- Home
- volume 13 (2009)
- numéro 4
- Factors affecting intake by grazing ruminants and related quantification methods: a review
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Factors affecting intake by grazing ruminants and related quantification methods: a review

Editor's Notes
Received 3 September 2008; accepted 10 March 2009
Résumé
Facteurs de variation de l’ingestion des ruminants au pâturage et principales méthodes permettant son estimation : revue de synthèse. L'objectif de cette étude est de discuter les principaux facteurs de variation de l’ingestion des ruminants au pâturage ainsi que les principales méthodes d’estimation de ce paramètre. Le niveau d’ingestion dépend, simultanément, de nombreux facteurs liés, par exemple, à la capacité du tube digestif de l'animal, à la couverture de ses besoins en nutriments, à la concentration des éléments nutritifs des plantes fourragères. Les aspects post-ingestifs interviennent également, ainsi que les caractéristiques morphologiques des plantes broutées. L’environnement dans lequel évolue l’animal, par le biais de l’abondance des ressources alimentaires, du climat, des processus d’apprentissage, peut également influencer le niveau d’ingestion. L’aspect multi-factoriel du contrôle de l’ingestion limite le nombre d'études sur l'estimation de ce paramètre en situation de pâturage. Les méthodes les plus couramment utilisées sont souvent lourdes à mettre en œuvre, couteuses en temps et en argent et parfois peu représentatives des conditions réelles de pâturages. De plus, elles manquent souvent de précision. Actuellement, la méthode des n-alcanes, marqueurs internes présents dans les cires cuticulaires des plantes, apparait comme l’une des meilleures voies pour estimer simultanément l’ingestion et la digestibilité de l’herbe pâturée. Toutefois, cette méthode reste difficile à appliquer sur de longues périodes et en situation de pâturage extensif. Si des bases de données suffisamment solides sont mises en place, la spectroscopie dans le proche infrarouge (SPIR) pourrait se révéler être une technique intéressante pour estimer rapidement la consommation d’herbe par les ruminants au pâturage. Plus spécialement, la SPIR appliquée aux matières fécales semble prometteuse dans le cadre de l’estimation de ce paramètre. Cette analyse rapide pourrait être considérée comme une alternative intéressante pour caractériser, qualitativement et quantitativement, le régime alimentaire des ruminants au pâturage.
Abstract
The aim of this review is to discuss the factors affecting intake of grazing ruminants and its main quantification methods. Level of intake depends on many factors linked, for instance to the gut capacity, to the animal’s requirements covering, or to the forage quality. The post-ingestive feedback of the intake, the morphological characteristics of grazed plants and the environment such as climate, characteristics of feed resources, are also factors of interest to explain some intake variations. Intake is a multi-factorial phenomenon. There are few studies on the estimation of that parameter. Methods and techniques developed to measure intake are often laborious and expensive, sometimes unrepresentative of the true grazing conditions and often lacking of accuracy. Currently, the n-alkanes, natural markers presents in the plants, appear as one of the best way to predict at the same time intake and digestibility of ingested diet. However, the method remains hard to apply for long periods and in free ranging schemes. If sufficiently robust databases and calibrations are developed, Near Infrared Spectroscopy (NIRS) appears as an interesting technique to predict rapidly intake and digestibility of grazed grass. Particularly, NIRS applied to faeces appears promising as related in recent studies. It could be considered as a good alternative for assessing the diet, in quantity and in quality, of grazing or ranging ruminants.
Table of content
1. Introduction
1Grass is the most economical herbivores feed during the grazing season. Therefore, in vivo digestibility and voluntary intake worth being estimated simultaneously in order to optimize grazing ruminant productions.
2These last few years, digestibility appears as the more studied parameter. In vivo digestibility of forages is commonly obtained by digestibility trials carried out on adult sheep (Demarquilly et al., 1995). The method is costly, time and labor consuming and is only used to assess the digestibility of unknown feedstuffs. To estimate more rapidly and easily the in vivo digestibility, different alternative methods exist and are well documented. Some of them are based on regressions between in vivo digestibility and forages characteristics such as cellulose (Lecomte et al., 1992), plant morphological characteristics (Demarquilly et al., 1981) or plant physiological stage (Valente et al., 2000). If these methods are sufficiently accurate to estimate the in vivo digestibility of pure grass sward, they are less appropriate for mixed forages due to the presence of various plant species differing in terms of chemical composition or morphological development stage. To solve this problem, in vitro techniques have been developed (Adegosan et al., 2000). The “rumen fluid pepsin” method of Tilley and Terry (1963) appears as the oldest but is poorly reproductible (Wainman et al.1, 1981, cited by Adegosan et al., 2000). In order to face this problem, enzymatic mixtures that simulate ruminal activities have replaced rumen fluid (Jarrige et al., 1969; De Boever et al., 1988; 1996; Aufrère et al., 1996 ; 2007). The cellulase-method is actually the most used to estimate the in vivo digestibility of a large range of forage.
3The gaz test method (Menke et al., 1979) measuring the volume of gas produced by the fermentation of forage in presence of rumen fluid is also an interesting tool to estimate in vivo digestibility, particularly of tropical forages (Stern et al., 1997; Babatounde, 2005).
4Indirect methods based on some faeces characteristics allow easily and enough accurately to predict in vivo digestibility of grazed grass. For instance, linear or quadratic relations linking faecal nitrogen and in vivo digestibility have been developed for temperate (Bartiaux-Thill et al., 1986; Peyraud, 1998) and tropical forages (Boval et al., 1996; Bouazizi et al., 1999). In the same way, indigestible internal plant markers such as lignin (Fahey et al., 1983), indigestible acid detergent fibres (Sunvold et al., 1991), and n-alkanes, naturally present in cuticular waxes of plant (Dove et al., 1991; 1996) and consequently in faeces, are also used to estimate the in vivo digestibility of grazing animals.
5Due to their complexity, few studies are carried on to the estimation of voluntary intake of grazing ruminants and actually, it is very difficult to estimate this parameter easily and with a sufficient precision. When it is done, intake is usually measured for one ruminant’s species on one type of pasture. Indeed, even if, according to the definition of Baumont et al. (2000) “intake is the maximum quantity of feed that can be eaten by an animal when this is supplied ad libitum as the sole feed” and, so, seems easy to quantify, its study is more complex. In reality, according to Illius et al. (1996) intake can be considered as a “psychological” phenomenon, involving the integration of many signals, and reflects the flexibility of a biological system evolved to cope with variability in food supply, composition and animal states. So, plant properties, associated for example to the presence of toxins, the taste or smell, are important parameters impacting diet selection and ingestive behavior of grazing ruminants and so the intake’s level (Provenza et al., 2003b).
6The aim of this review is to discuss the factors affecting the intake and food choice of grazing ruminants and the main methods used to determine them.
2. Intake’s variations of grazing ruminants
2.1. Intake expression
7Ways of expressing level of intake are numerous. Commonly, forage dry or organic matter intake is expressed in weight unit per animal and per day but the expression cannot be used to compare animal species or forages between them. For this purpose, intake may be expressed by kg of body weight raised to an exponent that can vary between 0.54 and 1.00 (Meissner et al., 1995). The choice of the exponent is a function of forage’s quality. With low quality forage, intake capacity of the animal appears more linked to the fill gut capacity and to the rate of passage of forage. For such forages, the exponent is 1.00 and intake is expressed per kg of body weight or in percent of body weight (Demment et al., 1985).
8Intake of good quality forages seems more controlled by physiological mechanisms. Intake of such forages is usually expressed per kg of metabolic weight (body weight raised to 0.75). The assumption is that intake is linked to energy requirements that are proportional to 0.75 power of body weight (Klieber, 19612 cited by Allison, 1985).
9Nevertheless, a recent study (Sauvant et al., 2006) has shown that, to compare intake’s level across forages and animal species, the best unit remains the dry matter in percent of body weight (DMI % BW). On this basis, relations between intake and passage rate of particles through the rumen or the energy digestibility appear independent of animal species. This is not the case when intake is expressed per kg of metabolic weight.
2.2. Level of intake at grazing
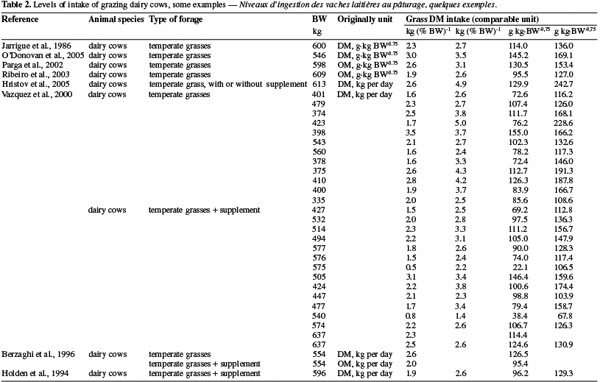
10Tables 1 and 2 summarize some examples of intake’s levels for ruminants (dairy cow, beef cattle, small ruminants) grazing various forages. A first observation is that intake’s level related to animal is highly variable and linked to the characteristic of the forages.
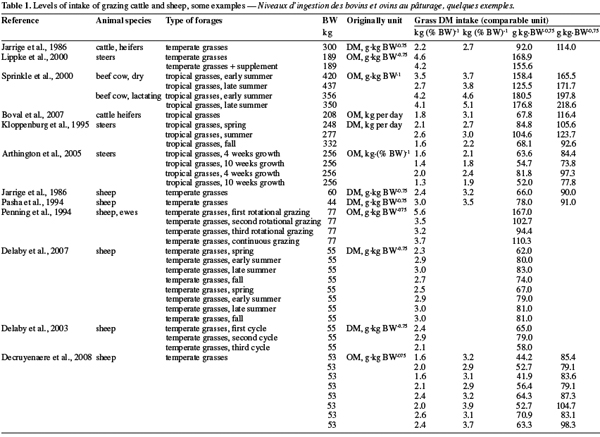
11According to the French references, the ad libitum intake of a reference grass (15% of crude protein, 77% of organic matter digestibility on a dry matter basis) is 75, 95 and 140 g of dry matter per kg of metabolic weight respectively for a standard sheep, a standard cattle (heifers) and a standard lactating dairy cow. On this basis, it is possible to calculate the “Fill Unit” (“unité d’encombrement”) of various forages (INRA, 2007).
12However huge intake’s level variability also occurs between breed or individuals within a given breed (Scott et al., 1999; Pearson et al., 2005). As an example, Dorper sheeps are less selective grazers, consume more shrubs and bushes and ingest a larger number of different plant species, than Merinos sheep (Brand, 2000).
13The way of expressing intake’s level also contributes to this variability. As described by Sauvant et al. (2006), if DMI, expressed in % of body weight, appears higher for small ruminants (sheep and goats) than for cattle (dairy or suckling cattle), this is the reverse when intake is expressed in terms of metabolic weight.
14Compared to temperate forages, voluntary intake of tropical forages is often lower but not highly different (2.03 kg DM per % BW vs 1.95 kg DM per % BW respectively for temperate and tropical forages), as confirmed in the meta-analysis of Assoumaya et al. (2007), despite, on a chemical point of view, temperate forages contain often higher proteins and lesser fibers. The difference decreases when the crude protein contents of both forages are similar. To explain the few difference, Assoumaya et al. (2007) indicated that tropical forages are usually longer chewed than temperate ones leading to a more efficient reduction of forages particles size compensating their lower apparent nutritive value.
3. Factors affecting intake regulation
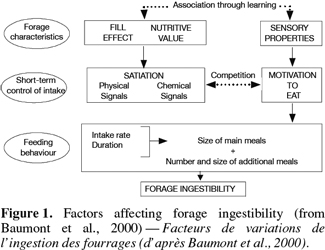
15The control of intake is multi-factorial (Rhind et al., 2002; Forbes, 2003). It depends, at the same time, on plants characteristics in relation to the gut capacity, to the animal’s requirements and nutrient concentrations of forages, to the post-ingestive feedback of the intake and the learning process, to the morphological characteristics of grazed plants, and on the environment such as climate, abundance and frequency of feed resources, etc. The figure 1 illustrates the complexity of forage intake regulation (Baumont et al., 2000).
3.1. Role of the ruminal fill
16The fill gut capacity, in relation to forages characteristics, can be considered as a main factor of regulation of voluntary intake. Intake appears limited by the maximal volume that the digestive tract can reach (Allison, 1985; Allen, 1996), even if herbivores are able to progressively modify the volume of their rumen and to increase the transit rate of digesta when the quality of forage decreased (Johnson et al., 1991; 1992; Van Soest3, 1994 cited by Schettini et al., 1999). This has been confirmed by the introduction, into the rumen, of tennis balls, water filled bags, or artificial fibres. More the ruminal ballast is bulky, in volume or in weight, more the intake decreases with or without digestibility modification (Schettini et al., 1999). In a recent study, Gregorini et al. (2007) confirm that ruminal fill can affect grazing behavior in terms of bite mass, bite depth and bite area. In this way, it is the short-term intake that is affected by the ruminal fill.
17In link to the ruminal fill, forage dry mater content can influence the voluntary intake. If dry matter of forage is lower than 20%, as in young grazed grass, the volume of water in the rumen increases and has depressive effect on the intake level, this in spite of a high forage digestibility (Pasha et al., 1994; Meissner et al., 1995). The age of plant regrowth is also a factor of variation. When plant growths and ages, protein content decreases, cell walls and tissues lignification increase with, as a consequence, an increase of forage retention time in the rumen, limiting voluntary intake (Jung et al., 1995; Baumont et al., 2000; Arthington et al., 2005). According to Parga et al. (2002), the daily herbage intake of lactating dairy cows decreases by 8.4% when comparing short and high age of grass regrowth. Jung et al. (1995) and Vazquez et al. (2000) confirmed that the level of dry matter intake was negatively correlated to the hemicellulose and cellulose (NDF) content but as described by the low coefficient of correlation (r = -0.65 and = -0.31), NDF alone appears as a bad predictor of intake.
3.2. Role of animal nutriment requirements and nutrient concentrations in forage
18The nutrients concentrations of forages are factors of interest in the regulation of food intake. On a "requirement theory" basis, the animal eats in order to maximize its production potential under some constraints such as its gut volume and diets quality (Yearsley et al., 2001). According to this theory, intake regulation would be based on the meeting of energy needs (Van Wieren, 1996; Kyriazakis, 2003). In this way, Peyraud et al. (1996) and Faverdin et al. (2007) have demonstrated that intake is positively linked to the body weight and to the level of production of dairy cows and so linked to the animal requirements. In a meta-analysis performed by Vazquez et al. (2000), factors linked to dairy cow needs and performances such as animal body weight, change in body weight and milk yield explain 71% of the total variations observed in dry matter intake. Hristov et al. (2005) confirmed that dry matter intake is strongly correlated to both nutrient digestibility and animal requirements. As a consequence, ruminants eating very fibrous forages are generally unable to cover their energy needs (Jung et al., 1995).
19In link to energy needs cover, animal physiological state appears to be an important factor controlling voluntary intake. As an example, lactating dairy or suckler cows, with their higher energy requirements, graze more selectively (selecting more bites on green grasses) and more intensively (expressing longer grazing periods) than dry cows (Gibb et al., 1999; Farruggia et al., 2006). As reported by Johnson et al. (1991; 1992), it exists a critical rumen fill above which dry matter intake is limited. This critical rumen fill could be different related to the physiological status of animal. So, the introduction of ballast into the rumen of dairy cows when the energy requirements are high (beginning of lactation), involved an important decrease of the intake level (0.043 to 0.099 kg dry matter per liter of added bulk). To attempt the energy need, cows in early lactation compensate with increasing the ruminal volume, decreasing the volume of digesta and increasing the passage rate of digesta. If the same experiment is repeated with dairy cows later in their lactation; when energy requirements are lower, intake does not seem to be modified by the introduction of the ballast in the rumen.
20The pregnancy state is also a factor controlling the voluntary intake. In the predictive equation of Faverdin et al. (2006), intake capacity of dairy cows is proportional to lactation state, age and maturity of cow, and to a “pregnancy indicator” that explains the decrease of intake during the last weeks of pregnancy.
21Animals can also regulate their intake in link to forages nutrient concentration. In this way, Cooper et al. (1995) have shown that, if sheep have the choice between two diets with different energy levels, they choose the diet that presents the highest energy density. If sheep have not the choice, they adapte their intake until their requirements are met.
22The energy – protein balance of the diet can also influence the level of intake and the diet selection. So, lambs that can select between pairs of diets, varying between 7.8 and 23.5% of crude protein, present a maximal level of intake with the diets containing between 14.1 and 17.2% of crude proteins (Kyriazakis et al., 1993). According to these observations, a judicious forage supplementation could contribute to improve diets nutritional balance and so, to increase the total voluntary intake (Berzaghi et al., 1996; Lippke et al., 2000; Vazquez et al., 2000).
3.3. Role of the intake’s peri and post-ingestive feedback
23The level of intake can be conditioned by other characteristics of the forage, such as flavor (taste and smell), appearance, texture and by the post-ingestive feedback occurring after its intake. In other terms: “if forage tastes good, animals tend to eat it more” (Baumont, 1996). The flavor – feedback interaction depends directly on chemical characteristics of feed, animal nutritional status and animal past or recent experiences. According to Provenza (2003a), the training of the animals can be made in various ways: “animals learn from their mother, before and after their birth, they learn from their pairs, they learn by testing new food, accepting them or rejecting them according to the consequences induced by this intake”. This can explain why the preference is never fixed. The post-ingestive feedback can evolve in relation to new experiences performed by the animal (Atwood et al., 2001).
24In the same way, ruminants are able to learn about the toxicity of a feed. For grazing and more for browsing ruminants, numerous plants contain secondary metabolites like tannins, terpenes that can affect digestibility, cause emetic effect and, as a consequence, reduce the voluntary intake. Ruminants are able to use these information to modulate or to avoid the intake of such plants if it is necessary. For instance, browsing goats tend to reduce their preference for browses sprayed with lithium chloride, chemical component associated with a high negative post-ingestive feedback (Ginane et al., 2005; Duncan et al., 2006); tannins may reduce the grazing of some forages legumes such Lotus pedunculatus by negatively affecting the ruminal fermentations (Reed, 1995); terpenes can inhibit the cellulolytic activity of rumen micro-organisms and so limite the intake of such plants (Nagy et al., 19684 cited by Provenza et al., 2003b).
25Finally, regulation of intake appears mediated by different signals, under the form of metabolites and hormones, emitted by the central nervous system and the peripheral organs like liver, pancreas, intestinal tracts, that can be regarded as mediators of appetite (Rhind et al., 2002). The role of leptin (hormone secreted by fatty cells), of cholocystokinine (hormone secreted by the intestinal mucous membrane), and of insulin in the control of satiety, in the ruminants, has been widely documented (Forbes, 1996; 2003). In the same way, during the digestion process, the rumen environment (pH and osmolarity) can also explain the variation of voluntary intake (Faverdin, 1999).
26As demonstrated by Cooper et al. (1995), sheep are able to make short-term changes in diet selection to maintain good ruminal fermentations and thereby a good well being sensation.
3.4. Role of plant morphological characteristics
27Sward characteristics, in terms of blade morphology, such as hair occurrence, thickness of cuticle (Loney et al., 2006), leaves size (Barre et al., 2006), of stems physical properties, of dead materials ratio can stimulate, limit or inhibit animal foraging behavior (Provenza, 2003a). More especially, these parameters have a huge influence on bite size and intake rate. So, Hodgson (1985)5, cited by Prache et al. (1997), reported that, in relation to grass characteristics, bite size can varied from 10 to 400 mg MO for sheep and from 70 to 610 mg MO for cattle. Under grazing, there is a close relationship between leaf proportion (Parga et al., 2002; O’Donovan et al., 2005), green leaf mass (Penning et al., 1994; Smit et al., 2005a), sward density (Prache et al., 1997) and dry matter intake.
28Explanations of the influence of plant morphological characteristics on intake are numerous. According to Benvenutti et al. (2006), stems can have a barrier effect on bite size and instantaneous intake rate. The higher is stems density, the smaller is the bite area and the slower is the biting rate. This leads to a decrease of the instantaneous intake rate. Boval et al. (2007) confirm that stem length and stem proportion in the sward have a negative impact on biting rate with correlation of, respectively, -0.67 and -0.40.
29Sward composition, in terms of plant species, can also influence the level of intake. Indeed, compared to grasses, legumes as white clover are often associated to higher level of intake (Ribeiro et al., 2003). Penning et al. (1995), Baumont (1996), Assoumaya et al. (2007) have explained that forage legumes are faster reduced in small particles than grasses and that less time is needed to take and masticate a similar bite for clover than for grass.
3.5. Role of the environment
30Intake during grazing does not depend only on diet quality. Short-term intake rate is also directly correlated to forage distribution and availability (Garcia et al., 2003). This can partly explain the lower level of intake observed under tropical rangeland, where forages resources can be scattered and/or heterogeneous with, as consequence, a reduction of the biting frequency and intake rate due to the time spent to go from one favorite site to another (Roguet et al., 1998). Nevertheless, animal can partly compensate biting rate decrease by increasing its grazing time. According to Gibb et al. (1999), when sward availability, measured through sward height, decreases, cows increase their total grazing times, their total jaw movement and their total number of bites in order to maintain their daily intake. These observations are confirmed by Boval et al. (2007) on tropical forages.
31Climatic conditions can also play an important role. Ruminant graze essentially during daylight and, in temperate climate, they make 6 to 8 meals with 2 main meals, one at the beginning and one at the end of the day. If temperature is higher than 25°C, they adapt their grazing behavior (early morning, late evening or night grazing) in order to avoid the warmest periods with, as possible consequence, a decrease of the time spent grazing and a decrease of daily intake (Baumont et al., 2000).
32Environment herbivores learning also plays an important role in resources utilization. On the one hand, animals have a memory of food allowance, location and distribution as related in the review of Dumont et al. (2003). On the other hand, the rearing practices can explain that the ability of cows to graze specific environment such as mountain slopes depends more on the rearing than on the animal breed (Meuret et al., 2006).
33Interaction between animals in the herd is sometimes cited to explain difference in animal grazing behavior. In this way, Sibbald et al. (2000) report that, on homogeneous vegetation, total time spent grazing by Scottish blackface sheep is higher when space allowance is high (200 m2 per head vs 50 m2 per head) without impact on herbage intake or digestibility. They conclude that the relation between time spent grazing and space allowance can be used to explain the extra activity required to maintain the group cohesion when space allowance increases.
4. Intake measurement under grazing
34Since numerous years, intake under grazing has been estimated through different methods. They can be classified into two categories: direct or indirect methods.
4.1. Direct measurements
35Direct methods are essentially based on the herbage mass measurement. In most cases, intake is estimated by the method of difference as used by Macoon et al. (2003) and Smit et al. (2005b). The method implies the knowledge of herbage mass before and after grazing. The herbage mass is usually estimated by cutting and weighing the grass harvested on a defined area. A “sward height meter” or “rising plate meter” or “disk meter”, measuring compressed sward surface height, may also be used to estimate grass density and quantity. The difference method is easy to apply and gives reliable results if grazing period is short (one or two days at the maximum) and stocking rate is high (ideally all grass of the grazing area must be consumed). If grazing period is longer, the error of estimation, linked to the grass re-growth during this period, is the major disadvantage of these methods. To reflect the effect of grass regrowth, herbage mass and regrowth is then measured in cages that exclude grazing animals (exclosure cages). By successive cuttings, the grazing is simulated and the herbage mass accumulation is measured. But without urinary and dung restitution, without specific defoliation linked to the grazing, the measured grass accumulation is often very different in grazed or non grazed area (Frame, 1993). The precision of the cutting methods is essentially based on the sampling methodology and a good precision is required at all step of the protocol to avoid the addition of errors of measure. This difference technique is mainly used to measure the intake of animals herd (Smit et al., 2005b).
36Instantaneous intake can also be directly estimated through live weight differences (Coates et al., 2000). With that method, it is possible to measure intake only over very short period (1 hour as an example). The accuracy of the measure is strongly dependent on the precision of balance and of the weight loss related to the dung and urine excretion during the period of measure.
37Another method of intake estimation is based on the hypothesis that the knowledge of animal requirements and performances is a good reflect of the nutritive values of ingested diet. The method is often used to determine the potential of intake of dairy cows as described by Faverdin et al. (2007). According to Macoon et al. (2003), for grazing supplemented dairy cows, to determine grass intake from animal performances is reliable and less expensive than other methods. With beef cattle, Minson et al. (1987) can estimate intake from live weight and rate of growth with a good accuracy (residual standard deviation of 8.7% of the mean). The difficulty of the method is, precisely, the determination of the true herbivore requirements. This is particularly true under tropical rangeland where many external factors like displacements, feed researches must to be added to basal animal needs (Allison, 1985).
4.2. Indirect measurements
38Intake of grazing ruminants can be estimated by indirect methods such as the markers techniques, ratio techniques, the recording of animal behavior and other empirical models.
39The markers technique implies the determination of natural indigestible plant components such as lignin, alkanes, or insoluble ashes which are excreted in faeces. The n-alkanes method developed by Mayes et al. (1986) appears actually as the best one to estimate intake under grazing. The method, based on the determination of the concentration, in plant and faeces, of natural alkanes and synthetic alkanes allows to calculate intake from:
40I = (Fi/Fj) x Dj / (Hi - (Fi/Fj) x Hj)
41where I = intake; Fi and Fj = concentration of natural and synthetic alkanes in faeces; Dj = dose rate of synthetic alkanes; Hi and Hj = concentration of natural and synthetic alkanes in forage.
42Ratio technique implies the determination of two parameters: forage digestibility and faecal output that allows estimating intake:
43If D = 100 x ((I – F) / I)
44I = F / (1-D/100)
45where D = forage digestibility coefficient (%); I = intake (weight unit per day); F = total faecal excretion (weight unit per day) (Lippke, 2002).
46Methods to estimate digestibility are numerous and well documented as reported in the review of Adegosan et al. (2000).
47Several methods exist for the determination of faecal output. The total collection of faeces is one of them but the method is difficult to apply at grazing. To collect faeces, animal must be harnessed with faecal bag or tethered, so such collection method can perturb grazing behavior (Lippke, 2002). Moreover a quantity of faeces difficult to estimate can escape from the collection bags and is a source of error in the estimation of intake (Adegosan et al., 2000).
48Another method to estimate faecal output is the use of indigestible external markers as chromium oxide, ytterbium. As for the total collection of faeces, the dosing of the markers requires a daily manipulation of the animal that can be a problem at grazing. The development of the controlled release device technique, that pulses automatically a daily amount of external markers in the rumen throughout the trial period, allows to limit the animal manipulation and seems sufficiently accurate to give a good estimate of faecal output (Compère et al., 1992; Berry et al., 2000; Ferreira et al., 2004).
49For the markers technique as for the ratio technique, one difficulty remains that the sampling of forage must be as representative as possible of the ingested diet. It is on this sample that indigestible natural markers or digestibility must be determined. For the n-alkanes the fact that plant organs and plant species present different n-alkanes profiles (Cortes et al., 2005) is a major source of error in the estimation of intake. In the same way, if digestibility is determined on a non representative sample of grazed grass, the estimation of intake will be biased. The hand plucking method, that simulates the biting of the herbivore, may be used to sampling grazed grass. Unfortunately, the reproductibility of the measure is linked to the calibration between animals and operator observations. Such calibration appears easier to set up with cattle than with sheep and goats that have a more selective grazing behavior (Wallis DeVries, 1995). The use of oesophageal fistulated animals appears disliked in regards to animal welfare and can modify herbivore behavior, as reported in several studies (Coates et al., 1987; Jones et al., 1992).
50Intake can be indirectly estimated by studying the grazing behavior. Indeed, intake is the product of three parameters: grazing time, biting rate and bite mass. Grazing time and biting rate can be measured by visual observation (Rook et al., 2004). The method is easier to apply and does not require costly equipments. Nevertheless the presence of the observer can perturb animal behavior at grazing, so it is primordial to accustom animals to the observer in order to avoid any behavior modification (Agreil et al., 2004).
51The recording of animal activities such as displacement, rumination, intake times have been largely tested and used to determine grazing time and biting rate (Laca et al., 2000). These recording methodologies require expensive materials and the harnessing of the animal with recording apparatus can disturb its behavior. Such techniques are difficult to apply with wild herbivores and on heterogeneous rangeland. As for the two firstly described methods, the major source of error of the measure remains the determination of the biting mass which may be estimated trough the use of oesophageally fistulated animals.
52Another alternative is the micro-histological analysis of plant residues contents in faeces or stomach and intestinal tract. The method is often used to approach the intake of wild ruminants. The main disadvantages of this technique are that, excepted for faeces collection, it requires the slaughter of the animal and that the identification of the ingested plant fraction, till the species level, is very difficult due to the digestion process (Holecheck et al., 1982a). Moreover, the quantitative estimation of the different ingested plant fractions is very few reliable as the quantity of plant fragments found in faeces or in stomachs are not, due to differential digestibility, directly proportional to the quantity of the ingested plant fractions.
53During these 30 last years many empirical models have been developed to estimate forage intake at pasture. These models are based on multiple regression between intake level and some characteristics of plant (OM yield, fibers content, digestibility, part of legumes, etc.), animal (live weight, average daily gain, milk production, stage of lactation, milk composition, pregnancy, etc.) and environment (temperature, rainfall, etc.). Most of these models derive from dairy cows experimental data (Holter et al., 1997; Delagarde et al., 2005). They are often specific to the relatively short range of grazing and experimental conditions used to develop them.
4.3. Potential of Near Infrared Reflectance Spectroscopy (NIRS)
54As related before, estimating intake of grazing ruminants is difficult. One solution could be the use of NIRS. During these 20 last years, the potential of NIRS analysis to characterize the nutritive value of grazed grass has been widely used. This indirect method is based on the establishment of calibration databases linking a NIRS spectra (light absorbencies at different wavelengths) to values, such as chemical or biological composition, obtained by reference measurement in laboratory. The most developed NIRS calibrations allow to estimate firstly in vivo digestibility and secondly intake from the analysis of different organic substrates as forage, oesophageal extruda or faeces. In most cases, digestibility and intake reference values stem from chemical analysis of forages or from animal trials.
55As reported in many earlier studies (Norris et al., 1976; Holecheck et al., 1982b; Biston et al., 1989; Lippke et al., 1989; De Boever et al., 1996), NIRS analysis of forage appears as accurate as other methods, like chemical, rumen fluid and enzymatic ones, to predict in vivo digestibility of a large range of forage. The size and the representativity of the databases regrouping various forage species, sward characteristics and localizations, or growing stage explain certainly the robustness of the NIRS technology.
56In the same time, studies have highlighted that NIRS applied to faeces could be more or even accurate than classical method to predict diet characteristics of grazing ruminants (Coleman et al., 1989; Stuth et al., 1989; Coleman et al., 1993; Leite et al., 1995; Decruyenaere et al., 2002). Indeed, chemical composition of faeces can reflect biological and chemical characteristics of ingested forage as well as the physiological status of the ruminant, two parameters of intake’s regulation. This chemical composition can be detected by NIRS and linked to intake and digestibility. So, for instance, the importance of fat wavelength to estimate digestibility and intake could be linked to higher microbial growth in the rumen and so to a higher proportion of microbes linked to the faecal forage residues (Decruyenaere et al., 2009).
57Stuth et al. (1989) and Lyons et al. (1992) have shown that in vivo grass digestibility of grazing ruminant under rangeland can be estimated by NIRS applied to faeces with the same accuracy as the one obtained with conventional analysis methods (standard error of calibration = 0.033). Garnsworthy et al. (2004) have concluded that direct prediction of dry matter intake of dairy cows by NIRS applied to faeces or by n-alkanes methods have similar accuracy (error of estimation = 0.36 and 0.44 kg DM per day from n-alkanes and 0.48 kg DM per day from faecal NIRS). More recently, Boval et al. (2004), Landau et al. (2004), Li et al. (2007) and Decruyenaere et al. (2009) have confirmed the interest of faecal NIRS to assess diet characteristics of respectively cattle, dairy goats and sheep.
58The main constraint of NIRS technique is the cost of the analytical equipment and the necessity to develop large reference databases that must be frequently updated to develop robust calibrations covering the different field situations. Moreover, as the robustness of the calibration is directly linked to the accuracy of the method used to obtain the reference values, a special attention has to be paid on this aspect. Actually, the digestibility trials and the n-alkanes techniques would be the best ones to produce in vivo digestibility and intake reference values for matching with faecal or forage NIRS spectra (Coates et al., 2000). The need of independent set of samples to validate the calibrations is also pointed as a disadvantage of the NIRS. Nevertheless, for small samples set, the cross validation technique can be used to evaluate the accuracy of the model (De Boever et al., 1995). To improve the potential of faecal NIRS to estimate in vivo digestibility and intake of grazing ruminants, it will be necessary to create larger database regrouping the greatest possible references in terms of forages and animal species.
5. Conclusion
59The voluntary intake of grazing ruminants depends on multiple factors linked to herbage and animal characteristics. In relation to the complexity of the phenomenon, it is very difficult to estimate them continuously along a grazing season and with a sufficient accuracy. The knowledge of intake level and diets quality for grazing ruminants is however essential to improve herd management in balancing sward availability to animal needs. Ideally, the methods developed to reach such a target must be accurate, applicable at the individual or herd scales and easy to use. Today, this target remains difficult to reach. Indeed, methods and techniques developed to measure diet quality are often laborious and expensive, sometimes unrepresentative of the true grazing conditions and often they lack of accuracy (Lippke, 2002), due to the numerous factors of variation of these digestibility and intake parameters.
60Currently, the n-alkanes technique appears as one of the best to predict at the same time intake and digestibility of ingested diet. However, the method remains difficult to apply for long period and in free ranging schemes.
61If sufficiently robust databases and calibrations are developed, NIRS applied to faeces is a rapid and non destructive technology that can predict, simultaneously, the digestibility and intake of a large set of similar samples. Faecal NIRS appears promising as related in recent studies and could be considered as a good alternative for assessing the diet, in quantity and in quality, of grazing or ranging ruminants.
Bibliographie
Adegosan A.T., Givens D.I. & Owens E., 2000. Measuring chemical composition and nutritive values in forages. In: ’t Mannetje L. & Jones R.M., eds. Field and laboratory methods for grassland and animal production research. Wallingford, UK: CAB International, 263-278.
Agreil C. & Meuret M., 2004. An improved method for quantifying intake rate and ingestive behaviour of ruminants in diverse and variable habitats using direct observation. Small Ruminant Res., 54(1-2), 99-113.
Allen M.S., 1996. Physical constraints on voluntary intake of forages by ruminants. J. Anim. Sci., 74, 3063-3075.
Allison C.D., 1985. Factors affecting forage intake by range ruminants: a review. J. Range Manage., 38(4), 305-311.
Arthington J.D. & Brown W.F., 2005. Estimation of feeding value of four tropical forage species at two stages of maturity. J. Anim. Sci., 83, 1726-1731.
Assoumaya C., Sauvant D. & Archimède H., 2007. Etude comparative de l’ingestion et de la digestion des fourrages tropicaux et tempérés. INRA Prod. Anim., 20(5), 383-392.
Atwood S.B, Provenza F.D., Wiedmeier R.D. & Banner R.E., 2001. Influence of free-choice vs mixed-ration diets on food intake and performance of fattening calves. J. Anim. Sci., 79, 3034-3040.
Aufrère J. & Graviou D., 1996. Prévision de la digestibilité des fourrages et aliments concentrés et composés des herbivores par une méthode enzymatique pepsine-cellulase. Rapport BIPEA Fourrages 14. Gennevilliers, France : BIPEA.
Aufrère J. et al., 2007. Prévision de la digestibilité des fourrages par la méthode pepsine-cellulase. Le point sur les équations proposées. INRA Prod. Anim., 20(2), 129-136.
Babatounde S., 2005. Etude de prédiction de la valeur alimentaire de graminées et de légumineuses fourragères en zone tropicale humide du Bénin. Thèse de doctorat : Faculté universitaire des Sciences agronomiques de Gembloux (Belgique).
Barre P. et al., 2006. Morphological characteristics of perennial ryegrass leaves that influence short-term intake in dairy cows. Agron. J., 98, 978-985.
Bartiaux-Thill N. & Oger R., 1986. The indirect estimation of the digestibility in cattle of herbage from Belgian permanent pasture. Grass Forage Sci., 41, 269-272.
Baumont R., 1996. Palatabilité et comportement alimentaire chez le ruminant. INRA Prod. Anim., 9(5), 349-358.
Baumont R., Prache S., Meuret M. & Morhand Fehr P., 2000. How forage characteristics influence behaviour and intake in small ruminants: a review. Livestock Prod. Sci., 61(1), 15-28.
Benvenutti M.A., Gordon I.J. & Poppi D.P., 2006. The effect of the density and physical properties of grass stems on the foraging behaviour and instantaneous intake rate by cattle grazing an artificial reproductive tropical sward. Grass Forage Sci., 61, 272-281.
Berry N.R. et al., 2000. The accuracy of intake estimation based on the use of alkane controlled-release capsules and faeces grab sampling in cows. Ann. Zootech., 49, 3-13.
Berzaghi P., Herbein J.H. & Polan C.E., 1996. Intake, site, and extent of nutrient digestion of lactating cows grazing pasture. J. Dairy Sci., 79(9), 1581-1589.
Biston R., Dardenne P. & Demarquilly C., 1989. Determination of forage in vivo digestibility by NIRS. In: Proceedings of the 16th International grassland congress, 4-11 October 1989, Nice, France, 895-896.
Bouazizi A. & Majdoub A., 1999. Prédiction des quantités ingérées et de la digestibilité du régime sélectionné par des ovins sur parcours semi-aride tunisien. Fourrages, 157, 77-87.
Boval M. et al., 1996. Evaluation d'indicateurs fécaux pour prédire la digestibilité et les quantités ingérées de Dichanthium sp. par des bovins créoles. Ann. Zootech., 45, 121-134.
Boval M. et al., 2004. Faecal Near Infrared Reflectance Spectroscopy (NIRS) to assess chemical composition, in vivo digestibility and intake of tropical grass by Creole cattle. Anim. Feed Sci. Technol., 114(1), 19-29.
Boval M., Fanchone A., Archimède H. & Gibb M.J., 2007. Effect of structure of a tropical pasture on ingestive behaviour, digestibility of diet and daily intake by grazing cattle. Grass Forage Sci., 62, 44-54.
Brand T.S., 2000. Grazing behaviour and diet selection by Dorper sheep. Small Ruminant Res., 36(2), 147-158.
Coates D.B., Schachenmann P. & Jones R.J., 1987. Reliability of extrusa samples collected from steers fistulated at the oesophagus to estimate the diet of resident animals in grazing experiments. Aust. J. Exp. Agric., 27, 739-745.
Coates D.B. & Penning P., 2000. Measuring animal performances. In: ’t Mannetje L. & Jones R.M., eds. Field and laboratory methods for grassland and animal production research. Wallingford, UK: CABI Publishing, 353-402.
Coleman S.W., Stuth J.W. & Holloway J.W., 1989. Monitoring the nutrition of grazing cattle with near-infrared analysis of faeces. In: Proceedings of the 16th International grassland congress, 4-11 October 1989, Nice, France, 881-882.
Coleman S.W. & Murray I., 1993. The use of near-infrared reflectance spectroscopy to define nutrient digestion of hay by cattle. Anim. Feed Sci. Technol., 44, 237-249.
Compère R. et al., 1992. Comparaison de deux méthodes utilisant le Cr2O3 pour estimer la quantité de fèces émises par les moutons : pellets de luzerne et bolus CAPTEC-CRD. Bull. Rech. Agron. Gembloux, 27, 365-383.
Cooper D.B. & Kyriazakis I., 1995. Diet selection in sheep: the role of the rumen environment in the selection of a diet from two feeds that differ in their energy density. Br. J. Nutr., 74, 39-54.
Cortes C., Damanesco J.C., Bechet G. & Prache S., 2005. Species composition of ryegrass (Lolium perenne) and tall fescue (Festuca arundinacea) mixtures using various combinations of n-alkanes. Grass Forage Sci., 60, 254-261.
De Boever J.L. et al., 1988. The use of pepsin cellulase technique to predict digestibility metabolizable and net energy of forages. Anim. Feed Sci. Technol., 19, 247-260.
De Boever J.L., Cottyn B.G., Vanacker J.M. & Boucque C.V., 1995. The use of NIRS to predict the chemical composition and the energy value of compound feeds for cattle. Anim. Feed Sci. Technol., 51, 243-253.
De Boever J.L. et al., 1996. Prediction of the feeding value of grass silages by chemical parameters, in vitro digestibility and near-infrared reflectance spectroscopy. Anim. Feed Sci. Technol., 60(1-2), 103-116.
Decruyenaere V. et al., 2002. Improvement and validation of the NIRS analysis applied to faeces to measure grass intake in pasture. In: Durand J.L., Emile J.C., Huygue Ch. & Lemaire G., eds. Proceedings of the 19th General meeting of the European Grassland Federation on multi-function grasslands, quality forages, animal products and landscapes, 29.05.2002, La Rochelle, France. British Grassland Society, Reading, UK: 196-197.
Decruyenaere V. et al., 2009. Evaluation of green forage intake and digestibility in ruminants using near infrared reflectance spectroscopy (NIRS): developing a global calibration. Anim. Feed Sci. Technol., 148, 2-4, 138-156.
Delaby L. & Pecatte J.R., 2003. Valeur alimentaire des prairies d’association ray-grass anglais/trèfle blanc utilisées entre 6 et 12 semaines de repousse. In: Actes des 10e Journées autour des Recherches sur les Ruminants, Rencontres Recherches Ruminants, 3-4.12.2003, Paris, France. Paris : INRA Editions, 389.
Delaby L., Pecatte J.R, Aufrère J. & Baumont R., 2007. Description et prévision de la valeur alimentaire de prairies multi-espèces. Premiers résultats. In: Actes des 14e Journées autour des Recherches sur les Ruminants, Rencontres Recherches Ruminants, 5-6.12.2007, Paris, France. Paris : INRA Editions, 249.
Delagarde R. & O’Donovan M., 2005. Les modèles de prévision de l’ingestion journalière d’herbe et de la production laitière des vaches au pâturage. INRA Prod. Anim., 18(4), 241-253.
Demarquilly C. & Jarrige R., 1981. Panorama des méthodes de prévision de la digestibilité et de la valeur énergétique des fourrages. In : Demarquilly C., ed. Prévision de la valeur nutritive des aliments des ruminants. Paris : INRA, 41-59.
Demarquilly C., Chenost M. & Giger S., 1995. Pertes fécales et digestibilités des aliments et des rations. In : Jarrigue R. et al., eds. Nutrition des ruminants domestiques, ingestion et digestion. Paris : INRA, 601-647.
Demment M.W. & Van Soest P.J., 1985. A nutritional explanation for body-size patterns of ruminant and non-ruminant herbivores. Am. Nat., 125, 641-672.
Dove H. & Mayes R.W., 1991. The use of plant wax alkanes as marker substance in studies of nutrition herbivores: a review. Aust. J. Agric. Res., 42, 913-952.
Dove H. & Mayes R.W., 1996. Plant wax components: a new approach to estimating intake and diet composition in herbivores. J. Nutr., 126, 13-26.
Dumont B. & Gordon I.J., 2003. Diet selection and intake within sites and across landscapes. In: Herrera-Camacho C.A. & Sandoval-Castro J., eds. Proceedings of the 6th International symposium on the nutrition of herbivores, 9-24 October 2003, Universidad Autonoma de Yucatan, Merida, Mexico, 175-194.
Duncan A.J. et al., 2006. How do herbivores trade-off the positive and negative consequences of diet selection decisions? Anim. Behav., 71(1), 93-99.
Fahey G.C. & Jung H.G., 1983. Lignin as marker in digestion studies: a review. J. Anim Sci., 57, 220-225.
Farruggia A. et al., 2006. Diet selection of dry and lactating beef cows grazing extensive pastures in late autumn. Grass Forage Sci., 61, 347-353.
Faverdin P., 1999. The effect of nutrients on feed intake in ruminants. Proc. Nutr. Soc., 58, 523-531.
Faverdin P., Delaby L. & Delagarde R., 2006. Prévision de l’ingestion des vaches laitières au cours de la lactation. In: Actes des 13e Journées autour des Recherches sur les Ruminants, Rencontres Recherches Ruminants, 6-7.12.2006, Paris, France. Paris : INRA Editions, 85-88.
Faverdin P., Delaby L. & Delagarde R., 2007. L’ingestion d’aliments par les vaches laitières et sa prévision au cours de la lactation. INRA Prod. Anim., 20(2), 151-162.
Ferreira L.M.M. et al., 2004. Estimation of feed intake by cattle using controlled-release capsules containing n-alkanes or chromium sesquioxide. J. Agric. Sci., 142, 225-234.
Forbes J.M., 1996. Integration of regulatory signals controlling forage intake in ruminants. J. Anim. Sci., 74, 3029-3035.
Forbes J.M., 2003. The multifactorial nature of food intake control. J. Anim. Sci., 81(E. Suppl. 2), 139-144.
Frame J., 1993. Herbage mass. In: Davies A., Baker R.D., Grand S.A. & Laidlaw A.S., eds. Sward measurement handbook. 2nd ed. Hurley, Berkshire, UK: British Grassland Society, 39-68.
Garcia F., Carrère P., Soussana J.F. & Baumont R., 2003. How do severity and frequency of grazing affect sward characteristics and the choices of sheep during the grazing season? Grass Forage Sci., 58, 138-150.
Garnsworthy P.C. & Unal Y., 2004. Estimation of dry-matter intake and digestibility in group-fed dairy cows using near infrared reflectance spectroscopy. Anim. Sci., 79, 327-334.
Gibb M.J., Huckle C.A., Nuthall R. & Rook A.J., 1999. The effect of physiological state (lactating or dry) and sward surface height on grazing behaviour and intake by dairy cows. Appl. Anim. Behav. Sci., 63(4), 269-287.
Ginane C. et al., 2005. Herbivore diet selection in response to simulated variation in nutrient rewards and plants secondary compounds. Anim. Behav., 69(3), 541-550.
Gregorini P., Gunter S.A., Masino C.A. & Beck P.A., 2007. Effect of ruminal fill on short-term intake rate and grazing dynamics of beef heifers. Grass Forage Sci., 62, 346-354.
Holden L.A., Muller L.D. & Fales S.L., 1994. Estimation of intake in high producing Holstein cows grazing grass pasture. J. Dairy Sci., 77, 2332-2340.
Holechek J.L., Vavra M. & Piepper R.D., 1982a. Botanical composition determination of range herbivores diets: a review. J. Range Manage., 35(3), 309-315.
Holechek J.L., Shenk J.S., Vavra M. & Arthun D., 1982b. Prediction of forage quality using near-infrared reflectance spectroscopy on oesophageal fistula samples from cattle on mountain range. J. Anim. Sci., 55, 971-975.
Holter J.B., West J.W. & Mc Gilliard M.L., 1997. Predicting ad-libitum dry matter intake and yield of Holstein cows. J. Dairy Sci., 80, 2188-2199.
Hristov A.N., Price W.J. & Shafii B., 2005. A meta-analysis on the relationship between intake of nutrients and body weight with milk volume and milk protein yield in dairy cows. J. Dairy Sci., 88, 2860-2869.
Illius A.W. & Jessop N.S., 1996. Metabolic constraints on voluntary intake in ruminants. J. Anim. Sci., 74, 3052-3062.
INRA, 2007. Alimentation des bovins, ovins et caprins. Besoins des animaux, valeurs des aliments. Versailles, France: Editions Quæ.
Jarrige R. & Thivend P., 1969. Action d’une cellulase fongique sur les membranes et son intérêt pour prévoir la digestibilité des plantes fourragères. Ann. Biol. Anim. Biochim. Biophys., 9, 171-190.
Jarrige R. et al., 1986. The INRA «fill unit» system for predicting the voluntary intake of forage-based diet in ruminants: a review. J. Anim. Sci., 63, 1737-1758.
Johnson T.R. & Combs D.K., 1991. Effects of prepartum diet, inert rumen bulk, and dietary polyethylene glycol on dry matter intake of lactating dairy cows. J. Dairy Sci., 74, 933-944.
Johnson T.R. & Combs D.K., 1992. Effects of inert rumen bulk on dry matter intake in early and midlactation cows fed diets differing in forage content. J. Dairy Sci., 75, 508-519.
Jones R.J. & Lascano C.E., 1992. Oesophageal fistulated cattle can give unreliable estimates of the proportion of legume in the diets of resident animals grazing tropical pastures. Grass Forage Sci., 47, 128-132.
Jung H.G. & Allen M.S., 1995. Characteristics of plant cell walls affecting intake and digestibility of forages by ruminants. J. Anim. Sci., 73, 2774-2790.
Kloppenburg P.B, Kiesling H.E., Kirksey R.E. & Donart G.B., 1995. Forage quality, intake and digestibility of yearlong pastures for steers. J. Range Manage., 48, 542-548.
Kyriazakis I., 2003. What are ruminant herbivores trying to achieve through their feeding behaviour and food intake. In: Herrera-Camacho C.A. & Sandoval-Castro J., eds. Proceedings of the 6th International symposium on the nutrition of herbivores, 9-24 October 2003, Universidad Autonoma de Yucatan, Merida, Mexico, 153-173.
Kyriazakis I. & Oldham J.D., 1993. Diet selection in sheep: the ability of growing lambs to select a diet that meets their crude protein (nitrogen x 6.25) requirements. Br. J. Nutr., 69, 617-629.
Laca E.A. & Wallis de Vries M.F., 2000. Acoustic measurement of intake and grazing behaviour of cattle. Grass Forage Sci., 55, 97-104.
Landau S., Glasser T., Dvash L. & Perevolotsky A., 2004. Faecal NIRS to monitor diet of mediterranean goats. S. Afr. J. Anim. Sci., 34(1), 76-80.
Lecomte Ph., Dardenne P. & Agneessens R., 1992. Prédiction de la digestibilité de la matière organique des fourrages verts par la méthode enzymatique à la pepsine cellulase et par spectrométrie dans le proche infrarouge. Rev. Agric., 45(1), 77-81.
Leite E.R. & Stuth J.W., 1995. Fecal NIRS equations to assess diet quality of free ranging goats. Small Ruminant Res., 15(3), 223-230.
Li H. et al., 2007. Faecal near infrared reflectance spectroscopy to predict diet quality for sheep. Small Ruminant Res., 68(3), 263-268.
Lippke H., 2002. Estimation of forage intake by ruminants on pasture. Crop Sci., 42(3), 869-872.
Lippke H., Barton II F.E. & Ocumpaugh W.R., 1989. Near infrared reflectance spectroscopy for estimation of digestible organic matter intake and body weight gain. In: Proceedings of the 16th International grassland congress, 4-11 October 1989, Nice, France, 893-894.
Lippke H., Forbes T.D.A. & Ellis W.C., 2000. Effect of supplement on growth and forage intake by stocker steers grazing wheat pasture. J. Anim. Sci., 78(6), 1625-1635.
Loney P.E., Mc Arthur Y.C., Potts B.M. & Jordan G.J., 2006. How does ontogeny in a Eucalyptus species affect patterns of herbivory by Brushtail Possums? Funct. Ecol., 20(6), 982-988.
Lyons R.K. & Stuth J.W., 1992. Faecal NIRS equations for predicting diet quality of free-ranging cattle. J. Range Manage., 45, 238-244.
Macoon B. et al., 2003. Comparison of three techniques for estimating the forage intake of lactating dairy cows at pasture. J. Anim. Sci., 81, 2357-2366.
Mayes R.W., Lamb C.S. & Colgrove P.M., 1986. The use of dosed and herbage n-alkanes as markers for determination of herbage intake. J. Agric. Sci. Camb., 107, 161-170.
Meissner H.H. & Paulsmeier D.V., 1995. Plant compositional constituents affecting between-plant and animal species prediction of forage intake. J. Anim. Sci., 73, 2447-2457.
Menke K.H. et al., 1979. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci., 93, 217-222.
Meuret M., Débit S., Agreil C. & Osty P.L., 2006. Eduquer ses veaux et ses génisses : un savoir empirique pertinent pour l’agroenvironnement en montagne ? Nat. Sci. Soc., 14, 343-352.
Minson D.J. & Mc Donald C.K., 1987. Estimating forages intake from the growth of beef cattle. Trop. Grassl., 21, 116-122.
Norris K.H., Barnes R.F., Moore J.E. & Shenk J.S., 1976. Predicting forage quality by infrared reflectance spectroscopy. J. Anim. Sci., 43, 889-897.
O’Donovan M. & Delaby L., 2005. A comparison of perennial ryegrass cultivars differing in heading date and grass ploidy with spring calving dairy cows grazed at two different stocking rate. Anim. Res., 54, 337-350.
Parga J., Peyraud J.L. & Delagarde R., 2002. Age of regrowth affects grass intake and ruminal fermentations in grazing dairy cows. In: Durand J.L., Emile J.C., Huyghe C. & Lemaire G., eds. Proceedings of the 19th General meeting of the European Grassland Federation on multi-function grasslands, quality forages, animal products and landscapes, 29.05.2002, La Rochelle, France. British Grassland Society, Reading, UK: 256-257.
Pasha T.N., Prigge E.C., Russell R.W. & Bryan W.B., 1994. Influence of moisture content of forage diets on intake and digestion by sheep. J. Anim. Sci., 72(9), 2455-2463.
Pearson R.A., Archibald R.F. & Muirhead R.H., 2005. A comparison of the effect of forage type and level of feeding on the digestibility and gastrointestinal mean retention time of dry forages given to cattle, sheep, ponies and donkeys. Br. J. Nutr., 95(1), 88-98.
Penning P.D., Parsons A.J., Orr R.J. & Hooper G.E., 1994. Intake and behaviour responses by sheep to changes in sward characteristics under rotational grazing. Grass Forage Sci., 49, 476-486.
Penning P.D. et al., 1995. Intake and behaviour responses by sheep, in different physiological states, when grazing monocultures of grass or white clover. Appl. Anim. Behav. Sci., 45(1-2), 63-78.
Peyraud J.L., 1998. Techniques for measuring faecal flow, digestibility and intake of herbage in grazing ruminants. Techniques for investigating intake and ingestive behaviour by farm animals. In: Davies A.M.C. & Giangiacomo R., eds. Proceedings of the 9th European intake workshop, IGER North Wyke, UK, 39-48.
Peyraud J.L., Comeron E.A., Wade M.H. & Lemaire G., 1996. The effect of daily herbage allowance, herbage mass and animal factors upon herbage intake by grazing dairy cows. Ann. Zootech., 45(3), 201-217.
Prache S. & Peyraud J.L., 1997. Préhensibilité de l’herbe pâturée chez les bovins et les ovins. INRA Prod. Anim., 10(5), 377-390.
Provenza F.D., 2003a. Foraging behavior: managing to survive in a world of change. Washington, DC: USDA.
Provenza F.D. et al., 2003b. Linking herbivore experience, varied diets, and plant biochemical diversity. Small Ruminant Res., 49, 257-274.
Reed J.D., 1995. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci., 73, 1516-1528.
Rhind S.M., Archer Z.A. & Adam C.L., 2002. Seasonality of food intake in ruminants: recent developments in understanding. Nutr. Res. Rev., 15, 43-65.
Ribeiro H.M.N., Delagarde R. & Peyraud J.L., 2003. Inclusion of white clover in strip-grazed ryegrass swards: herbage intake and milk yield of dairy cows at different ages of sward regrowth. Anim. Sci., 77, 499-510.
Roguet C., Dumont B. & Prache S., 1998. Sélection et utilisation des ressources fourragères par les herbivores : théories et expérimentations à l’échelle du site et de la station alimentaires. INRA Prod. Anim., 11(4), 273-284.
Rook A.J. et al., 2004. Bite dimensions and grazing movements by sheep and cattle grazing homogeneous perennial ryegrass swards. Appl. Anim. Behav. Sci., 88, 227-242.
Sauvant D., Assoumaya C., Giger-Reverdin S. & Archimède H., 2006. Etude comparative du mode d’expression du niveau d’alimentation chez les ruminants. In: Actes des 13e Journées autour des Recherches sur les Ruminants, Rencontres Recherches Ruminants, 6-7.12.2006, Paris, France. Paris : INRA Editions, 103.
Schettini M.A., Prigge E.C. & Nestor E.L., 1999. Influence of mass and volume of ruminal contents on voluntary intake and digesta passage of forage diet in steers. J. Anim. Sci., 77, 1896-1904.
Scott L.L. & Provenza F.D., 1999. Variation in food selection among lambs: effects of basal diet and foods offered in a meal. J. Anim Sci., 77, 2391-2397.
Sibbald A.M., Shellard L.J. & Smart T.S., 2000. Effect of space allowance on the grazing behaviour and spacing of sheep. Appl. Anim. Behav. Sci., 70(1), 49-62.
Smit H.J. et al., 2005a. Effects of perennial ryegrass (Lolium perenne L.) cultivars on herbage production, nutritional quality and herbage intake of grazing dairy cows. Grass Forage Sci., 60, 297-309.
Smit H.J., Taweel H.Z., Tas B.M. & Elgersma A., 2005b. Comparison of techniques for estimating herbage intake of grazing dairy cows. J. Dairy Sci., 88, 1827-1836.
Sprinkle J.E. et al., 2000. Digesta kinetics, energy intake, grazing behaviour, and body temperature of grazing beef cattle differing in adaptation to heat. J. Anim. Sci., 78, 1608-1624.
Stern M.D., Bach A. & Calsamiglia S., 1997. Alternative techniques for measuring nutrient digestion in ruminants. J. Anim. Sci., 75, 2256-2276.
Stuth J.W., Kapes E.D. & Lyons R.K., 1989. Use of near infrared spectroscopy to assess nutritional status of cattle diets on rangeland. In: Proceedings of the 16th International grassland congress, Nice, France, 889-890.
Sunvold G.D. & Cochran R.C., 1991. Technical note: evaluation of acid detergent lignin, alkaline peroxide lignin, acid insoluble ash, and indigestible acid detergent fiber as internal markers for prediction of alfalfa, bromegrass, and prairie hay digestibility by beef steers. J. Anim. Sci., 69, 4951-4955.
Tilley J.H.A. & Terry R.A., 1963. A two stage technique for in vitro digestion of forage crops. Grass Forage Sci., 18, 104-111.
Valente M.E., Borreani G., Peiretti P.G. & Tabacco E., 2000. Codified morphological stage for predicting digestibility of Italian ryegrass during spring cycle. Agron. J., 92, 967-973.
Van Wieren S.E., 1996. Do large herbivores select a diet that maximizes short-term energy intake rate? Forest Ecol. Manage., 88, 149-156.
Vazquez O.P. & Smith T.R., 2000. Factors affecting pasture intake and total dry matter intake in grazing dairy cows. J. Dairy Sci., 83, 2301-2309.
Wallis DeVries M.F., 1995. Estimating forage intake and quality in grazing cattle: a reconsideration of the hand-plucking method. J. Range Manage., 48, 370-375.
Yearsley J., Tolkamp B.J.N. & Illius A.W., 2001. Theoretical developments in the study and prediction of food intake. Proc. Nutr. Soc., 60, 145-156.
Notes
To cite this article
About: Virginie Decruyenaere
Walloon Agricultural Research Centre (CRA-W). Farming Systems Section. Rue du Serpont, 100. B-6800 Libramont-Chevigny (Belgium). E-mail: decruyenaere@cra.wallonie.be
About: André Buldgen †
Univ. Liege - Gembloux Agro-Bio Tech. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
About: Didier Stilmant
Walloon Agricultural Research Centre (CRA-W). Farming Systems Section. Rue du Serpont, 100. B-6800 Libramont-Chevigny (Belgium).






