- Accueil
- volume 13 (2009)
- numéro 3
- Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): a review
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): a review

Notes de la rédaction
Received on November 19, 2008, accepted on March 31, 2009
Résumé
Synthèse bibliographique sur le comportement de recherche et de ponte des femelles de syrphes aphidiphages (Diptère: Syrphidae). Les syrphes aphidiphages utilisent deux mécanismes différents au cours de leur recherche et comportement de ponte, un mécanisme orienté visant à trouver une source de nourriture qui assure à la femelle d’avoir l’énergie nécessaire pour sa mobilité et la maturation de ses organes reproducteurs, et l’autre visant à trouver un site de ponte propice. Les femelles de syrphes prédateurs ont une forte mobilité qui leur permet de distribuer les œufs sur de larges territoires, et de localiser les colonies de pucerons plus tôt dans la saison que les autres prédateurs aphidiphages. Le résultat net est que la plupart des œufs de syrphe ont tendance à être déposés à proximité de colonies de pucerons. Cependant, le choix du site d’oviposition par les femelles peut être crucial pour la survie larvaire parce que les larves de syrphe ne peuvent pas se déplacer sur de longues distances pour la recherche de nourriture. C’est pourquoi les femelles gravides devraient montrer une préférence pour les sites présentant une grande valeur nutritive et un faible risque de prédation et de compétition. Pour les syrphes aphidiphages, plusieurs facteurs influencent le choix du site de ponte, parmi lesquels l’habitat, la plante hôte, l’espèce de puceron, la taille de la colonie de pucerons, les substances sémiochimiques émises par les pucerons et leur association avec les plantes-hôtes, la présence de compétiteurs intra- ou interspécifiques et l'âge de la femelle. Dans cette revue bibliographique, nous recensons les informations disponibles sur ces facteurs afin de comprendre les mécanismes de décision des femelles de syrphes prédateurs au cours du comportement de ponte, ce qui constitue une étape importante avant d’utiliser ces prédateurs dans la lutte biologique contre les pucerons.
Abstract
Aphidophagous hoverflies forage according two different host-finding mechanisms: they forage for suitable food sources (for their energy-expensive hovering flight, and for protein to mature their reproductive system), and for suitable oviposition sites. Syrphids are highly mobile, enabling them to lay eggs over large areas, and to locate aphid colonies earlier in the season than other aphidophaga. The result is that most syrphid eggs tend to be laid close to aphid colonies. The choice of oviposition sites may be crucial for offspring performance because the neonate larvae have limited dispersal ability. Selection of aphid patches should therefore reflect nutritional value, risk of predation and competition pressure. Several factors are known to affect the choice of oviposition site: habitat, host plant, aphid species, aphid availability, semiochemicals, the presence of intra- or interspecific competitors and female age. We review here the available information on these factors in order to understand the mechanisms of decision-making by syrphid females during their egg-laying behavior, a crucial aspect of their effective use in strategies of the biological control of aphids.
Table des matières
1. Introduction
1Although a good number of insects do not feed as adults, for the majority, most of their adult life is dedicated to actions related both to the acquisition of food and to reproduction, and crucial decisions must be taken concerning these two activities. Oviposition behavior is a vital component of many aspects of insect biology (e.g. population dynamics, life history and biological control of insect pests). One major aspect of oviposition behavior is host selection. Offspring are often obliged to feed on the host chosen by females during their egg-laying behavior. Optimality theory as applied to oviposition predicts that female choice should reflect a preference for oviposition sites with high expected fitness for their offspring, usually in the form of high nutritional value, low risk of predation and competition pressure (Mangel, 1987), good growth, survival, and future reproductive potential, etc.: eggs deposited in unsuitable hosts are likely to die or result in inferior adults (Nufio et al., 2004; Singer et al., 2004). This relationship is especially important for insect species where neonate offspring are relatively sessile and have limited mobility to forage (Thompson, 1988; Peckarsky et al., 2000).
2During foraging and oviposition behavior, entomophagous insects are confronted with a diversity of environmental situations in which they may adopt different behavioral strategies. By their actions, they can influence the structure and population dynamic of their hosts or prey and of other predators/parasitoids present in the same guild and of the overall community (Jervis et al., 1996). They can also mediate interactions between insect herbivores and their host plants, thereby constituting a selective factor on herbivore host plant preference (Price et al., 1980). Thus when an individual attempts to forage, it must decide where to feed or oviposit for potential prey or hosts, what type of prey or hosts to accept and, when to move to a new habitat (Barnard, 1983). The outcome of these decisions can greatly influence the survival and fitness of predators and parasitoids. In trying to understand what influences the decision processes of foraging insects, ecologists have increasingly turned to optimal foraging theory (Stephens et al., 1986; Scheirs et al., 2002).
3How do these ideas work out in the relationship between predatory hoverflies and their aphid prey? Aphids are considered to be major pests in most agricultural ecosystems (van Emden et al., 2007). They have distinctive characteristics that make them highly suitable in some ways and highly challenging in others as prey for insect predators. On the one hand, aphids have small and soft bodies, and their higher growth and development rates enable them to occur at high densities. On the other, aphid colonies are ephemeral and unpredictable over both space and times, requiring special adaptations to be able to take advantage of them. Aphidophagous predators such as predatory hoverflies therefore need appropriate tactics and strategies to locate aphid infestation quickly, and to exploit the opportunities and overcome the challenges posed by this particular group of prey.
4Aphidophagous hoverflies have long been recognized as important aphid natural enemies (Chambers, 1988). The larvae of species such as Episyrphus balteatus DeGeer are predators on more than 100 species of aphids worldwide (Sadeghi et al., 2000b). Because of their high reproductive rates and voracities (Chambers et al., 1986; Poehling, 1988; Gilbert, 1993; Tenhumberg et al., 1995) and suitable oviposition behavior (Kan et al., 1986; Kan, 1988a; Sadeghi, 2000), they can have a significant impact in the suppression of aphid population growth and abundance, but good evidence is rare.
5The ability to detect aphids and oviposit close to aphid colonies plays a major role in the effectiveness of predatory hoverflies. A high rate of prey search is considered to be one of the most desirable attributes of biological control agents (Jervis et al., 1996; Murdoch et al., 1996). The relatively sessile nature of neonate syrphid larvae does not allow them to exploit aphid prey on different host plants (Chandler, 1969). Moreover, they do not perceive aphids before contact or only at very short distance (Bargen et al., 1998). The female’s ability to find and oviposit within the future foraging range of its progeny is therefore a critical determinant of potential biocontrol performance.
6The reason for the poor progress in developing a foraging theory for insect predators is that most studies have concentrated on the most voracious stage, the larva, rather than the adult. Thus for a complete understanding of insect predator-prey dynamics, it is necessary to determine the behavior that maximizes predator fitness, and this involves studying the foraging and oviposition behavior of female predators (Ferran et al., 1993). Gilbert (1993) has described the importance of predatory hoverflies and their natural history, biology and ecology. Recent reviews have largely discussed information on prey-predatory hoverflies interactions (Rojo et al., 2003), and the degree of specialization of aphidophagous syrphids (Gilbert, 2005). However, there is no review on the searching and oviposition behavior of aphidophagous hoverflies. Here, we summarize available information about the foraging and oviposition behavior of aphidophagous hoverflies, and the behavioral mechanisms of decision-making by syrphid female during their egg-laying behavior.
2. Factors influencing foraging and oviposition behavior of aphidophagous hoverflies
7The choice of habitat, host plant, aphid species, aphid colony size, visual and chemical stimuli, oviposition site, must all be considered during searching and egg-laying behavior of syrphid predators. This review will discuss largely the main factors influencing searching and ovipostion behavior of predatory hoverflies.
2.1. Habitat
8Searching for resources is one of the most important activities of gravid female insects (Bell, 1990). According to classical foraging theories, foragers maximize energetic gains by selectively exploiting patches rich in resources and by minimizing foraging time in poor patches (Stephens et al., 1986). Adults predatory syrphids are frequent flower visitors since they feed only on nectar and pollen: nectar serves principally as a source of energy to sustain their strong flight and to extend longevity, while pollen allows maturation of the reproductive system in both sexes (Schneider, 1948; 1969; Gilbert, 1981; Chambers, 1988). Additionally, flowers can also provide optical cues such as size, color, shape and scent influencing the searching behavior of syrphid predators (Kan, 1988a; 1988b; Haslett, 1989; Lunau, 1993; Sutherland et al., 1999). Floral cues are important signals in helping foraging hoverflies to find and select a floral feeding site, and the foraging activity of hoverflies in crops can be enhanced by a continuous supply of flowers with easily available pollen sources such as Asteraceae and Umbelliferae in field margins (Ruppert et al., 1991; Colley et al., 2000; Morris et al., 2000). For example, cereal fields are usually characterized by shortage of food for flower visitors, and alternative agricultural practices that favor wild flowers (i.e. set-aside, herbicide-free buffer zones, conservation strips) may lead to improved attraction of adult hoverflies (van Emden, 2003). Ambrosino (2006) showed that the presence of floral resources in Oregon broccoli fields enhanced the predatory potential of hoverflies on aphids. This seems to indicate that young syrphid females probably focus on flower foraging during the first week after emergence before switching to searching for aphids and oviposition sites. Thereafter, they will travel between floral and aphid patches to maintain egg production (van Rijn et al., 2006).
9The important second stage of the foraging behavior of syrphid females is to locate a suitable oviposition site. Hoverfly females are known to exhibit high mobility, enabling them to distribute eggs over large areas (Schneider, 1948; Chambers, 1988), and to locate aphid colonies earlier in the season than other aphidophaga (Hagen et al., 1968; Horn, 1981; Dixon, 2000). The key questions facing searching hoverfly females can be summed up simply:
10- when and where in the course of their search should they oviposit?
11- what are the cues and behavioral mechanisms involved in choosing their oviposition site?
12An elegantly simple model of hoverfly oviposition behavior emerged during the 1960s. Female aphidophagous hoverflies are highly mobile and their ability to select a potentially successful oviposition site therefore merely depends upon the availability of aphid-infested plants (Dixon, 1959; Chandler, 1968a; 1968b; Schneider, 1969). This model has served since as the standard general explanation for the degree of discrimination exhibited by syrphid females in selecting an oviposition site, and the various stimuli which induce oviposition responses in particular hoverfly species. Laboratory and field experiments and observations over the past several decades have generally supported this model. The net result of searching and oviposition behavior is that syrphid eggs tend to be laid close to aphid colonies (Chandler, 1968a; 1968b; Chambers, 1988; Dixon, 2000; Scholz et al., 2000; Ambrosino et al., 2007), enabling the emerging young larvae to locate the food sources immediately. Syrphid predators may conduct an intensified local search after locating aphids. Field and laboratory observations have shown that a female approaches an infested plant in a straight line, and then hovers, moving slowly around plants until it reaches a position opposite and close to an aphid colony, where it hovers a short time before alighting with the ovipositor extended. Finally, the ovipositor is bent ventrally and drawn over the substrate and an egg is laid (Dixon, 1959; Schneider, 1969; Scholz et al., 2000; Sutherland et al., 2001; Almohamad et al., 2008c).
13Oviposition sites are very variable, and are related both to the number and location of eggs deposited. Syrphid eggs are often laid singly, either close to or within aphid colonies, although some species lay eggs in batches distant from the colony or even on uninfested plants (Chambers, 1988). In the latter case, young larvae may survive by cannibalizing conspecific eggs.
14In the field, aphids of different species have been found with syrphid eggs actually attached to them, which demonstrates how close eggs can be laid to aphids (Dixon, 1959). In certain melanostomine and all Platycheirus species except Platycheirus scutatus (Meigen), eggs are equally often deposited on plants without as on those with aphids (Chandler, 1968a; Gilbert, 1986), and eggs of the latter species are laid in batches of two to four, instead of singly (Gilbert, 1986). The net effect is that these species exploit small aphid colonies that do not attract species such as Syrphus ribesii L. and Eupeodes (Metasyrphus) corollae Fabr.: the first larva to hatch can cannibalize the others and then search for aphids, and females lay in advance of aphid attack. In species such as Pipizella varipes Meig., females select as oviposition sites the base of stems of Pastinaca sativa L. (Apiaceae) plants, the roots of which are infested with aphids just below soil level, although the aphids are not visible to ovipositing flies (Dixon, 1959). Choice of oviposition site varies markedly according to hoverfly species, even in similar conditions: female Eupeodes luniger Meigen, laid over 50% of their eggs touching aphids (Brevicoryne brassicae L.) on Brussels sprouts (Brassica oleracea L. cv. 'gemmifera') and less than 1% on uninfested plants, whereas female Platycheirus manicatus Meig. laid less than 5% touching aphids and over 50% on uninfested plants (Chandler, 1968c). Epistrophella emarginata (Say) oviposits on the petioles of leaves and Syrphus knabi Shannon usually on the upper surface of the lamina (Curran, 1925). Laboratory observations have also demonstrated that E. balteatus females have a distinct preference of position to lay their eggs on Vicia faba L. plants: 91% were found on the bottom side of infested leaflets, 2% on the upper side, and 7% of eggs mainly on the top of plant (Scholz et al., 2000).
2.2. Host plant
15According to Cortesero et al. (2000), host plant effects on the efficiency of insect natural enemies can occur in various ways, such as by mediating host/prey accessibility and availability, providing host/prey finding cues, influencing host/prey suitability and providing supplemental food resources.
16Several studies have shown that host plant factors play important roles in the selection of oviposition site by aphidophagous hoverflies (Dixon, 1959; Chandler, 1968b; Sanders, 1983a; 1983b; Sadeghi et al., 2000a; Almohamad et al., 2007a). Most syrphid species are known to lay their eggs close to aphid-infested plants, whereas other species (i.e. Melanostoma spp. and Platycheirus species) tend to lay their eggs freely on uninfested plants. Thus, the existence of species that oviposit in the absence of aphids may be valuable in biological control, and provides a useful tool for the investigation of non-aphid oviposition stimuli. Some host-plant factors affecting oviposition were clearly shown in the study of Chandler (1968b): plant species, plant appearance and substrate of plant surface were all important. Species such as Platycheirus spp. preferred waxy over glossy varieties of Brussels sprouts (Brassica oleracea) if they were uninfested, but this preference was much less marked if the plants were infested; Eupeodes spp. preferred glossy plants when both types were uninfested, but not if the plants were infested; Melanostoma spp. preferred waxy plants irrespective of the presence or absence of aphids; Sphaerophoria spp. responded more like Platycheirus than Eupeodes. Other species could seemingly discriminate and select plants on the basis of their appearance, although different contact stimuli may also have mediated oviposition. The nature of the plant surface substrate affects the number of eggs laid per patch (batch size) in species of Melanostoma and Platycheirus (Chandler, 1968b).
17It has been also suggested that there is a balance between aphid and host plant factors governing syrphid oviposition. If the aphid stimulus is reduced, by scarcity or absence, or if the female is old, host plant factors become more important (Dixon, 1959; Chandler, 1967; 1968b; Schneider, 1969; Sadeghi et al., 2000c). Evidence for this is discussed in several studies. For example, Platycheirus manicatas females oviposit selectively on healthy Brussels sprouts or bean plants adjoining those heavily infested with aphids (cabbage aphid Brevicoryne brassicae L., and bean aphid Aphis fabae Scopoli, respectively) (Chandler, 1968b). Oviposition responses to host plants with low aphid infestations may be especially good at keeping aphids at low densities.
18Plant chemistry (allelochemicals or secondary plant metabolites) can also affect the foraging and oviposition behavior. Studies in the literature have largely focused on host plant chemistry effects on the suitability of aphid prey for overall performance and subsequent fecundity, but few studies have compared the performance of syrphid larvae feeding on one aphid species but from different host plants (Schmutterer, 1972; Rüzicka, 1975; Sadeghi et al., 2000b; Hindayana, 2001; Vanhaelen et al., 2001; 2002; Almohamad et al., 2007a). Oviposition responses to different host plants associated with one aphid species have received little attention, with only two studies. Vanhaelen et al. (2001) demonstrated that E. balteatus females significantly prefer to oviposit on white mustard plants (Sinapis alba L. containing high glucosinolate (GLS) levels) rather than on oilseed rape plants (Brassica napus L. containing low GLS levels), both of which were infested with the same aphid species (Myzus persicae Sulzer). GLS compounds are well known allelochemicals of the Brassicaceae, with a strong influence on both the phytophages and entomophages of the community (Francis et al., 2001). Almohamad et al. (2007a) recently showed that potato plants Solanum tuberosum L. were preferred by ovipositing E. balteatus females over Black Nightshade plants Solanum nigrum L. infested with the same aphid species (M. persicae). The importance of volatile compounds (e.g. E-(β)-farnesene: EβF) emitted from these aphid-host plant combinations may explain these oviposition preferences. Further investigations are needed to understand better oviposition activity in relation to plant allelochemicals, and the consequent effect of this on offspring performance.
19Some physical plant characteristics (e.g. presence of trichomes) have also been shown to influence the acceptance of aphid/host plant as oviposition site. Field observations have demonstrated that that nettle (Urtica dioica L.) infested with Microlophium carnosum Buckton was poorly accepted by ovipositing E. balteatus females (Sadeghi et al., 2000a), but it is unclear whether physical aspects of the plant were influential. A variety of factors affect the evolved rank hierarchy of suitability: the host plant as a habitat for larvae; the intrinsic suitability of the aphid as food (which may vary with host plant: Hodek, 1993). Thus the survival of E. balteatus larvae on nettles in nature may be low because of the physical effects of this host plant itself on the larvae, which must be able to move on its surface. Nettle aphids are also known to be especially adept at avoiding capture (Sadeghi et al., 2000a). All these reasons could underlie the fact that the combination M. carnosum / U. dioica was the least preferred aphid by E. balteatus.
20Other host plant factors (e.g. floral characters, color) are found to have important impact on searching and oviposition behavior. Several researches conducted in both North America and Europe indicate that aphidophagous species such as E. balteatus exhibit considerable positive and negative selectivity for native flowering species (Cowgill et al., 1993; Branquart et al., 2000; Fitzgerald et al., 2004). Branquart et al. (2000) showed that adults have a strong flower preference for pollen and nectar produced by native plants with large inflorescences and flat corollas (i.e. Apiaceae, Asteraceae, Ranunculaceae and Rosaceae). These authors also suggested that several polyphagous species such as E. balteatus, Melanostoma mellinum L., Eupeodes corollae, Sphaerophoria scripta L. and Platycheirus spp. can access pollen and nectar in flowers with small tubular corollas, an important asset for colonizing open and ephemeral habitats. Indeed, flowers are considered to have important effects on distribution and oviposition in neighbouring aphid – infested plants. For example, work from New Zealand has shown that syrphids move into adjacent crops (brassica crops) from rich floral patches (of Phacelia tanacetifolia Benth.: Hydrophyllaceae), where they oviposit, and the subsequent larvae can cause a decrease in aphid populations (White et al., 1995). MacLeod (1999) also demonstrated that species such as E. balteatus were significantly more abundant on arable field margins with rich floral resources than those with no additional floral resources; and yet another study showed that the presence of floral resources in Oregon broccoli fields enhanced predatory potential (Ambrosino, 2006). As result, managing hedgerows and field margins to create florally rich habitats to attract and retain syrphids is an option farmers can consider to encourage them into fields as part of a system of integrated pest management.
21In conclusion of this section, host plant factors are likely to be very important in the foraging and oviposition behavior of aphidophagous hoverflies.
2.3. Aphid species
22Aphid species differ in their profitability and suitability for insect predators. Hodek (1993) has distinguished several types of prey for aphidophagous predators. One such division was suitable vs unsuitable prey. Suitable prey could function either as essential, enabling larval development and egg production, or as alternative, enabling just survival or accumulation of energetic reserves for overwintering (Hodek et al., 1996). Unsuitable prey, which can include toxic species, can be either rejected or accepted. This classification arose from finding that several aphid species were accepted but unsuitable, (i.e. they were inadequate for larval development or oviposition). Michaud (2005) also stressed that the suitability of prey sometimes differed for larval development and adult reproduction.
23Aphidophagous hoverflies are likely to encounter diverse aphid species when foraging for an oviposition site. Selection among aphid species should reflect a preference for high expected offspring performance (Scheirs et al., 2002). Ovipositing females do appear to discriminate among different food types, and appear to have a rank order hierarchy of preference for aphid prey species or aphid-host plant combinations. Females become less selective with increasing age, but the rank hierarchy is preserved (Sadeghi et al., 2000a; 2000b). The hierarchy-threshold model (Courtney et al., 1989; Sadeghi et al., 2000a; 2000b) can be applied to a gravid female syrphid searching among a set of possible prey (A to E) (Figure 1). In this model, females possess an intrinsic evolved degree of preference for each food type, producing a rank order of preference among prey that does not change throughout an individual’s lifetime; individuals accepting a low-ranking food type will also accept all higher ranking types; and actual acceptance of an encountered type depends on whether the stimulus of that food type exceeds the current motivational threshold (which can vary with factors such as age or egg-load). The model is particularly useful because it synthesizes two disparate strands of adaptive explanations of specialization (Berenbaum, 1990), one involving slow processes of evolutionary change based usually on various sorts of trade-off and coevolution, and the other invoking optimal foraging and concentrating on the behavioral flexibility of the individual in response to variation in ecological conditions, acting via “rules of thumb”.
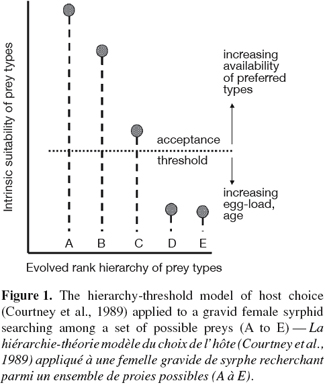
24Field observations have shown that gravid females of generalists such as E. balteatus and S. ribesii exhibit significant preferences in the distribution of their eggs among various aphids in natural habitats (Budenberg et al., 1992; Sadeghi et al., 2000a; Almohamad et al., 2007a). Even greater selectivity may reasonably be predicted in specialists such as Xanthandrus (Lyon, 1968) and Platycheirus fulviventris Macquart (Rotheray et al., 1987). Because most species are oligophagous (Gilbert et al., 1990), predatory hoverflies are clearly like insect herbivores in that most species are relatively specialized (Schoonhoven et al., 1998). However, it has been also reported that there are varying degrees of specialization among individuals within the populations of at least one generalist, E. balteatus (Sadeghi et al., 1999). Some individual females differ from others in their preferences, and at the individual level there appeared to be life-history trade-offs in performance with these preferences. Thus part of the female population of E. balteatus seems to be specialized to particular aphids as prey; the rest of the population may also be specialized, but to aphid species not tested in the study, or may consist of truly generalized individual females.
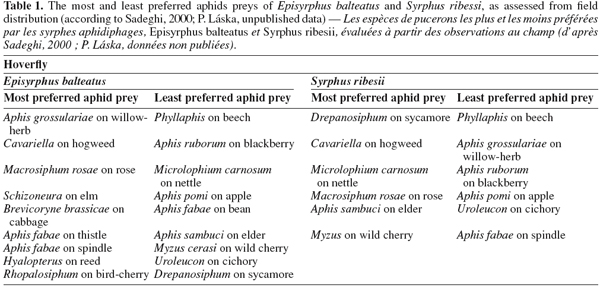
25There are rather few studies that investigate oviposition preferences in response to different aphid species. A good example of such a study is that of Budenberg et al. (1992), who found that E. balteatus females lay their eggs in response to some aphid species such as rose-grain aphid Metopolophium dirhodum Walker and pea aphid Acyrthosiphon pisum Harris, but not to others such as the nettle aphid M. carnosum. This preference is expressed in response to honeydew alone. In another study, A. pisum and Macrosiphum rosae L. were clearly more preferred hosts of E. balteatus and S. ribesii females, and M. carnosum and Aphis ruborum (blackberry aphid) were the least preferred (Sadeghi et al., 2000a), results consistent with the field distribution of larvae (Table 1). In the study of Almohamad et al. (2007a), the foraging and oviposition behavior of E. balteatus females was evaluated in response to different aphid species (A. pisum, A. fabae and Megoura viciae Buckton) infesting one host plant (V. faba). A. pisum and M. viciae were equally attractive, whereas A. fabae was less attractive.
26In addition to their ability to reduce aphid abundance, aphid predators can also cause changes in prey characteristics by inducing defensive responses that help prey avoid being consumed; these often come at a cost to some other aspect of prey biology. Aphids possess a range of defenses against predators, including morphological, social, chemical, and behavioral defenses (Losey et al., 1998). These behavioral responses may affect suitability for syrphid females. A beautiful work on aphid defense against syrphid predators is a study by Shibao (1998). He clearly demonstrated that gravid female Eupeodes confrater (Wiedemann) adjusted their oviposition behavior in response to soldier density in its prey, the bamboo aphid Pseudoregma bambucicola (Takahashi). This aphid has huge colonies and a soldier caste for colony defense who pierce the eggs and neonate larvae of aphid predators. A gravid female of E. confrater circles the colony carefully: if she finds soldiers present, then she lays a batch of eggs on a spider’s web nearby, up to 1 m away. The first larva to emerge cannibalizes the rest of the batch to provide the energy to crawl to the colony: having a meal or two before meeting a soldier will make all the difference between surviving and succumbing. If the gravid female does not encounter any soldiers in her search, then she lays single eggs in the colony, as is normal for most aphidophagous syrphids. Another species, Eupeodes hakiensis (Matsumura), has adapted to dealing with the aggressive soldier instars of their Ceratovacuna aphid prey by evolving a hard impenetrable eggshell, and larval behavior that leads them to forage only at the edge of the colony, moving away when not feeding (Mizuno et al., 1997).
27Aphid size may also be an important characteristic in determining oviposition choice. Kan (1988a; 1988b) noted that aphid size is critical for the newly eclosed first-instar larva, and part of the reason for ovipositing in young colonies may be to make available small and tender aphids for the first few meals of the first instar. However, small size is not good in the longer term, over the entire developmental period: the higher mortality and longer development time for E. balteatus larvae feeding on apple aphid (Aphis pomi) may be related to the small size of individuals of this aphid, which imposes extra capture costs on older larvae and makes it a least-preferred aphid by hoverfly females (Table 1). Further consequence is that normal colony size and density of aphid species may be one reason why blackberry aphids (which often occur at very low densities) are low in the oviposition preference hierarchy (Table 1). Newly emerged larvae must have enough food to develop successfully, and periods of food deprivation during the larval stage can result in dwarfed adults (Rüzicka et al., 1976) with lowered fecundity or even sterility (Cornelius et al., 1980). Michaud et al. (2001) showed that Pseudodorus clavatus F. could hamper the population growth of the brown citrus aphid by decreasing production of the winged form.
2.4. Prey availability
28A predator that responds numerically to increasing aphid numbers and oviposits accordingly is thought to be ideal for suppressing pest populations before they reach damaging levels (Murdoch et al., 1996). Predatory hoverfly larvae exploit temporary aphid colonies as food resources in crops and on a wide range of herbaceous plants (Salveter, 1996). Aphid colonies are ephemeral, patchily distributed resources (Dixon, 1959; Kan, 1988a; 1988b), suddenly disappearing due to predation, parasitism, fungal epizootics, declining host-plant quality, changes in weather, or dispersal. Syrphid larvae therefore face a potentially unstable food supply, and hence it may be important to be able to locate aphid infestations quickly. According to Horn (1981), adult syrphids appear to be especially adept at locating aphid colonies because of their strong flight and ability to hover and inspect foliage for aphids. For example, E. balteatus females are able to find even small and isolated aphid colonies (Itô et al., 1977). High levels of oviposition can therefore occur relatively early, and large numbers of larvae can hatch before aphid populations have attained rapid growth rates (Tenhumberg et al., 1992; Ambrosino et al., 2007).
29Several studies have demonstrated that oviposition varies with the size of aphid infestations (Dixon, 1959; Chandler, 1968b; Kan, 1988b; Bargen et al., 1998; Scholz et al., 2000; Belliure et al., 2001; Sutherland et al., 2001; Almohamad et al., 2006; Ambrosino et al., 2007). This behavior has been attributed to a "buy futures" tactic of oviposition whereby foraging females are selecting aphid colony sizes based on their future potential rather than their immediate value (Kan, 1988b). Different species have indeed different optimum aphid population sizes for oviposition. A very good example is the study of Chandler (1968b) on the relation between aphid infestation and syrphid oviposition in field. He found that Platycheirus manicatus preferred about 100 aphids per plant, Platycheirus scutatus about 1,000, S. ribesii about 2,000, whereas Sphaerophoria scripta had no obvious preference. Other species such as Eupeodes luniger preferred small numbers of large aggregates to a large number of smaller ones, whereas E. balteatus preferred the opposite. Other studies have also demonstrated that E. balteatus prefers smaller aphid colonies, or aphid colonies with a high proportion of early aphid instars (Kan et al., 1986; Kan, 1988a; 1988b; Hemptinne et al., 1993). In the study of Ambrosino et al. (2007), the numbers of eggs were very low on broccoli plants with fewer than 50 aphids, and none were seen on leaves that had more than 400 aphids. Thus the tendency of the different species to select aphid populations of different sizes and distribute their eggs accordingly could reflect adaptations that reduce interspecific competition.
30There is no evidence of a peak in hoverfly oviposition at higher aphid numbers at the plant level. Other factors may influence this, for example the quantity of volatile compounds emitted from aphids (such as EβF: Almohamad et al., 2008b), and their liquid secretions (such as honeydew: Budenberg et al., 1992; Sutherland et al., 2001). EβF has an attractive effect on E. balteatus females and acts as an oviposition stimulant (Almohamad et al., 2008c), and honeydew acts as a contact kairomone and oviposition stimulant (Budenberg et al., 1992; Sutherland et al., 2001). Sutherland et al. (2001) also reported that females demonstrated more gustatory and oviposition responses to honeydew-treated areas. With aphid alarm pheromone (EβF), honeydew might also provide females with information about aphid colony size.
31Syrphid eggs and larvae are also more exposed to cannibalism and/or the risk of starvation if the aphid colony on which they are feeding disappears before they complete their development. This could happen when too many eggs are laid in the colony or too late in the development of the colony, i.e. when the aphids are preparing to disperse. Evaluation of the aphid colony by females, and directed prey location by larvae, would therefore be favored, resulting in lower larval mortality and subsequently higher reproductive success (Kindlmann et al., 1993; Almohamad et al., 2007b). Thus females manifest evolved behavioral mechanisms in response to aphid colony size that enables them to forage for an oviposition site that will support the development of their offspring.
2.5. Semiochemicals
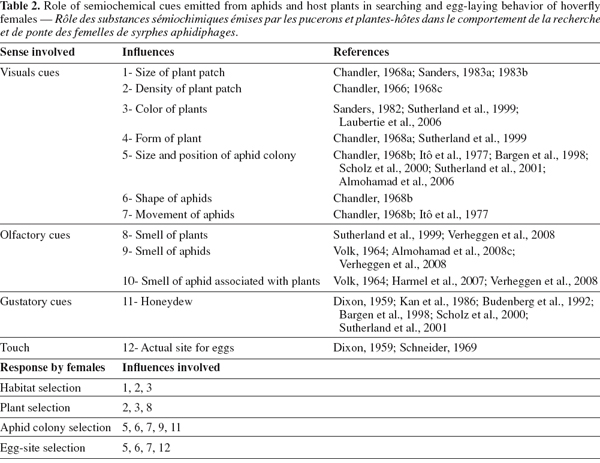
32Choice of oviposition site is described as a process of recognition, often depending on the development phase of the searching insect and on the cues available (Schoonhoven et al., 1998). Host choice involves a number of actions, from initial perception of the host, through testing stages by different sensory systems, until the final decision of rejection, or acceptance: i.e. laying eggs (Bernays, 1996), all of which may involve semiochemicals mediating these actions (Dicke, 1999; Ninkovic et al., 2001; Harmel et al., 2007; Verheggen et al., 2008). These chemical signals emitted from plants or aphid host plant can be considered a part of the indirect defense of plants against herbivores (Harmel et al., 2007). Studies in the literature have largely focused on the role of semiochemicals emitted by aphid prey or associations with their host plants on various aphid natural enemies, including ladybeetles, and parasitic hymenoptera (Du et al., 1998; Francis et al. 2004). Little information is available about the role of semiochemicals in searching behavior and acceptance of oviposition sites by predatory hoverflies (Laubertie et al., 2006; Almohamad et al., 2008a; Verheggen et al., 2008).
33Field and laboratory experiments have shown that females are able to find even small and isolated aphid colonies (Chambers, 1991). Behavioral observations show that they do not approach aphid-infested plants directly, but slowly scan close to non-infested plants and non-infested parts of infested plants in search for aphids, and only remain stationary directly front of aphid-infested plants (Dixon, 1959; Scholz et al., 2000). This suggests that foraging behavior is not simply a random search for prey, but is instead guided by specific volatiles or substrate-linked semiochemicals. Thus oviposition is almost certainly elicited by both olfactory and visual cues. Female Eupeodes corollae and E. balteatus respond positively to stimuli originating from aphid honeydew, and probably also to ones from aphid siphunculus secretion. Such stimuli may act both as long-distance kairomones and oviposition stimuli after the location of a plant with prey (Volk, 1964; Budenberg et al., 1992; Bargen et al., 1998; Shonouda et al., 1998; Sutherland et al., 2001). Additionally, female E. corollae respond to structural characters of plants, having a preference for vertical rather than horizontal surfaces and preferring darker to lighter strips (Sanders, 1983a; Chambers, 1988). E. balteatus females also respond to leaf color (Sutherland et al., 2001).
34In aphidophagous hoverflies, it has been suggested that there are four stages in the location and acceptance of an oviposition site. During these stages, a range of different ovipositional cues (visual, auditory, olfactory and gustatory) are used (Table 2). In the first stage of searching behavior, females use long-range optical cues, including the size, density and color of the stand of vegetation, to help them find suitable oviposition sites (Chandler, 1966; Sanders, 1982; Lunau, 1993; Sutherland et al., 1999; Laubertie et al., 2006). Short-range optical cues are then thought to operate in the second stage, which involves aphid-colony recognition (Dixon, 1959; Sanders, 1983a, 1983b; Kan et al., 1986; Sutherland et al., 2001). Several studies have shown that females oviposit in response to volatile compounds emitted from aphids and their liquid secretions such as honeydew (Dixon, 1959; Budenberg et al., 1992; Almohamad et al., 2008b; Shonouda, 1998; Verheggen et al., 2008).
35The third (penultimate) stage involves the processing of olfactory stimuli. There is an apparent dichotomy in behavioral responses to olfactory stimuli, identified by Chandler (1968c):
36– phytozetic species, such as Melanostoma mellinum, rely more on plant-derived stimuli than on aphid location;
37– aphidozetic species, such as E. balteatus, use aphid-derived chemicals to locate their prey and subsequent oviposition sites.
38There are few published works on the role of chemical odors in hoverfly attraction, but a very good example is the study of Verheggen et al. (2008), who tested the olfactory responses of E. balteatus to several aphid and plant volatiles, including terpenoids (mono-and sesquiterpenes) and green leaf volatiles ((Z)-3-hexenol, (E)-2-hexenol, (E)-2-hexenal and hexanal). They found that monoterpenes induced significant responses, whereas sesquiterpenes were inactive, except for the aphid-alarm pheromone (E)-β-farnesene. Some chemical volatiles ((Z)-3-hexenol and EβF) caused orientation toward the host plant, and stimulated egg-laying, suggesting that oviposition site selection depends on the perception of odors released from aphids, plants, or aphids in association with particular host plants. Francis et al. (2005) showed that E. balteatus larvae are guided by olfactory cues from aphids to locate their aphid prey. Almohamad et al. (2008c) found that E. balteatus females respond positively to the odor of (E)-β-farnesene, but not to the odor of geranyl acetone.
39In the final stage, gustatory stimuli (proboscis extention) are used in response to aphid liquid secretions such as honeydew, and they then exhibit an abdominal protraction or oviposition (Dixon, 1959; Budenberg et al., 1992). Honeydew is also known to serve as an important oviposition stimulus for E. balteatus females (Budenberg et al., 1992; Bargen et al., 1998; Sutherland et al., 2001).
2.6. Intraguild interactions (the presence of intra- and interspecific competitors)
40In addition to their ability to reduce aphid populations effectively, aphidophagous hoverflies do not exist in isolation but generally are part of larger complexes within the aphidophage guild (Rosenheim et al., 1995; Hindayana et al., 2001; Lucas, 2005). Syrphids can act as intraguild (IG) - predators against other aphid predators (Hindayana et al., 2001; Fréchette et al., 2007), and parasitoids (Kindlmann et al., 1992; Meyhöfer et al., 2002; Almohamad et al., 2008a). Apart from prey effects, intra-and interspecific competition may be an important factor regulating performance. Interactions between coexisting syrphid species that share the same aphid prey resource in a patchy habitat often result in intraguild predation, and larvae engage in conspecific and heterospecific predation of eggs and larvae (Benestad Hågvar, 1972; Branquart et al., 1997; Hindayana et al., 2001; Fréchette et al., 2007). The effects of such interactions in a guild may either lead to stabilizing of prey-predator populations (Godfray et al., 1992) or adversely affect the foraging and oviposition performance of individual predators (Rosenheim et al., 1995; Agarwala et al., 2003).
41Syrphid larvae are much less mobile than adults (Chandler, 1969). Additionally, several studies on intraguild predation among syrphid species and other predators have demonstrated that syrphid eggs and larvae are vulnerable to cannibalism (Branquart et al., 1997), and are highly susceptible to predation by other aphid predators such as the ladybird Coccinella septempunctata L., lacewing Chrysoperla carnea Stephens and gall midge Aphidoletes aphidomyza Rondani (Hindayana et al., 2001; Fréchette et al., 2007). Ovipositing hoverfly females would therefore benefit by developing an avoidance of intra- and interspecific individuals present in the same colonies in order to reduce the predation risk to their offspring. Recently it has been discovered that female aphidophages adapt their oviposition behavior in the presence of conspecific and heterospecific competitors. These studies have largely focused on chrysopids (Ruzicka, 1996), coccinellids (Doumbia et al., 1998; Agarwala et al., 2003) and the gall midge (Ruzicka et al., 1998), but there are some on syrphids. A very good example is the study of Scholz et al. (2000) on E. balteatus, that demonstrated that ovipositing females avoid aphid colonies in which conspecific eggs are already present, and the oviposition-deterring stimuli were still active when the eggs were removed. Similar oviposition avoidance was shown by E. balteatus females to the presence of conspecific larvae (Völkl, 1990). Recent studies have demonstrated that the stimuli permitting this discrimination probably derive from syrphid eggs or larvae (Almohamad et al., unpublished data).
42The presence of heterospecific competitors can influence foraging and oviposition. In the study of Almohamad et al. (2008a), foraging and oviposition behavior of E. balteatus females are affected by the presence of parasitoids: females laid significantly fewer eggs in colonies with mummified aphids than in unparasitized or parasitized colonies. They also showed oviposition avoidance response to the presence of Harmonia axyridis larvae (Almohamad et al., unpublished data). Thus the presence of intra- and interspecific individuals (i.e. intraguild predators) is likely to influence the choices made by ovipositing syrphids.
2.7. Effect of female age
43Female age, through time limitation, may be an important factor determining a forager’s decision; when an organism is close to the end of its life it may be more advantageous for it to accept a poor quality oviposition site than it is for a young organism (Mangel, 1987). This decline in selectivity with age has much empirical support. For example, aphidophagous ladybirds Adalia bipunctata (L.) were less selective when older, or when they had previously experienced poor quality patches (Fréchette et al., 2004). Weisser (1994) demonstrated that the parasitoid Lysiphlebus cardui Marshall becomes less selective (for aphid age) as it ages. However, in the field, Heimpel et al. (1996) found no evidence that age affected the oviposition behavior of the parasitoid Aphytis aonidiae (Mercet).
44The age effect is so general that it is incorporated into the hierarchy threshold model (Courtney et al., 1989; Sadeghi et al., 2000a; 2000b), but the influence of age is not well-documented in aphidophagous hoverflies: so far we know of only three studies (Chandler, 1967; Guest, 1984; Sadeghi et al., 2000a). Young females of E. balteatus and S. ribesii exhibit a marked hierarchical preference for particular species of aphids and do not oviposit on uninfested plants, but they lose discrimination as they get older (Sadeghi et al., 2000a); Guest (1984) showed that E. balteatus females increasingly lay eggs away from aphids as they age. In contrast, the distance between the nearest aphid and the egg decreased with female age in Eupeodes luniger (Chandler, 1967), and older female E. balteatus and S. ribesii laid more eggs on uninfested plants than did young ones, indicating that ageing decreased responses to aphid-related stimuli more than to plant-related ones.
2.8. Effect of egg load and host deprivation
45The hierarchy threshold model of host choice has two components: an inherent, fixed (in each individual) rank order of preference of hosts, and a variable threshold of acceptability that depends in part on internal factors such as egg load (i.e. the number of mature eggs in the ovaries) (Sadeghi et al., 2000c). This biological factor is found to be a source of variation in host choice by ovipositing females. Minkenberg et al. (1992) concluded that the role of egg-load, egg-load dynamics and the function of egg-load response will lead to a more complete understanding of variation in oviposition behavior. Host deprivation is also used to investigate the effect of the egg load on oviposition behavior (Fitt, 1986). Sadeghi et al. (2000c) reported that E. balteatus and S. ribesii (L.) females do not waste their mature eggs when facing a shortage of hosts or when there are no suitable aphids. Dixon (1959) also showed that female E. corollae could retain mature eggs in the absence of aphids, but eventually some eggs were laid. Females could retain mature eggs for several weeks in the absence of suitable oviposition site. Prolonged retention reduced fecundity but increased longevity (Lyon, 1965).
3. Conclusion
46We conclude that several factors have been shown to be involved in the selection of oviposition site by aphidophagous hoverflies. These factors include habitat, host plant physical characteristics (i.e. floral characters), the aphid species, aphid colony size and density, semiochemicals emitted from aphids or their association with host plants, the presence of intra- or interspecific competitors and female age. Females show evolved behavioral mechanisms in response to these factors that enable them to forage for an oviposition site that will support the development of their offspring. This review highlights much that has been learned, but also emphasizes that much remains to be learned about the mechanisms of decision-making by individual females to assess aphid patch quality during their egg-laying behavior. Detailed information about searching and oviposition behavior provide an essential foundation for designing effective biological control, and for better understanding when, where and how syrphids can suppress aphid populations.
Bibliographie
Agarwala B.K., Bardhanroy P., Yasuda H. & Takizawa T., 2003. Effects of conspecific and heterospecific competitors on feeding and oviposition of a predatory ladybird: a laboratory study. Entomol. Exp. Appl., 106(3), 219-226.
Almohamad R., Verheggen F.J., Francis F. & Haubruge E., 2006. Evaluation of hoverfly Episyrphus balteatus DeGeer (Diptera: Syrphidae) oviposition behaviour toward aphid-infested plants using a leaf disc system. Commun. Agric. Appl. Biol. Sci., 71(2 Pt B), 403-412.
Almohamad R., Verheggen F.J., Francis F. & Haubruge E., 2007a. Predatory hoverflies select their oviposition site according to aphid host plant and aphid species. Entomol. Exp. Appl., 125(1), 13-21.
Almohamad R., Verheggen F.J., Francis F. & Haubruge E., 2007b. Aphid density influences oviposition behaviour and larval performance in predatory hoverfly. In: Proceedings of 16th International plant protection congress, 15-18 October 2007, Glasgow, Scotland, UK. Vol. 1. Glasgow, Scotland, UK, 306-307.
Almohamad R. et al., 2008a. Discrimination of parasitized aphids by a hoverfly predator: effect on larval performance, foraging and oviposition behavior. Entomol. Exp. Appl., 128(1), 73-80.
Almohamad R. et al., 2008b. Emission of alarm pheromone by non-preyed aphid colonies. J. Appl. Entomol., 132(8), 601-604.
Almohamad R., Verheggen F.J., Francis F. & Haubruge E., 2008c. Impact of aphid colony size and associated induced plant volatiles on searching and oviposition behaviour of a predatory hoverfly. B. J. Entomol., 10, 17-26.
Ambrosino M.D., 2006. Enhancing the predatory potential of hoverflies on aphids in Oregon broccoli fields with floral resources. PhD thesis: Oregon State University, Corvallis (OR, USA).
Ambrosino M.D., Jepson P.C. & Luna J.M., 2007. Hoverfly oviposition response to aphids in broccoli fields. Entomol. Exp. Appl., 122(2), 99-107.
Bargen H., Saudhof K. & Poehling H.M., 1998. Prey finding by larvae and adult females of Episyrphus balteatus. Entomol. Exp. Appl., 87(10), 245-254.
Barnard C.J., 1983. Animal behaviour: ecology and evolution. Beckenham, UK: Croom Helm.
Bell W.J., 1990. Searching behavior patterns in insects. Annu. Rev. Entomol., 35, 447-467.
Belliure B. & Michaud J.P., 2001. Biology and behaviour of Pseudodorus clavatus (Diptera: Syrphidae), an important predator of citrus aphids. Ann. Entomol. Soc. Am., 94(1), 91-96.
Benestad Hågvar E., 1972. The effect of intra- and interspecific larval competition for food (Myzus persicae) on the development at 20°C of Syrphus ribesii and Syrphus corollae (Diptera, Syrphidae). Entomophaga, 17(1), 71-77.
Berenbaum M., 1990. Evolution of specialization in insect-umbellifer associations. Annu. Rev. Entomol., 35, 319-343.
Bernays E.A., 1996. Selective attention and host-plant specialization. Entomol. Exp. Appl., 80(1), 125-131.
Branquart E., Hemptinne J.-L., Bauffe C. & Benfekih L., 1997. Cannibalism in Episyrphus balteatus (Dipt.: Syrphidae). Entomophaga, 42(1-2), 145-152.
Branquart E. & Hemptinne J.-L., 2000. Selectivity in the exploitation of floral resources by hoverflies (Diptera: Syrphidae). Ecography, 23(6), 732-742.
Budenberg W.J. & Powell B., 1992. The role of honeydew as an oviposition stimulant for two species of syrphids. Entomol. Exp. Appl., 64(1), 57-61.
Chambers R.J., 1988. Syrphidae. In: Minks A.K. & Harrewijn P., eds. Aphids, their biology, natural enemies, and control. Amsterdam, The Netherlands: Elsevier, 259-270.
Chambers R.J., 1991. Oviposition by aphidophagous hoverflies (Diptera: Syrphidae) in relation to aphid density and distribution in winter wheat. In: Polgár L., Chambers R.J., Dixon A.F.G. & Hodek I., eds. Behaviour and impact of aphidophaga. Proceedings of the 4th meeting of the IOBC ecology of aphidophaga, Sept. 1990, Gödöllö, Hungary. The Hague: SPB Academic Publishing, 115-121.
Chambers R.J. & Adams T.H.L., 1986. Quantification of the impact of hoverflies (Diptera: Syrphidae) on cereal aphids in winter wheat: an analysis of field populations. J. Appl. Ecol., 23, 895-904.
Chandler A.E.F., 1966. Some aspects of host plant selection in aphidophagous Syrphidae. In: Hodek I. Ecology of aphidophagous insects. The Hague: Junk; Prague: Academia, 113-115.
Chandler A.E.F., 1967. Oviposition responses by aphidophagous Syrphidae (Diptera). Nature, 213, 736.
Chandler A.E.F., 1968a. Some host-plant factors affecting oviposition by aphidophagous Syrphidae (Diptera). Ann. Appl. Biol., 61(3), 415-423.
Chandler A.E.F., 1968b. The relation between aphid infestations and oviposition by aphidophagous Syrphidae (Diptera). Ann. Appl. Biol., 61(3),425-434.
Chandler A.E.F., 1968c. Some factors influencing the occurrence and site of oviposition by aphidophagous Syrphidae (Diptera). Ann. Appl. Biol., 61(3), 435-446.
Chandler A.E.F., 1969. Locomotory behavior of first instar larvae of aphidophagous Syrphidae (Diptera) after contact with aphids. Anim. Behav., 17, 673-678.
Colley M.R. & Luna J.M., 2000. Relative attractiveness of potential beneficial insectary plants to aphidophagous hoverflies (Diptera: Syrphidae). Environ. Entomol., 29(5), 1054-1059.
Cornelius M. & Barlow C.A., 1980. Effect of aphid consumption by larvae on development and reproductive efficiency of a flowerfly, Syrphus corollae (Diptera:Syrphidae). Can. Entomol., 112, 989-992.
Cortesero A.M., Stapel J.O. & Lewis W.J., 2000. Understanding and manipulating plant attributes to enhance biological control. Biol. Control, 17, 35-49.
Courtney S.P., Chen G.K. & Gardner A., 1989. A general model for individual host selection. Oikos, 55, 55-65.
Cowgill S.E., Wratten S.D. & Sotherton N.W., 1993. The selective use of floral resources by the hoverfly Episyrphus balteatus (Diptera: Syrphidae) on farmland. Ann. Appl. Biol., 122, 223-231.
Curran C.H., 1925. Contribution to a monograph of the American Syrphidae from north of Mexico. Kansas Univ. Sci. Bull., 15, 1-216.
Dicke M., 1999. Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol. Exp. Appl., 91(1), 131-142.
Dixon A.F.G., 2000. Insect predator-prey dynamics: ladybird beetles and biological control. Cambridge, UK: Cambridge University Press.
Dixon T.J., 1959. Studies on the oviposition behaviour of Syrphidae (Diptera). Trans. R. Entomol. Soc. Lond., 111, 57-80.
Doumbia M., Hemptinne J.-L. & Dixon A.F.G., 1998. Assessment of patch quality by ladybirds: role of larval tracks. Oecologia, 113, 197-202.
Du Y. et al., 1998. Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J. Chem. Ecol., 24(8), 1355-1368.
Ferran A. & Dixon A.F.G., 1993. Foraging behavior of ladybird larvae (Coleoptera: Coccinellidae). Eur. J. Entomol., 90(4), 383-402.
Fitt G.P., 1986. The influence of a shortage of hosts on the specificity of oviposition behaviour in species of Dacus (Diptera, Tephritidae). Physiol. Entomol., 11(2), 133-143.
Fitzgerald J.D. & Solomon M.G., 2004. Can flowering plants enhance numbers of beneficial arthropods in UK apple and pear orchards? Biocontrol Sci. Techn., 14(3), 291-300.
Francis F., Lognay G., Wathelet J.P. & Haubruge E., 2001. Effects of allelochemicals from first (Brassicaceae) and second (Myzus persicae and Brevicoryne brassicae) trophic levels on Adalia bibunctata. J. Chem. Ecol., 27(2), 243-256.
Francis F., Lognay G., Gaspar C. & Haubruge E., 2004. Olfactory responses to aphids and host plant volatile releases: (E)-β-farnesene an effective allomone for the predator Adalia bipunctata. J. Chem. Ecol., 30(4), 741-755.
Francis F., Martin T., Lognay G. & Haubruge E., 2005. Role of (E)-β-farnesene in systematic aphid prey location by Episyrphus balteatus larvae. Eur. J. Entomol., 102(3), 431-436.
Fréchette B., Dixon A.F.G, Alauzet C. & Hemptinne J.-L., 2004. Age and experience influence patch assessment for oviposition by an insect predator. Ecol. Entomol., 29(5), 578-583.
Fréchette B., Rojo S., Alomar O. & Lucas E., 2007. Intraguild predation between syrphids and mirids: who is the prey? Who is the predator? Entomophaga, 52(2), 175-191.
Gilbert F., 1981. Foraging ecology of hoverflies: morphology of the mouthparts in relation to feeding on nectar and pollen in some common urban species. Ecol. Entomol., 6(3), 245-262.
Gilbert F., 1986. Hoverflies. Naturalists’ Handbook 5. Cambridge, UK: Cambridge University Press.
Gilbert F., 1993. Hoverflies. Naturalists' Handbooks 5. 2nd ed. Surrey, UK: Richmond Press.
Gilbert F., 2005. Syrphid aphidophagous predators in a food-web context. Eur. J. Entomol., 102(3), 325-333.
Gilbert F. & Owen J., 1990. Size, shape, competition, and community structure in hoverflies (Diptera: Syrphidae). J. Anim. Ecol., 59(1), 21-39.
Godfray H.C.J. & Pacala S.W., 1992. Aggregation and the population dynamics of parasitoids and predators. Am. Nat., 140(1), 30-40.
Guest P.J., 1984. Oviposition strategies of aphidophagous syrphids. PhD thesis: Imperial College of Science and Technology, London (UK).
Hagen K.S. & van den Bosch R., 1968. Impact of pathogens, parasites and predators on aphids. Annu. Rev. Entomol., 13, 325-384.
Harmel N. et al., 2007. Role of terpenes from aphid-infested potato on searching and oviposition behavior of Episyrphus balteatus. Insect Sci., 14(1), 57-63.
Haslett J.R., 1989. Adult feeding by holometabolous insects: pollen and nectar as complementary nutrient sources for Rhingia campestris (Diptera: Syrphidae). Oecologia, 81(3), 361-363.
Heimpel G.E., Rosenheim J.A. & Mangel M., 1996. Egg limitation, host quality, and dynamic behavior by a parasitoid in the field. Ecology, 77(8), 2410-2420.
Hemptinne J.-L., Dixon A.F.G., Doucet J.-L. & Petersen J.E., 1993. Optimal foraging by hoverflies (Diptera: Syrphidae) and ladybirds (Coleoptera: Coccinellidae): mechanism. Eur. J. Entomol., 90(4), 451-455.
Hindayana D., Mayhöfer R., Scholz D. & Poehling H.M., 2001. Intraguild predation among the hoverfly Episyrphus balteatus DeGeer (Diptera: Syrphidae) and other aphidophagous predators. Biol. Control, 20(3), 236-246.
Hodek I., 1993. Habitat and food specifity in aphidophagous predators. Biocontrol Sci. Techn., 3, 91-100.
Hodek I. & Honek A., 1996. Ecology of the Coccinellidae. Dordrecht, The Netherlands; Boston, MA, USA; London, Kluwer Academic Publishers.
Horn D.J., 1981. Effect of weedy backgrounds on colonization of collards by green peach aphid, Myzus persicae, and its major predators. Environ. Entomol., 10(3), 285-289.
Itô K. & Iwao S., 1977. Oviposition behavior of a syrphid, Episyrphus balteatus, in relation to aphid density on the plant. Jpn. J. Appl. Entomol. Zool., 21, 130-134.
Jervis M. & Kidd N., 1996. Insect natural enemies. Practical approaches to their study and evaluation. London: Chapman & Hall.
Kan E., 1988a. Assessment of aphid colonies by hoverflies. I. Maple aphids and Episyrphus balteatus (DeGeer) (Diptera: Syrphidae). J. Ethol., 6(1), 39-48.
Kan E., 1988b. Assessment of aphid colonies by hoverflies. II. Pea aphids and 3 syrphid species; Betasyrphus serarius (Wiedemann), Metasyrphus frequens Matsumura and Syrphus vitripennis (Meigen) (Diptera: Syrphidae). J. Ethol., 6(1), 135-142.
Kan E. & Sasakawa M., 1986. Assessment of the maple aphid colony by the hoverfly Episyrphus balteatus (DeGeer) (Diptera: Syrphidae). J. Ethol., 4, 121-127.
Kindlmann P. & Ruzicka Z., 1992. Possible consequences of a specific interaction between predators and parasites of aphids. Ecol. Modelling, 61(3-4), 253-265.
Kindlmann P. & Dixon A.F.G., 1993. Optimal foraging in ladybird beetles (Coleoptera: Coccinellidae) and its consequences for their use in biological control. Eur. J. Entomol., 90(4), 443-450.
Laubertie E.A., Wratten S.D. & Sedcole J.R., 2006. The role of odour and visual cues in the pan-trap catching of hoverflies (Diptera: Syrphidae). Ann. Appl. Biol., 148(2), 173-178.
Losey J.E. & Denno R.F., 1998. The escape response of pea aphids to foliar-foraging predators: factors affecting dropping behaviour. Ecol. Entomol., 23(1), 53-61.
Lucas E., 2005. Intraguild predation among aphidophagous predators. Eur. J. Entomol., 102(3), 351-364.
Lunau K., 1993. Interspecific diversity and uniformity of flower colour patterns as cues for learned discrimination and innate detection of flowers. Experientia, 49(11), 1002-1010.
Lyon J.P., 1965. Influence of some factors on the expression of the potential for reproduction in aphidophagous Syrphidae. Ann. Epiphyt., 16, 397-398.
Lyon J.P., 1968. Contribution to the biological study of Xanthandrus comptus Harris. Ann. Epiphyt., 19, 683-693.
MacLeod A., 1999. Attraction and retention of Episyrphus balteatus DeGeer (Diptera: Syrphidae) at an arable field margin with rich and poor floral resources. Agric. Ecosystems Environ., 73(3), 237-244.
Mangel M., 1987. Oviposition site selection and clutch size in insects. J. Math. Biol., 25(1), 1-22.
Meyhöfer R. & Klug T., 2002. Intraguild predation on the aphid parasitoid Lysiphlebus fabarum (Marshall) (Hymenoptera: Aphidiidae): mortality risks and behavioral decisions made under the threats of predation. Biol. Control., 25(3), 239-248.
Michaud J.P., 2005. On the assessment of prey suitability in aphidophagous Coccinellidae. Eur. J. Entomol., 102(3), 385-390.
Michaud J.P. & Belliure B., 2001. Impact of syrphid predation on production of migrants in colonies of the brown citrus aphid, Toxoptera citricida (Homoptera: Aphididae). Biol. Control, 21(1), 91-95.
Minkenberg O.P.J., Tatar M. & Rosenheim J.A., 1992. Egg load as a major determinant of variability in insect foraging and oviposition behavior. Oikos, 65, 134-142.
Mizuno M., Itioka T., Tatematsu Y. & Ito Y., 1997. Food utilization of aphidophagous hoverfly larvae (Diptera: Syrphidae, Chamaemyiidae) on herbaceous plants in an urban habitat. Ecol. Res., 12(3), 239-248.
Morris M.C. & Li F.Y., 2000. Coriander (Coriandrum sativum) ‘companion plants’ can attract hoverflies, and may reduce pest infestation in cabbages. N. Z. J. Crop Hortic. Sci., 28, 213-217.
Murdoch W.W. & Briggs CJ., 1996. Theory for biological control: recent developments. Ecology, 77, 2001-2013.
Ninkovic V., Al Abassi S. & Pettersson J., 2001. The influence of aphid-induced plant volatiles on ladybird beetle searching behavior. Biol. Control, 21(2), 191-195.
Nufio C.R. & Papaj D.R., 2004. Superparasitism of larval hosts by the walnut fly, Rhagoletis juglandis, and its implications for female and offspring performance. Oecologia, 141(3), 460-467.
Peckarsky B.L., Taylor B.W. & Caudill C.C., 2000. Hydrologic and behavioral constraints on oviposition of stream insects: implications for adult dispersal. Oecologia, 125(2), 186-200.
Poehling H.M., 1988. Influence of cereal aphid control on specific predators in winter wheat (Homoptera: Aphididae). Entomol. Gen., 13, 163-174.
Price P.W. et al., 1980. Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst., 11, 41-65.
Rojo S. et al., 2003. A world review of predatory hoverflies (Diptera, Syrphidae : Syrphinae) and their prey. Alicante, Spain: Centro Iberoamericano de la Biodiversidad, Universidad de Alicante.
Rosenheim J.A. et al., 1995. Intraguild predation among biological control agents: theory and evidence. Biol. Control, 5(3), 303-335.
Rotheray G.E. & Dobson J., 1987. Aphidophagy and the larval and pupal stages of the syrphid Platycheirus fulviventris (Macquart). Entomol. Gaz., 38, 245-251.
Ruppert V. & Molthan J., 1991. Augmentation of aphid antagonists by field margins rich in flowering plants. In: Polgár L., Chambers R.J, Dixon A.F.G. & Hodek I., eds. Behaviour and impact of aphidophaga. Proceedings of the 4th meeting of the IOBC ecology of aphidophaga, September 1990, Gödöllö, Hungary. The Hague: SPB Academic Publishing, 243-247.
Růžička Z., 1975. The effects of various aphids as larval prey on the development of Metasyrphus corollae (Dipt.: Syrphidae). Entomophaga, 20, 393-402.
Růžička Z., 1996. Oviposition-deterring pheromone in Chrysopidae (Neuroptera): intra- and interspecific effects. Eur. J. Entomol., 93,161-166.
Růžička Z. & Havelka J., 1998. Effects of oviposition-deterring pheromone and allomones on Aphidoletes aphidimyza (Diptera: Cecidomyiidae). Eur. J. Entomol., 95, 211-216.
Růžička Z. & Gonzales Cairo V., 1976. The effect of larval starvation on the development of Metasyrphus corollae (Diptera). Vestn. Cesk. Spol. Zool., 40, 206-213.
Sadeghi H., 2000. Oviposition preference and larval performance in hoverflies. PhD thesis: Nottingham University (UK).
Sadeghi H. & Gilbert F., 1999. Individual variation in oviposition preference, and its interaction with larval performance, in an insect predator. Oecologia, 118(4), 405-411.
Sadeghi H. & Gilbert F., 2000a. Oviposition preferences of aphidophagous hoverflies. Ecol. Entomol., 25(1), 91-100.
Sadeghi H. & Gilbert F., 2000b. Aphid suitability and its relationship to oviposition preference in predatory hoverflies. J. Anim. Ecol., 69(5), 771-784.
Sadeghi H. & Gilbert F., 2000c. The effect of egg load and host deprivation on oviposition behaviour in aphidophagous hoverflies. Ecol. Entomol., 25(1), 101-108.
Salveter R., 1996. Populationsaufbau aphidophager Schwebfliegen. (Diptera: Syrphidae) in der Agrarlandschaft. Ph.D. thesis: University Berne (Switzerland).
Sanders W., 1982. Der Einfluß von Farbe und Beleuchtung des Umfeldes auf die Eiablagehandlung der Schwebfliege. Syrphus corollae Fabr. Z. Angew. Zool., 69, 283-297.
Sanders W., 1983a. The searching behaviour of gravide Syrphus corollae Fabr. (Dipt: Syrphidae) and its depending on the optical cues. Z. Angew. Zool., 70, 235-247.
Sanders W., 1983b. The searching behaviour of gravide Syrphus corollae Fabr. (Dipt: Syrphidae) in relation to variously designed plant models. Z. Angew. Zool., 70, 449-462.
Scheirs J. & De Bruyn L., 2002. Integrating optimal foraging and optimal oviposition theory in plant-insect research. Oikos, 96(1), 187-191.
Schmutterer H., 1972. Zur Beutespezifität polyphager, räuberischer Syrphiden Ostafrikas. Z. Angew. Entomol., 71, 278-286.
Schneider F., 1948. Beitrag zur Kenntnis der Generationsverhältnisse und Diapause räuberischer Schwebfliegen (Syrphidae, Dipt.). Mitt. Schweiz. Entomol. Ges., 21, 249-285.
Schneider F., 1969. Bionomics and physiology of aphidophagous syrphidae. Annu. Rev. Entomol., 14, 103-124.
Scholz D. & Poehling H.M., 2000. Oviposition site selection of Episyrphus balteatus. Entomol. Exp. Appl., 94(2), 149-158.
Schoonhoven L.M., Jermy T. & van Loon J.J.A., 1998. Insect-plant biology: from physiology to evolution. London: Chapman & Hall.
Shibao H., 1998. An offensive and defensive battle between the hoverfly Eupeodes confrater and the soldier-producing aphid Pseudoregma bambucicola. The Insectarium, 35(8), 224-233.
Shonouda M.L., Bombosch S., Shalaby A.M. & Osman S.I., 1998. Biological and chemical characterization of a kairomone excreted by the bean aphids, Aphis fabae Scop. (Homoptera: Aphididae), and its effect on the predator Metasyrphus corollae Fabr. II. Behavioural response of the predator M. corollae to the aphid kairomone. J. Appl. Entomol., 122(1), 25-28.
Singer M.S., Rodrigues D., Stireman J.O. & Carrière Y., 2004. Roles of food quality and enemy-free space in host use by a generalist insect herbivore. Ecology, 85(10), 2747-2753.
Stephens D.W. & Krebs J.R., 1986. Foraging theory. Princeton, NJ, USA: Princeton University Press.
Sutherland J.P., Sullivan M.S. & Poppy G.M., 1999. The influence of floral character on the foraging behaviour of the hoverfly, Episyrphus balteatus. Entomol. Exp. Appl., 93(2), 157-164.
Sutherland J.P., Sullivan M.S. & Poppy G.M., 2001. Oviposition behaviour and host colony size discrimination in Episyrphus balteatus (Diptera: Syrphidae). Bull. Entomol. Res., 91, 411-417.
Tenhumberg B. & Poehling H.M., 1992. Investigation on density dependent responses of syrphids (Diptera: Syrphidae) in winter wheat. Mitt. Dtsch. Ges. Allg. Angew. Entomol., 8(1-3),140-146.
Tenhumberg B. & Poehling H.M., 1995. Syrphids as natural enemies of cereal aphids in Germany: aspects of their biology and efficacy in different years and regions. Agric. Ecosystems Environ., 52(1), 39-43.
Thompson J.N., 1988. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl., 47(1), 3-14.
van Emden H.F., 2003. Conservation biological control: from theory to practice. In: van Driesche R., ed. International symposium on biological control of arthropods. Morgantown, WV, USA: USDA Forest Service, 199-208.
van Emden H.F. & Harrington R., 2007. Aphids as crop pests. London, UK: Cabi Publishing.
Vanhaelen N., Haubruge E., Gaspar C. & Francis F., 2001. Oviposition preferences of Episyrphus balteatus. Med. Fac. Landbouww. Univ. Gent, 66(2a), 269-275.
Vanhaelen N., Gaspar C. & Francis F., 2002. Influence of prey host plant on a generalist aphidophagous predator, Episyrphus balteatus (Diptera: Syrphidae). Eur. J. Entomol., 99(4), 561-564.
van Rijn P.C.J., Kooijman J. & Wäckers F.L., 2006. The impact of floral resources on syrphid performance and cabbage aphid biological control. IOBC/WPRS Bull., 29(6), 149-152.
Verheggen F.J. et al., 2008. Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. J. Chem. Ecol., 34(3), 301-307.
Volk S., 1964. Untersuchungen Zur Eiablage von Syrphus corollae Fabr. (Diptera: Syrphidae). Z. Angew. Entomol., 54, 365-386.
Völkl W., 1990. Fortpflanzungsstrategien von Blattlausparasitoiden (Hymenoptera, Aphidiidae): Konsequenzen ihrer Interaktionen mit Wirten und Ameisen. PhD thesis: University of Bayreuth (Germany).
Weisser W.W., 1994. Age-dependent foraging behaviour and host-instar preference of the aphid parasitoid Lysiphlebus cardui. Entomol. Exp. Appl., 70(1), 1-10.
White A.J., Wratten S.D., Berry N.A. & Weigmann U., 1995. Habitat manipulation to enhance biological control of Brassica pests by hoverflies (Diptera: Syrphidae). J. Econ. Entomol., 88(5), 1171-1176.
Pour citer cet article
A propos de : Raki Almohamad
Univ. Liege - Gembloux Agro-Bio Tech. Department of functional and evolutionary Entomology. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: almohamad.r@fsagx.ac.be
A propos de : François J. Verheggen
Univ. Liege - Gembloux Agro-Bio Tech. Department of functional and evolutionary Entomology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Éric Haubruge
Univ. Liege - Gembloux Agro-Bio Tech. Department of functional and evolutionary Entomology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






