillium on apples. IOBC/WPRS Bull., 16, 106-110.
Jijakli M.H., Lepoivre P., Tossut P. & Thonard P., 1993. Biological control of Botrytis cinerea and Penicillium sp. on post-harvest apples by two antagonistic yeasts. Meded. Fac. Landbouwkd. Toegepaste Biol. Wet. Univ. Gent, 58, 1349-1358.
Jijakli M.H. & Lepoivre P., 1998. Characterization of an exo-β-1.3-glucanase produced by Pichia anomala strain K, antagonist of Botrytis cinerea on apples. Phytopathology, 88, 335-343.
Jijakli M.H., De Clercq D., Dickburt C. & Lepoivre P., 2002. Pre- and post-harvest practical application of Pichia anomala strain K, β-1,3-glucans and calcium chloride on apples: Two years of monitoring and efficacy. IOBC/WPRS Bull., 25, 29-32.
Lahlali R., Serrhini M.N. & Jijakli M.H., 2004. Efficacy assessment of Candida oleophila (strain O) and Pichia anomala (strain K) against major post-harvest diseases of citrus fruits in Morocco. Commun. Appl. Biol. Sci., 69, 601-609.
Lahlali R., Serrhini M.N. & Jijakli M.H., 2005. Studying and modelling the combined effect of water activity and temperature on growth rate of P. expansum. Int. J. Food Microbiol., 103, 315-322.
Lahlali R., Serrhini M.N., Friel D. & Jijakli M.H., 2006. In vitro effects of water activity, temperature and solutes on the growth rate of P. italicum Whmer and P. digitatum Sacc. J. Appl. Microbiol., 101, 628-636.
Lahlali R., Serrhini M.N., Friel D. & Jijakli M.H., 2007. Predictive modelling of temperature and water activity (solutes) on the in vitro radial growth of Botrytis cinerea Pers. Int. J. Food Microbiol., 114, 1-9.
Liew S.L., Ariff A.B., Raha A.R. & Ho Y.W., 2005. Optimization of medium composition for the production of a probiotic microorganism, Lactobacillus rhamnosus, using response surface methodology. Int. J. Food Microbiol., 102, 137-142.
Massart S. & Jijakli M.H., 2006. Identification of differentially expressed genes by cDNA-amplified fragment length polymorphism in the biocontrol agent Pichia anomala (strain Kh5). Phytopathology, 96, 80-86.
Myers R.H. & Montgomery D.C., 2002. Response surface methodology: process and product optimization using designed experiments. New York, USA: John Wiley & Sons Inc.
Panagou E.Z., Skandamis P.N. & Nychas G.-J.E., 2003. Modelling the combined effect of temperature, pH and aw on the growth rate of Monascus ruber, a heat-resistant fungus isolated from green table olives. J. Appl. Microbiol., 94, 146-156.
Pujol M. et al., 2003. Monitoring system for the biocontrol agent Pichia anomala strain K using quantitative competitive PCR-ELOSA. Plant Pathol., 53, 103-109.
Ratkowsky D.A., 2003. Model fitting and uncertainy. In: McKellar R.C. & Lu X., eds. Modeling microbial response in foods. Boca Raton, FL, USA: CRC Press, 151-196.
Ross T., 1996. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol., 81, 501-508.
Samapundo S. et al., 2005. Predictive modelling of the individual and combined effect of water activity and temperature on the radial growth of Fusarium verticilloides and F. Proliferatum on corn. Int. J. Food Microbiol., 105, 35-52.
Teixidõ N. et al., 1998a. Ecophysiological responses of the biocontrol yeast Candida sake to water, temperature and pH stress. J. Appl. Microbiol., 84, 192-200.
Teixidõ N., Vinãs I., Usall J. & Magan N., 1998b. Improving ecological fitness and environmental stress tolerance of the biocontrol yeast Candida sake by manipulation of intracellular sugar alcohol and sugar content. Mycol. Res., 102, 1409-1417.
Teixidõ N., Usall J. & Vinas I., 1999. Efficacy of pre-harvest and post-harvest Candida sake biocontrol treatments to prevent blue mould on apples during cold storage. Int. J. Food Microbiol., 50, 203-210.
Van Eck J.H., Prior B.A. & Brandt E.V., 1993. The water relations of growth and polyhydroxy alcohol production by ascomycetous yeasts. J. Gen. Microbiol., 139, 1047-1054.
Para citar este artículo
Acerca de: Rachid Lahlali
Gembloux Agricultural University – FUSAGx. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Mohammed Bajji
Gembloux Agricultural University – FUSAGx. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Mohamed Najib Serrhini
Ecole Nationale d’Agriculture de Meknès. Department of Phytopathology. BP S/40 50001. MA-Meknès (Morocco).
Acerca de: Mohamed Haïssam Jijakli
Gembloux Agricultural University – FUSAGx. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: jijakli.h@fsagx.ac.be






