- Accueil
- volume 13 (2009)
- numéro spécial
- Electronic nose for determination of aflatoxins in maize
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Electronic nose for determination of aflatoxins in maize

Abstract
The aim of this study was to evaluate the potential for use of an electronic nose for rapid identification of maize samples contaminated with aflatoxins. Principal component analysis (PCA) and linear discriminant analysis (LDA) were used to investigate whether the electronic nose was able to distinguish between contaminated and non-contaminated maize samples. The obtained results indicate that the electronic nose was able to distinguish between maize samples according to the presence and absence of total aflatoxins. LDA was 100% correct in making prediction. These results could be used in further studies aimed at development of a robust classification model, which could be useful to screen for aflatoxins at concentration limits proposed by the European legislation.
1Some preliminary results of this study were presented at the " 2° Congresso nazionale: Le micotossine nella filiera agro-alimentare, Roma, 16-18 ottobre 2006 ".
1. Introduction
2As a cereal crop, maize is one of the most important food and feed commodities. However, mycotoxin contamination of maize represents a widespread problem. In fact, maize can be easily contaminated by toxigenic mould such as Aspergillus and Fusarium species that are important either as plant pathogens in the field or as the source of mycotoxin contaminants during storage. Several issues are associated with grain moulds and their secondary metabolites in maize, i.e. mycotoxins, including lowered grain quality, adverse effects on human health, and on animal health and reproduction (Fink-Gremmels, 1999; Hussein et al., 2001). Although numerous toxic fungal metabolites can be found in maize, attention has focused on the few mycotoxins that occur with greater frequency such as aflatoxins that are a group of secondary metabolites produced by the Aspergillus flavus and Aspergillus parasiticus. Aflatoxins B1, B2, G1, and G2 are a principal public health concern because of their pivotal role in the occurrence of primary liver cancer. Since 1993, aflatoxin B1 has been classified by the International Agency for Research on Cancer as carcinogenic to humans (group 1) (IARC, 1993). The European Commission fixed maximum levels for aflatoxin B1 (5.0 μg.kg-1) and total (B1, B2, G1, G2) aflatoxins (10.0 μg.kg-1) in " maize to be subjected to sorting or other physical treatment before human consumption or use as an ingredient in foodstuffs " (Commission Regulation (EC) No 1881/2006). Due to the high economic and sanitary impact on food safety and human/animal health, control of mycotoxin contamination is a primary objective of producers, manufacturers, regulatory agencies and researchers. Rapid methods for the determination of mycotoxins in cereals are highly needed in order to prevent the entry of mycotoxins into the food chain and thereby mitigate the human and animal risk. An electronic nose (EN) may represent a promising analytical tool to be used for an early detection of mould spoilage in grain. The underlined hypothesis for the potential use of electronic nose is that the growth and the biochemical pattern of mycotoxin-producing fungi cause chemical changes in the composition of volatile compounds (Olsson et al., 2002). Volatiles can be used as taxonomic markers of mycotoxigenic and non-mycotoxigenic fungi species (Magan et al., 2000). Electronic noses consist of non-specific chemical detectors, which interact with different volatile molecules and provide an electronic signal that can be utilized effectively as a fingerprint of the volatile molecules associated with the product. Identification and quantification of the odors by means of a pattern recognition system is possible (Feast, 2001). In this context, recent studies have demonstrated EN capability in order to discriminate between non-infected and infected samples with different species or different strain of toxigenic fungi, through the production of volatile secondary metabolites and to demonstrate the variation of the metabolic pathway due to the contamination of grain (Keshri et al., 2000; Magan et al., 2000; Falasconi et al., 2005; Paolesse et al., 2006; Presicce et al., 2006; Sahgal et al., 2007). Multivariate analysis for the extraction of additional information from EN data and evaluation of association of fungal content with mycotoxins give promising results on the capability of this technique as tool and model for classification and quantification of mycotoxins. Thus, chemometric models applied to EN analysis enabled correct classification of contaminated maize and wheat samples with aflatoxins and deoxynilvalenol (DON), respectively (Tognon et al., 2005; Cheli et al., 2007; Dell’Orto et al., 2007). Volatile compounds analysis by EN was used to detect ochratoxin A and deoxynivalenol in barley (Olsson et al., 2002). In this work, the potential use of an electronic nose combined with a multivariate statistic for rapid identification of maize samples contaminated with aflatoxins were investigated.
2. Material and methods
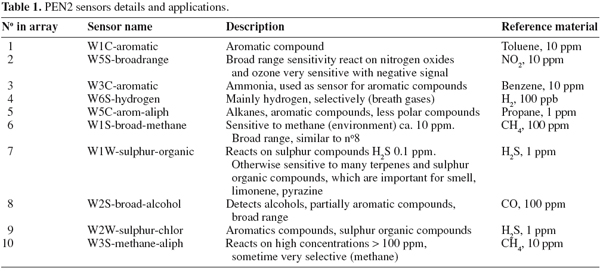
3Thirty maize meal samples containing aflatoxins were used in this study. Samples were analyzed by a commercial direct competitive enzyme-linked immunosorbent assay (ELISA) for the determination of total aflatoxins (B1, B2, G1 and G2) content. The assay is intended for use in grains, cereals, nuts, animal feeds and other commodities. Three aliquots of each sample were analyzed by the electronic nose PEN2 (Airsense Analytics GmbH, Schwerin, Germany) equipped with an enrichment and desorption unit (EDU2) and an automatic sampling device. Preliminarily, all the parameters involved in headspace sampling and analysis were optimized in order to obtain the best compromise between sensor responses and measurement time. Three grams of each sample were placed in airtight glass vials with a volume of 12 ml and the headspace inside was equilibrated for 24 h at room temperature. Afterwards, samples were exposed to a thermal desorption period, performed by EDU2 enricher/desorber unit (Air sense Analytics GmbH, Sherwin, Germany), and finally analyzed by the 10 MOS (Metal Oxide Semiconductor) sensors of the PEN2 electronic nose. Sensor details are reported in table 1. Electronic nose data were submitted to Principal Component Analysis (PCA) as explorative approach. Cross-validated Linear Discriminant Analysis (LDA) was adopted as classification model to make distinction from aflatoxins containing samples and aflatoxins free ones. Analysis was performed by SAS software (SAS Institute, 1999).
3. Results and discussion
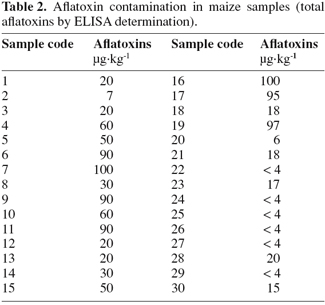
4Thirty maize samples were analyzed for aflatoxin content by ELISA and the results are given in table 2. In 24 maize samples aflatoxins concentration was in a range of 6 μg.kg-1-100 μg.kg-1, while 6 samples resulted under the detection limit of the assay (3 μg.kg-1).
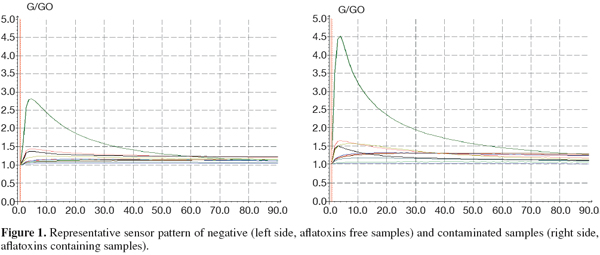
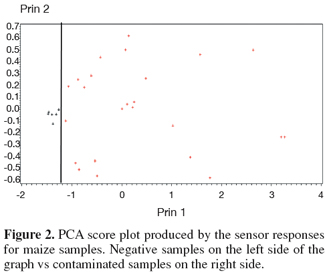
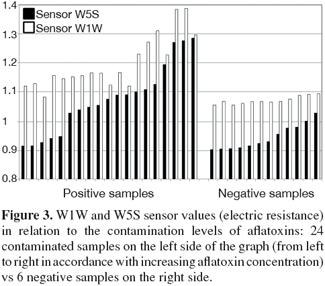
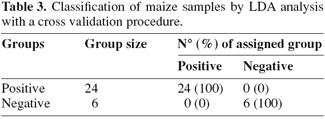
5The analysis of the maize samples by electronic nose showed that the sensor response curves stabilized after from 80 to 90 sec and therefore the signal of the sensors at 89 sec was used in analysis. Representative sensor pattern of negative and aflatoxin contaminated maize samples are presented in figure 1. PCA analysis applied to EN data showed that the first two components were able to explain 98.04% of total data variance. The corresponding plot of the two principal components is given in figure 2. A clear separation of the samples into two groups was found according to the presence and absence of total aflatoxins. The first group, in the left side of the principal component plot, corresponds to the negative samples. The second group, in the right side of the plot, corresponds to the contaminated maize samples, as determined by ELISA. Furthermore EN results showed that two MOS sensors (W1W - Sulphur-organic and W5S - Broadrange) were related with the concentration of aflatoxins quantified by ELISA (Figure 3). Signals from these two sensors gave higher response for aflatoxins containing than aflatoxins free maize samples, showing the best correlation with ELISA results, even if not direct indication of aflatoxins concentration can be provided. To classify the maize samples into contaminated and non-contaminated group, the LDA classification method was applied to the data set obtained by the sensor response (Table 3). LDA gave a recognition percentage of 100% of correct response in cross-validation prediction. Therefore LDA demonstrated the ability of EN in classification of positive samples from negative ones. These results are consistent with those reported by other authors, which used different cereals contaminated with other mycotoxins (Olsson et al., 2002; Tognon et al., 2005; Cheli et al., 2007; Dell’Orto et al., 2007).
6Results indicate that electronic nose may be successfully applied as rapid method for screening of maize samples contaminated with aflatoxins. Rapid methods usually refer to methods that take minutes to get a result. In our experimental conditions, sensors array response was available for statistics after approximately 30 min analysis. However for mycotoxin assessment, the speed of the method is not the only factor to be considered as other parameters are fundamental, such as user friendliness, reliability, non-destructivity, cost of analysis, possible use in a non-laboratory environment. After a setting up period, EN analysis can be carried out in completely automated way and no high level is required to perform the assay.
4. Conclusion
7The results of this viability study clearly indicate that it is possible to differentiate and classify maize samples contaminated and non-contaminated with aflatoxins by using an electronic nose equipped with 10 MOS. Despite the small number of samples, the electronic nose was able to detect a clear difference in volatile profile of maize in the presence and absence of aflatoxins using PCA analysis. By the use of LDA a correct classification (100%) of maize contaminated and non-contaminated with aflatoxins was achieved. Results indicate that electronic nose may be successfully applied as rapid and non-destructive method for screening of commodities contaminated with fungal toxins, in order to select samples that must undergo further accurate quantitative analysis. Further improvements of the model are needed in order to eliminate or minimize the component in the model not directly related to aflatoxins concentration, to evaluate the potentiality of classification below/above legal limits and maybe to develop robust regression models for prediction of aflatoxin content in maize samples. Electronic nose, which enables high sample throughput with no sample preparation, appears to be very promising as rapid and non invasive diagnostic tool for rapid screening of commodities contaminated with fungal toxins, in order to select samples which must undergo further accurate quantitative analysis. Potential exists for EN technology coupled with chemometric analysis and neural network system for the development of out of laboratory and on-line systems in order to monitor grain quality.
8Acknowledgements
9The Author would like to acknowledge COMAZOO s.c.a.r.l., Montichiari (Brescia, Italy) for providing maize samples and the analytical support in aflatoxin analysis. This study was supported by a FIRST 2006 Grant.
Bibliographie
Cheli F., Savoini G., Campagnoli A. & Dell’Orto V., 2007. Naso elettronico come "fit-for purpose approach" applicato alla determinazione di aflatossine nel mais. In: Miraglia M. & Brera C., eds. Rapporti ISTISAN 07/37. Roma: ISS, 253.
Commission Regulation (EC) No 1881/2006 of 19 December 2006. Off. J. Eur. Union, L 364, 20.12.2006, 5-24.
Dell’Orto V. et al., 2007. Impiego del naso elettronico abbinato a modelli chemometrici. In: Miraglia M. & Brera C., eds. Rapporti ISTISAN 07/37. Roma: ISS, 207.
Falasconi M. et al., 2005. Detection of toxigenic strains of Fusarium verticilloides in maize by electronic olfactory system. Sens. Actuators B, 108, 250-257.
Feast S., 2001. Potential application of electronic noses in cereals. Cereal Food World, 46(4), 159-161.
Fink-Gremmels J., 1999. Mycotoxins: their implications for human and animal health. Vet. Q., 21, 115-120.
Hussein H.S. & Brasel J.M., 2001. Toxicity, metabolism, and impact of mycotoxins on human and animals. Toxicology, 167, 101-134.
International Agency for Research on Cancer (IARC), 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monogr. Eval. Carcinog. Risk Chem. Hum., 56, 359-444.
Keshri G. & Magan N., 2000. Detection and differentiation between mycotoxigenic and non-mycotoxigenic strains of two Fusarium spp. using volatile production profiles and hydrolytic enzymes. J. Appl. Microbiol., 89(5), 825-833.
Magan N. & Evans P., 2000. Volatiles as an indicator of fungal activity and differentiation between species, and the potential use of electronic nose technology for early detection of grain spoilage. J. Stored Prod., 36, 319-340.
Olsson J., Borjesson T., Lundstedt T. & Schnuerer J., 2002. Detection and quantification of ochratoxin and deoxinivalenol in barley grain by GC-MS and electronic nose. Int. J. Food Microbiol., 72, 203-214.
Paolesse R. et al., 2006. Detection of fungal contamination of cereal grain samples by an electronic nose. Sens. Actuators B, 119, 425-430.
Presicce D.S. et al., 2006. Response evaluation of an E-nose towards contaminated wheat by Fusarium poae fungi. Sens. Actuators B, 118, 433-438.
Sahgal N., Needeman R., Cabanes F.J. & Magan N., 2007. Potential for detection and discrimination between mycotoxigenic and non-toxigenic moulds using volatile production pattern: a review. Food Addit. Contam., 24, 1161-1168.
SAS Institute, 1999. SAS/STAT. User’s Guide, Version 8. Cary, NC, U.S.A: SAS Institute.
Tognon G. et al., 2005. Implementation of the electronic nose for the identification of mycotoxins in durum wheat (Triticum durum). Vet. Res. Commun., 29(Suppl. 2), 391-393.
Pour citer cet article
A propos de : Federica Cheli
University of Milan. Veterinary Faculty. Department of Veterinary Science and Technology for Food Safety. Via Trentacoste, 2. I-20134 Milano (Italy). E-mail: federica.cheli@unimi.it
A propos de : Anna Campagnoli
University of Milan. Veterinary Faculty. Department of Veterinary Science and Technology for Food Safety. Via Trentacoste, 2. I-20134 Milano (Italy).
A propos de : Luciano Pinotti
University of Milan. Veterinary Faculty. Department of Veterinary Science and Technology for Food Safety. Via Trentacoste, 2. I-20134 Milano (Italy).
A propos de : Giovanni Savoini
University of Milan. Veterinary Faculty. Department of Veterinary Science and Technology for Food Safety. Via Trentacoste, 2. I-20134 Milano (Italy).
A propos de : Vittorio Dell’Orto
University of Milan. Veterinary Faculty. Department of Veterinary Science and Technology for Food Safety. Via Trentacoste, 2. I-20134 Milano (Italy).






