- Home
- Volume 14 (2010)
- numéro 1
- Development of entomotoxic molecules as control agents: illustration of some protein potential uses and limits of lectins (Review)
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Development of entomotoxic molecules as control agents: illustration of some protein potential uses and limits of lectins (Review)

Editor's Notes
Received on 28 November 2008; accepted on 10 June 2009
Résumé
Développement de molécules entomotoxiques comme agents de contrôle : utilisations potentielles de certaines protéines et limites des lectines (Revue). L'utilisation des pesticides à travers le monde a augmenté de façon spectaculaire ces vingt dernières années. Pourtant, les effets que ceux-ci peuvent avoir vis-à-vis de la santé humaine et de l'environnement ont déjà été largement mis en évidence par de nombreux auteurs. Pour réduire l'utilisation de substances toxiques, des efforts ont été concentrés sur le développement d'alternatives biologiques. Pendant plusieurs décennies, les toxines produites par la bactérie Bacillus thuringiensis (Bt) ont été le seul principe actif intégré dans des organismes génétiquement modifiés (OGM) de grandes cultures afin de conférer des résistances aux insectes ravageurs. Ces dernières années, plusieurs recherches ont été menées sur le développement de nouvelles molécules insecticides, notamment des protéines de défense produites naturellement dans certaines plantes. Parmi ces protéines se trouvent, entre autres, des inhibiteurs d'enzymes digestives et des lectines. Une des méthodes prometteuses pour accroître la résistance des plantes serait de valoriser en particulier le potentiel des lectines d'origine végétale ou fongique vis-à-vis des ravageurs.
Abstract
Worldwide use of pesticide has dramatically increased during the last two decades, which are known to cause a lot of problems to both environment and human health. Due to the harmful effects of insecticide on environment, human health, non target organism, water pollution and increasing insect resistance, recent efforts have been made are to reduce broad spectrum of toxicant added to the environment. It is necessary to balance agricultural needs with environmental and health issues when using insecticides. Currently, the use of entomotoxic proteins has been increased because of the growing need to obtain better food quality and to protect the environment. Bt toxins derived from bacteria, Bacillus thuringiensis (Bt) the main commercial genetically modified organism (GMO), or entomotoxic product were successfully used against a range of insect orders such as Lepidoptera, Coleoptera and Diptera. In recent years, several investigations were focused on other entomotoxic potential, including products based on some defense proteins present in plants. Among them some classes of proteins such as digestive enzyme inhibitors and lectins showed greater potential for exploitation in transgenic-based pest control strategies. Currently, some lectins from plant and fungal origin were successfully used against a wide range of important insect pests.
Table of content
1. Introduction
1Due to deleterious effects of chemical pesticides on the environment and non target organisms, recent efforts have been made to reduce the systematic application of pesticides and to replace them with other methods. Hence, there has been a need to discover new effective pests control methods conferring a protection to plants against insect pests (Conner et al., 2003; Majumder et al., 2005; Ferry et al., 2006; Macedo et al., 2006). First effort for this purpose was to develop and increase the use of protein toxins from Bacillus thuringiensis (Bt). This bacterial protein was widely used as bio-insecticide against a wide range of insect pests because it has little effect on the other organisms and environment. Indeed, this method was first started commercially in 1950s with Bt toxins as biological insecticides (Aronson, 1994; Federici, 2005). Bt preparations were initially sprayed directly for several decades on plants to protect them against a variety of insect pests, but in 1981 the genes encoding the pesticide proteins have also been successfully transferred into plant species such as maize, providing protection without the need for spraying (Schnepf et al., 1981; Federici, 2005).
2Currently, due to resistance of some insect pests to repeated Bt toxin applications (Tabashnik et al., 1990; McGaughey et al., 1992; Ferre et al., 2002; Janmaat et al., 2003), and none affected homopteran insect pests by Bt toxins, other supplemented methods, including the use of entomotoxic proteins were introduced (Bandyopadhyay et al., 2001; Carlini et al., 2002; Vasconcelos et al., 2004; Wang et al., 2006). To date, several different classes of plant proteins including lectins, ribosome-inactivating proteins, protease inhibitors and α-amilase inhibitors have been shown to be insecticidal effects towards a range of economically important insect pests by direct assay or by expression in transgenic plants (Ishimoto et al., 1989; Ryan, 1990; Chrispeels et al., 1998; Gatehouse et al., 1998; Ussuf et al., 2001).
3So far, one of the efficient strategies against insect pests has been exclusively based on lectins, mainly from the mannose and mannose/glucose. Some of these, such as Galanthus nivalis agglutinin (GNA), a mannose specific lectin and Canavalia ensiformis, agglutinin (ConA) a mannose/glucose specific lectin have been successfully engineered into a variety of crops including wheat, tobacco, sugarcane, rice and potatoes as resistant factor against some of their insect attacks (Powell et al., 1993; Gatehouse et al., 1996; Sauvion et al., 2004).
4More recently, in addition to the use of a broad range of plant lectins, another source of protein derived from fungi was successfully delivered via artificial diet against some of important insect pests, and were shown to have high deleterious effects on these insects, compared to plant lectins (Trigueros et al., 2003). One of the most important features of fungal lectins, compatible with the proposed defensive function, is their high toxic property to aphids' pests, compared to well-known plant lectins (Trigueros et al., 2003; Karimi et al., 2007).
5In this review, we attempted to analyze the literature data on the role played by entomotoxic proteins in the very complex relationships between plants and the insects that feed on plants.
2. Plant-insect interactions
6On one hand, plants and insects are some of the living organisms that are continuously interacting in a complex way. Plants developed different mechanisms to reduce insect attack, including specific responses that activate different metabolic pathways which considerably alter their chemical and physical aspects (Ryan, 1990; Smith et al., 2006). The best-known plant substances supposedly involved in defence mechanisms against phytophagous insects are ribosome-inactivating proteins (RIPs), protease inhibitors (PIs), amylase inhibitors (αAl-I) and lectins. They are particularly abundant in plant storage organs such as tubers and seeds (Ferrari et al., 1991; Aronson, 1994; Peumans et al., 1995; Koiwa et al., 1997; Halitschke et al., 2001; Murdock et al., 2002; Carlini et al., 2002; Bell et al., 2004).
7On the other hand, insects have developed several strategies to overcome plant defense barriers, allowing them to feed, grow and reproduce on their host plants. However, insects possess a powerful assemblage of enzymes that constitute their defense against chemical toxicants (Chrispeels et al., 1991; Scott et al., 2001). The better understanding of this complex interaction between plants and insects will allow us to achieve more effective methods for the biological control of insect pests with natural products by developing new plant varieties with enhanced genetically modified (GM) crop (Haruta et al., 2001; Halitschke et al., 2001).
3. Entomotoxic Proteins
8So far, extensive studies have been carried out to identify proteins with insecticidal properties against major economic pests. There are many proteins from different origins with direct insecticidal property and ability to express in transgenic plants. Among them some enzyme inhibitors and lectins are being evaluated for their ability to confer the broad-spectrum insect resistance in transgenic crop plants or by direct assays to control of insect pests (Carlini et al., 2002; Vasconcelos et al., 2004).
3.1. Ribosome-inactivating proteins
9Ribosome-inactivating proteins (RIPs) are a group of plant proteins that are capable of inactivating eukaryotic ribosome's and accordingly are called ribosome-inactivating proteins, which play an important role in plant defense and hence can be exploited in plant protection (Van Damme et al., 2001; Peumans et al., 2001; Sharma et al., 2004). Several lines of evidence support the idea that RIPs play a role in plant defense (Nielsen et al., 2001; Peumans et al., 2001). RIPs are subdivided on the basis of their molecular structure into three distinct groups. Type-I RIPs is composed of a single polypeptide chain and endowed with a variety of activities including immunosuppressive/anti-mitogenic, anti-tumor/anti-proliferative, and anti-viral activities (Ng et al., 1992; Barbieri et al., 1993; 1996). More recently, Bertholdo-Vargas et al. (2009) reported that type-I RIPs has also entomotoxic activity toward Lepidopteran insects. Type-II RIPs is a heterodimer consisting of two polypeptide chains (A & B chains), which A-chain is linked through a disulfide bridge to a B-chain. The A-chain has an N-glucosidase activity of the ribosomal ribonucleic acid (rRNA) and B-chain contains carbohydrate-binding domains and is also regarded as lectin. They are defence proteins that directly targeted plants eating organisms. Whereas, type-III RIPs has a single chain containing an extended carboxyl-terminal domain with unknown function (Barbieri et al., 1993; 1996; Van Damme et al., 2001; Peumans et al., 2001; Bass et al., 2004).
10Ricin, abrin and modeccin are well known examples of RIPs, which irreversibly inactivate ribosomes by removing a specific adenine from a highly conserved tetra-nucleotide loop present in the large ribosomal subunit (Endo et al., 1987; Barbieri et al., 1993). Some of these such as ricin (type-II RIPs) have high toxicity effect against a variety of insects, although these effects are variable on different insect orders (Gatehouse et al., 1990; Wei et al., 2004). More recently, Shahidi-Noghabi et al. (2009) demonstrated that expression of Sambucus niger agglutinin (SNA-I, type-II RIPs) from elderberry bark in transgenic tobacco has a deleterious effect on two important insect pests, the tobacco aphid Myzus nicotianae and the beet armyworm Spodoptera exigua. These information provide further support for RIPs having a role in the plant resistance to insect pest species.
11RIPs may be able to bind to specific sites on the cell surfaces; either exerting their toxic action at the membrane level or after uptake and internalization of the toxic polypeptide chain. Most RIPs specifically recognize galactosyl terminated glycoproteins on the cell surface and as such facilitate the entry of the RIPs onto the cell, where it can exert its enzymatic activity on ribosome or other cellular structures. Although the biochemical properties of the RIPs are well studied, but their exact mechanisms of action at the tissue level of RIPs-ingested insects are not well understood (Vervecken et al., 2000; Stirpe et al., 2006; Shahidi-Noghabi et al., 2008; 2009; Bertholdo-Vargas et al., 2009).
12Recently, one kind of RIPs from winter aconite, Eranthis hyemalis (Ranunculales: Ranunculaceae) has been shown to have high toxicity effect to mammals (Carlini et al., 2002).
3.2. Protease inhibitors
13Proteases inhibitors (PIs) are polypeptides that occur in a wide variety of plants, and are able to bind to insects mid-gut proteolytic enzymes, rendering them inactive by competitive inhibition (Laskowski et al., 1980), thus, providing a natural defense against herbivorous insects (Ryan, 1990). In plants, different roles for protease inhibitors have been suggested, including their action as regulators of endogenous proteolytic activity (Ryan, 1990), as participants in many developmental processes, as programmed cell death (Solomon et al., 1999), and as components associated with the resistance of plants against insects and pathogens (Etzler, 1986; Hilder et al., 1987; Lu et al., 1998; Pernas et al., 1999; Gatehouse et al., 1999; Ussuf et al., 2001; Rahbé et al., 2003a). PIs have been shown to function as plant defense molecules and were considered for use in preventing insect predation. They could be good candidate proteins to be used by direct assays or by expression in transgenic plants to the control of some insect pests (Green et al., 1972; Etzler, 1986; Hilder et al., 1987; Koiwa et al., 1998; Mosolov et al., 2001; Srinivasan et al., 2005).
14The defensive capacities of plant PIs rely on the inhibition of proteases present in insect guts, causing a reduction in the availability of amino acids necessary for their growth and development (De Leo et al., 1998; 2002; Hilder et al., 1999; Rahbé et al., 2003a; Zhu-Salzman et al., 2003). Insect digestive proteases can be classified as serine, cysteine, aspartic and metallo-proteases inhibitors (Richardson, 1977; Terra et al., 1994). Some of these, such as serine and cysteine proteases inhibitors are the most widely studied, and have been shown that have deleterious effects on two important orders of insect pests including Lepidopteran and Coleopteran, which may include reduced fecundity, increased mortality and decreased weight (Kuroda et al., 1996; Gruden et al., 1998; Murdock et al., 1988; Elden, 2000; Maqbool et al., 2001). A number of Coleopteran insects' mid-gut are slightly acidic and utilize cysteine and aspartic proteases to hydrolyse their dietary proteins, while most of Lepidopteran pests have alkaline mid-gut environment and use serine proteases as their major digestive enzymes (Kitch et al., 1986; Murdock et al., 1987; Terra et al., 1994).
15So far, cDNA sequences encoding different protease inhibitors have been expressed in the genome of different important plants such as potato, oilseed, rapeseed, tobacco, cereals and protective effects have been obtained in some cases, mainly against Lepidopteran, Coleopteran and Hemipteran pests (Leple et al., 1995; Urwin et al., 1995, 1998; Koiwa et al., 1997; 1998; Schuler et al., 1998; Lecardonnel et al., 1999; Ussuf et al., 2001; Maqbool et al., 2001; Ceci et al., 2003; Macedo et al., 2003; Rahbé et al., 2003a; 2003b). PIs have been used to induce enhance level of resistance to insect pests in transgenic plants, due to their small size, abundance, stability and high specificity for a particular class of digestive enzymes of insects (Ussuf et al., 2001; Abdeen et al., 2005).
16The greatest interest will probably be attracted to plants containing a combination of genes encoding protease inhibitors and other protective proteins. For example, the effect of Bt toxin was synergized by protease inhibitors. In such cases, the inhibitors can probably act not only as self-sufficient protective proteins, but can also protect other recombinant proteins from the deleterious effects of proteolytic enzymes (De Leo et al., 1998; Cloutier et al., 2000; Mosolov et al., 2001; Zhao et al., 2003; Christou et al., 2006).
17The action mode of PIs at the tissue level of PIs-ingestion insect are extremely selective and different types of those have different action mechanism and biological processes. Ramos et al. (2009) suggested that the toxic effect of the protease inhibitors induced the insect to eliminate its digestive enzymes in feces, complicating its digestion. In contrast, some insects such as Spodoptera littoralis (Lepidopteran), can overcome the deleterious effects of protease inhibitors by synthesizing different proteases that are insensitive to particular inhibitors (Paulillo et al., 2000; Brito et al., 2001; De Leo et al., 2001; Volpicella et al., 2003). Whereas, the exact action mechanism of PIs at the tissue level of insects is not well-known (Jongsma et al., 1997; Carlini et al., 2002; Amirhusin et al., 2007).
3.3. α-Amylase inhibitors
18Alpha-amylase inhibitors (αAl-I) are known as starch blockers because they contain substances that prevent dietary starches from being absorbed by the body. Microorganisms, higher plants, and animals produce a large number of different protein inhibitors of α-amylases in order to regulate the activity of these enzymes (Da Silva et al., 2000; Toledo et al., 2007). These inhibitors widely distributed in the most important digestive enzymes of many insects that feed exclusively on seed products during larval and/or adult life, thereby acting as insect anti-feedants (Chrispeels et al., 1991). Alpha-amylase inhibitors can be extracted from several types of plants, especially those in the legume family and also use as a defence mechanism against insect pests (Ishimoto et al., 1989; Kluh et al., 2005). In addition, amylase inhibitors are of great interest as potentially important tool of natural and engineered resistance factor against some of insect pests in transgenic plants (Gatehouse et al., 1998; Valencia et al., 2000; Yamada et al., 2001). The expression of the cDNA encoding αAl-I into some plants such as pea (Pisum sativum L.) and azuki bean (Vigna anguralis L.) against bruchid beetle pests (Coleoptera: Bruchidae) has been well documented for showing ability of these inhibitors to be used as plant resistance factors against some species of insect pests (Ishimoto et al., 1989; Yamada et al., 2001; Kluh et al., 2005).
3.4. Lectins
19Lectins are a class of proteins of non-immune origin that possess at least one non-catalytic domain that specifically and reversibly bind to mono- or oligosaccharides (Dixon, 1981; Peumans et al., 1995). They encompass different members that are diverse in their sequences, structures, binding site architectures, carbohydrate affinities, and specificities as well as their larger biological roles and potential applications. However, today the term lectin is broadly used to denote all types of carbohydrate-binding proteins that do not catalyse reactions with their ligands (Peumans et al., 1995; Van Damme et al., 1998; Chandra et al., 2006). Lectins bind mono- and oligosaccharides reversibly whith high specificity, defined in terms of the monosaccharide that inhibit lectin-induced agglutination or precipitation reactions. These glycoproteins are multivalent and possess more than one sugar binding site (Lis et al., 1998). They firstly were discovered more than 100 years ago by Stillmark (1888) and currently, they are extensively distributed in nature and several hundred of these molecules have been isolated from different organisms (Peumans et al., 1995; Van Damme et al., 1998). Different roles and functions have been ascribed to lectins. The principal functions of lectins are to act as recognition molecules within the immune system, protein storage, cell surface adhesion and they have been implicated in defence mechanisms against invading pathogens and predators, this function may be a major role within seeds and other peripheral tissues of the plants (Peumans et al., 1995; Van Damme et al., 1998; Rudiger et al., 2001; Trigueros et al., 2003). Lectins are broadly defined as carbohydrate-binding proteins other than enzymes or antibodies with the characteristic property of agglutinating blood cells (Van Damme et al., 1998).
20Various lectins have already been found to be toxic towards important members of insect orders, including Coleoptera (Gatehouse et al., 1984; Czapla et al., 1990), Lepidoptera (Czapla et al., 1990) and Homoptera (Powell et al., 1993; Sauvion et al., 1996). The harmful effects of lectins on biological parameters of insects include larval weight decrease, mortality, feeding inhibition, delay in total developmental duration, adult emergence and fecundity on the first and second generation (Powell et al., 1993; Habibi et al., 1993). Recently, a wide range of lectins from different organisms have been successfully examined for their negative effects on the life parameters of some economically insect pests (Foissac et al., 2000; Couty et al., 2001; Trigueros et al., 2003; Sauvion et al., 2004; Karimi et al., 2007; Shahidi-Noghabi et al., 2008; 2009).
21Sources of lectins. Lectins are extensively distributed in nature and several hundred of these molecules have been isolated from plants, fungi, viruses, bacteria, invertebrates and vertebrates (Peumans et al., 1995). Recently, one of the promising methods for plant resistance against insects attacks have increased the interest in the potential toxicity of plant and fungal lectins towards some of important insect pests (Foissac et al., 2000; Carlini et al., 2002; Trigueros et al., 2003; Sauvion et al., 2004; Karimi et al., 2007).
22Plant lectins. The best-characterized families of plant lectins are the Fabaceae, Poaceae and Solanaceae; especially some leguminous seeds have a remarkable amount of lectins. Lectins have been found in virtually, all kinds of vegetative tissues such as leaf, stem, bark, bulb, tuber, corm, rhizome, root, fruit, flower, ovary, phloem sap, latex, nodule, seed, stem and even in nectar (Van Damme et al., 1998). Plant lectins function as storage proteins and they have been implicated in defence mechanisms against invading pathogens (Powell et al., 1993; Peumans et al., 1995; Van Damme et al., 1998; Rudiger et al., 2001). The insecticidal activity of plant lectins against many important insects has been well documented to show their ability to be used as bio-pesticides (Table 1) (Gatehouse et al., 1995; Powell, 2001; Carlini et al., 2002). Among plant lectins presented in table 1 as entomotoxic lectins, GNA and ConA were more investigated and delivered successfully, via artificial diet and expressed in a range of crops, they have been shown to present deleterious effects on a range of important insect pests (Down et al., 1996; Fitches et al., 1997; 2001b; Rao et al., 1998; Foissac et al., 2000).
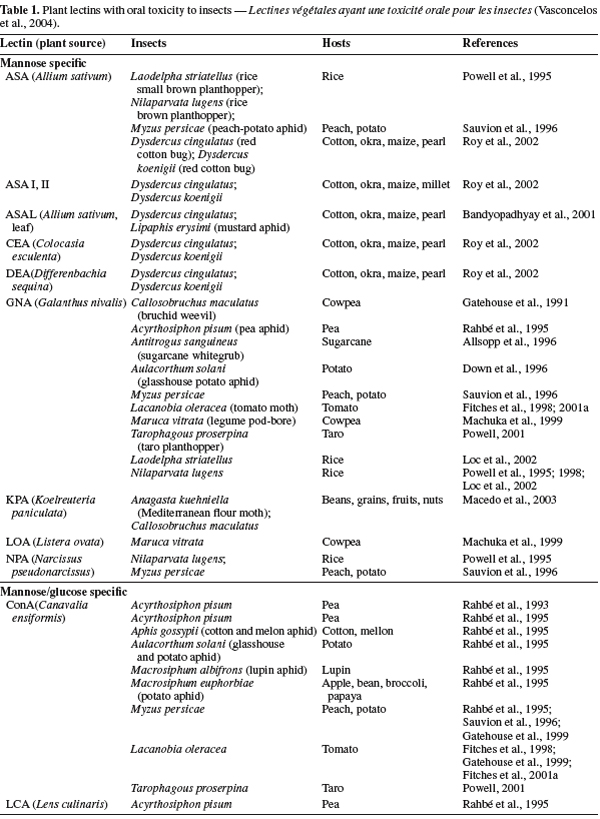
23GNA have been expressed in various crops including potato (Brich et al., 1999), tobacco (Hilder et al., 1987), wheat (Stoger et al., 1999), rice (Rao et al., 1998), sugarcane (Allsopp et al., 1996) and have been shown to exhibit significant anti-insect effects against a number of insect orders, including Lepidopteran (Fitches et al., 1997; Bell et al., 1999), Coleopteran (Leple et al., 1995) and Homopteran (Down et al., 1996; Gatehouse et al., 1996).
24ConA has shown also deleterious effect on some insect orders such as Homoptera (Powell, 2001) and Lepidoptera, when expressed in transgenic plants (Gatehouse et al., 1999). ConA affected survival; delayed development durations, larval weight and mortality of these orders that have ingested them. The effect of ConA consumption on these orders may fluctuate among different species because, some species are highly susceptible to ConA, and the effect on other may be less or moderate (Rahbé et al., 1993; Gatehouse et al., 1997; 1999; Sauvion et al., 2004) (Table 1).
25Fungal lectins. Mushrooms contain various potential interesting proteins, including lectins in their organs such as mycelium, spores and fruiting bodies (Wang et al., 1998; Wang et al., 2002; Ng, 2004). Many lectins have been derived from different fungi and partially isolated and characterized for their effects on mammalian physiology as antitumor and anticancer, but there are little information on their role on phytophagous insects (Wang et al., 2002; Trigueros et al., 2003). Until now, a range of mushroom lectins including Xerocomus chrysenteron lectin (XCL), Agaricus bisprous lectin (ABL) and Arthrobotrys oligospora lectin (AOL) have been isolated and all of them are well known for their reversible antiproliferative effects. But, only XCL has well shown significant negative effects on some insect orders, such as the Dipteran (Drosophila melanogaster) and Hemipteran (Acyrthosipon pisum), and exhibited a higher insecticidal activity than the GNA, which is one of the most lectins toxic to these insects (Trigueros et al., 2003). XCL incorporated with artificial diets has been shown highly toxic effect toward two important Homopteran pests, Myzus persicae and A. pisum when compared with control lectin, ConA (Karimi et al., 2006; 2007). XCL is produced by an edible mushroom and belongs to a group of lectins which first described for AOL, a Deuteromycetes and ABL, a Basidiomycete for sequence homology and sugar specificity, which are specific for N-acetyl-D-galactosamine and galactose (Trigueros et al., 2003; Francis et al., 2003). Recently, the entomotoxic proteins based on fungal lectin appear to be a promising biological control agent against some of the important insect pests.
26Lectin mechanism of actions. Entomotoxic lectins either directly or indirectly cause profound morphological and physiological modifications in the insect intestine. The investigations on possible mechanisms of lectin toxicity in the insects at the cellular level were initiated 24 years ago, when Gatehouse et al. (1984) firstly reported the binding of Phaseolus vulgaris lectin (PHA) to midgut epithelial cells of the cowpea weevil, Callosobruchus maculatus.
27In fact, the mode of action for each lectin at the tissue level is related to the occurrence of appropriate carbohydrate moieties on the organ surface and the ability of lectin to bind to them (Fitches et al., 2001a; 2001b). In general, the action mechanism of the lectin in insects could be the binding of them to the midgut epithelium causing disruption of the epithelial cells including elongation of the striated border microvilli, swelling of the epithelial cells into the lumen of the gut lead to complete closure of the lumen, and impaired nutrient assimilation by cells, allowing absorption of potentially harmful substances from intestine into circulatory system, fat bodies, ovarioles and throughout the haemolymph (Gatehouse et al., 1984; Powell et al., 1998; Habibi et al., 1998; 2000; Fitches et al., 1998; 2001b; Sauvion et al., 2004; Majumder et al., 2005). Consequently, the action mechanism of various lectins at the cellular levels clearly differs between different insect species, which are highly specific binding to oligosaccharides (Habibi et al., 2000; Fitches et al., 2001a; Sauvion et al., 2004).
28Lectin interaction in virus transmission. Plant viruses may be transmitted by number of routes including: seeds, vegetative propagation/grafting, mechanical and vectors. Many different groups of living organisms such as bacteria, fungi, nematodes, mites and insects can act as vectors and spread viruses from one plant to another (Gray, 1996). Currently, many of important plant viruses are transmitted by insects of the Hemiptera order (sap-sucking insects) including aphids, whiteflies, leafhoppers, planthoppers, and thrips (Gildow, 1993; Gray, 1996; Gray et al., 2003).
29Plant viruses transmitted by insects are divided into two broad categories with different transmission processes: circulative and non circulative viruses. Circulative viruses are usually defined as moving from the alimentary canal of a vector insect onto its hemocoel and are able to back out through the salivary secretory system, from which these viruses are introduced back into the plant host during insect feeding (Figure 1). Whereas, non circulative virus associated with the cuticular lining of the insect mouthparts or foregut and directly released as digestive secretions onto the plant when insect begins to feed (Gray, 1996; Gray et al., 1999; 2003; Hogenhout et al., 2008).
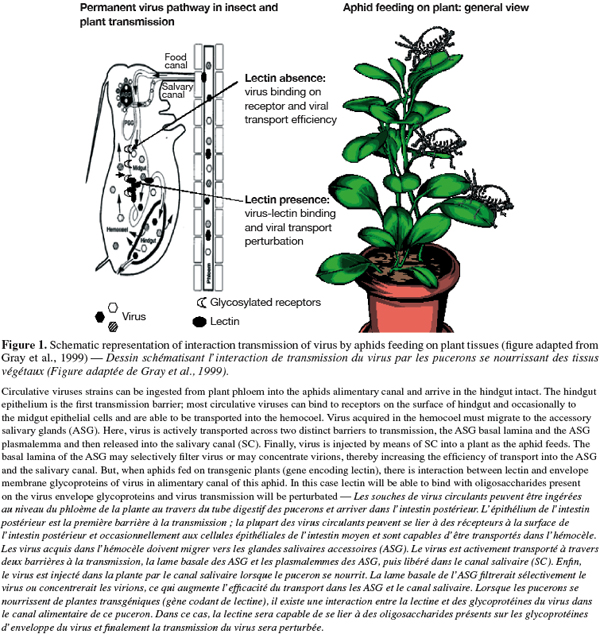
30The initiation of a successful virus infection cycle requires the attachment of virions to specific receptor molecules on the surfaces of host cells, with subsequent penetration of and entry into the host cells for the release of viral genomes for replication (Dimitrov, 2004; Marsh et al., 2006). Some electron microscopic observations have suggested that plant viruses enter their vector insect cells via receptor-mediated endocytosis; for example, luteoviruses were believed to move across the alimentary canal to hemocoel and salivary glands of aphid vectors by endocytosis phenomena (Garret et al., 1993; Gray et al., 2003). Electron microscopy, immunofluorescence studies, and experiments with various inhibitors support the hypothesis that Rice Dwarf Virus (RDV) enters vector insect cells through receptor-mediated, clathrin-dependent endocytosis and is sequestered in a low-pH-dependent endosomal compartment (Wei et al., 2007).
31Understanding the mechanisms of virus transmission is the key to developing effective strategies to block virus-vector interactions. The principal mechanisms of recognition of cells, proteins, tissues or signals involve the recognition of carbohydrates residues (Pereira et al., 2008). Some lectins are able to identify and bind various pathogens, including viral glycoproteins. By the recognition and the binding with viral glycoproteins, lectins are able to decrease the binding of virus with receptors (Thielens et al., 2002; Naidu et al., 2004) and subsequently, avoid the transport of virus from gut to hemocoel of insect vector (Nelson et al., 2005; Desoignies, 2008). Finally, in this case virus transmission will be suspended (Figure 1).
32However, Desoignies (2008) reported that transmission of Potato virus Y (PVY) through Myzus persicae was significantly reduced when this aphid fed on an artificial diet incorporating Pisum sativum lectin (PSL).
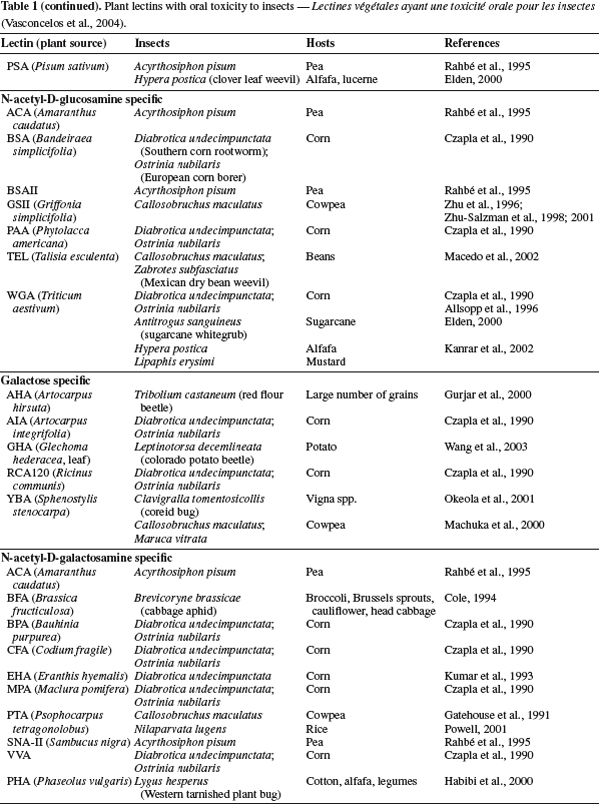
33Lectin applications
34Plants producing natural lectin. In general the plants that are surrounding the main crops are the first barrier to insect attacks. There are many plants such as cowpea, broad and green beans, pea that are able to produce natural lectins in their phloem (Van Damme et al., 1998). These plants are compatible as an intercrop with many crop plants. Therefore, the use of these plants surrounding the main crops is one of the potential ways to reduce insect damage and the virus transmission. In fact, when the insects feed on these plants which have injected lectin, they would not more be able to act as vectors of virus. Indeed, transmission of virus by these insects will decrease because of their receptor site has been blocked by lectin and virus is not able to pass the alimentary canal to reach onto salivary canal and injection again in plants (Gray, 1996; Gray et al., 2003; Desoignies, 2008).
35Gene isolation and transgenic plant proteins. Transgenic plants technology or genetically modified (GM) crops can be a useful tool in producing resistant crops, by introducing novel resistance genes into plants provides a sustainable alternative to the control of insect pests and phytovirus (Gatehouse et al., 1997; 1999; Gray et al., 2003). Currently, the two major groups of plant-derived genes used to confer insect resistance on crops are those of inhibitors of digestive enzymes (proteases and amylase inhibitors) and lectins have been introduced into crops genomes and are now being tested in field conditions (Gatehouse et al., 1993; Hilder et al., 1987; Hilder et al., 1999; Carlini et al., 2002; Schuler et al., 1998). Additionally, crops have been engineered to express a range of insect-plant resistance (Table 2), and have been shown to confer enhanced levels of resistance to different order insect pests including Lepidopteran (Gatehouse et al., 1997), and Homopteran (Down et al., 1996; Gatehouse et al., 1996), when expressed in wheat (Stoger et al., 1999), and rice (Rao et al., 1998) resulting in increased resistance to aphids and plant hoppers, respectively (Powell et al., 1998).
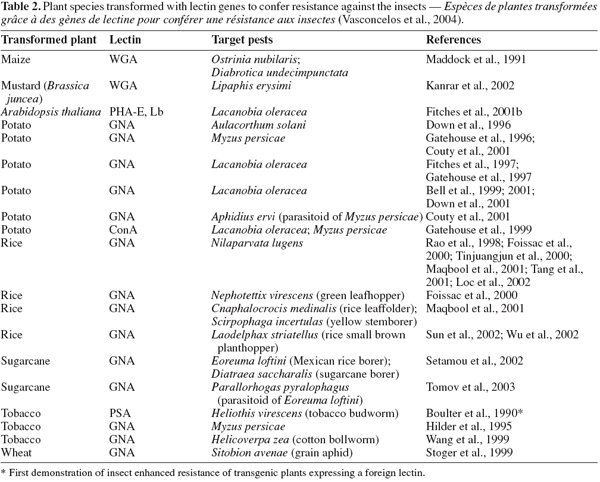
36Until now, many lectins from different origins such as Allium sativum agglutinin (ASA), Wheat germ agglutinin (WGA), Urtica dioica agglutinin (UDA), Phytohemagglutinin ( PHA), a lectin from the red kidney bean, Phaseolus vulgaris L., Pisum sativum lectin (PSL), ConA, XCL and GNA have been isolated and partially characterized for their effects on insect pests, but among them some of encoding insecticidal lectins such as GNA, ConA, WGA and PSA have been shown greater potential for expression into a variety of crops including wheat, tobacco, sugarcane, rice and potatoes as resistant factors against some of important insect pests (Maddock et al., 1991; Powell et al., 1993; 1995; Habibi et al., 1993; Hilder et al., 1995; Gatehouse et al., 1996; 1997; 1999; Down et al., 1996; Fitches et al., 1997; Rao et al., 1998; Kanrar et al., 2002; Setamou et al., 2002) (Table 2).
37Overall, transgenic plants expressing high levels of lectins exhibited some degree of resistance to the target insects. It is worth mentioning that the great interest on transgenic crop plants expressing the gene for GNA, ConA and WGA or other mannose-binding lectins such as ASA resides in the fact that although these lectins show toxicity against species of various insect orders, they are non toxic to mammals and birds (Powell et al., 1995; Down et al., 1996; Bandyopadhyay et al., 2001). Therefore GNA, ConA and WGA have been transferred and expressed in several crop plants (Table 2). Genetic engineering of crop plants has been developed as an alternative to chemical insecticides for plant protections against insect pests (Ranjekar et al., 2003).
38Synergistic effects on other proteins. One of the interest applications of lectin is its synergistic effect on the other entomotoxic proteins. In fact, most resistance phenomena of insects to toxic protein such as Bt toxin is due to their unique resistance gene (Cao et al., 2002). Therefore, expression of multiple resistance genes such as combination of Bt toxin and lectin gene in plants, can increase their resistance to insect pests. Maqbool et al. (2001) reported that rice plants carrying three insecticidal genes including cry1Ac, cry2A and the snowdrop lectin gene (encoding gene GNA) showed enhanced level of resistance to a range of different rice pests. According to Abdeen et al. (2005) and Amirhusin et al. (2004), anti-insect activities of inhibitor enzymes (protease & α-amilase inhibitors) were often increased, when incorporated with lectin compared with their individual effect. Therefore, lectins are able to use as control agents against insect pests and they have synergistic effects on other entomotoxic proteins.
4. Conclusion
39The aim of this literature review was to highlight the ability of some proteins including lectins as resistant factors against some important insect pests to reduce the use of massive chemical compounds. They have been demonstrated direct insecticidal activity to a wide range of insect pests and have a potential to express in transgenic crops to conferring insect-resistant plants. Currently, one of the promising methods for insect-resistance plants has increased the interest on potential toxicity of plant lectins towards some important insect pests, which targeted Homopteran insects which are not affected by Bt toxin and other protein inhibitors with enzymatic activity. In addition to the extensive use of plant lectins, other sources of lectins from fungi including XCL have been presented to control some insect pests. This fungal lectin has been shown with high toxicity effects on some insect pests, compared to plant lectins (ConA & GNA).
Bibliographie
Abdeen A. et al., 2005. Multiple insect resistance in transgenic tomato plants over-expressing two families of plant proteinase inhibitors. Plant Mol. Biol., 57, 189-202.
Allsopp P.G. & McGhie T.K., 1996. Snowdrop lectin and wheat germ lectins as antimetabolites for the control of sugarcane white grubs. Entomol. Exp. Appl., 80(2), 409-414.
Amirhusin B. et al., 2004. Soyacystatin N inhibits proteolysis of wheat alpha-amylase inhibitor and potentiates toxicity against cowpea weevil. J. Econ. Entomol., 97, 2095-2100.
Amirhusin B. et al., 2007. Protease inhibitors from several classes work synergistically against Callosobruchus maculatus. Ann. Rev. Plant Physiol. Plant Mol. Biol., 52, 785-816.
Aronson A.I., 1994. Bacillus thuringiensis and its use as a biological insecticide. In: Janick J., ed. Plant Breeding Reviews. New York, USA: Wiley, 19-45.
Bandyopadhyay S., Roy A. & Das S., 2001. Binding of garlic (Allium sativum) leaf lectin to the gut receptors of homopteran pests is correlated to its insecticidal activity. Plant Sci., 61(5), 1025-1033.
Barbieri L., Battelli M.G. & Stirpe F., 1993. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta, 154(3-4), 237-282.
Barbieri L. et al., 1996. Polynucleotide adenosine glycosidase activity of saporin-L1: effect on DNA, RNA and poly (A). Biochem. J., 319(Pt 2), 507-513.
Bass H.W. et al., 2004. Maize ribosome-inactivating proteins (RIPs) with distinct expression patterns have similar requirements for proenzyme activation. J. Exp. Bot., 55(406), 2219-2233.
Bell H.A. et al., 1999. The effect of snowdrop lectin (GNA) delivered via artificial diet and transgenic plants on Eulophus pennicornis (Hymenoptera: Eulophidae) a parasitoid of the tomato moth Lacanobia oleracea (Lepidoptera: Noctuidae). J. Insect Physiol., 45(11), 983-991.
Bell H.A. et al., 2001. Effect of dietary cowpea trypsin inhibitor (CpTI) on the growth and development of the tomato moth, Lacanobia oleracea (Lepidoptera: Noctuidae) and on the success of the gregarious ectoparasitoid, Eulophus pennicornis (Hymenoptera: Eulophidae). Pest Manage. Sci., 57(1), 57-65.
Bell H.A. et al., 2004. Oral toxicity and impact on fecundity of three insecticidal proteins on the gregarious ectoparasitoid Eulophus pennicornis (Hymenoptera: Eulophidae). Agric. Forest Entomol., 6(3), 215-222.
Bertholdo-Vargas R. et al., 2009. Type 1 ribosome-inactivating proteins-entomotoxic, oxidative and genotoxic action on Anticarsia gemmatalis (Hübner) and Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). J. Insect Physiol., 55(1), 51-58.
Boulter D. et al., 1990. Additive protective effects of incorporating two different higher plants derived insect resistance genes in transgenic tobacco plants. Crop Prot., 9(5), 351-354.
Brich A.N.E. et al., 1999. Tri-tropic interactions involving pest aphid's predatory 2-spot ladybirds and transgenic potatoes expressing snowdrop lectin for aphid resistance. Mol. Breed., 5(1), 75-83.
Brito L.O. et al., 2001. Adaptation of tobacco budworm Heliothis virescens to proteinase inhibitors may be mediated by synthesis of new proteinases. Comp. Biochem. Physiol. B, 128, 365-375.
Cao J. et al., 2002. Broccoli plants with pyramided cry1Ac and cry1C Bt genes control diamondback moths resistant to Cry1A and Cry1C proteins. Theor. Appl. Genet., 105, 258-264.
Carlini C.R. & Grossi-de-Sa M.F., 2002. Plant toxic proteins with insecticidal properties: a review on their potentialities as bio-insecticides. Toxicon, 40(11), 1515-1539.
Ceci L.R. et al., 2003. Selection by phage display of a variant mustard trypsin inhibitor toxic against aphids. Plant J., 33, 557-566.
Chandra N.R. et al., 2006. Lectindb: a plant lectin database. Glycobiology, 16(10), 938-946.
Chrispeels M.J. & Raikhel N.V., 1991. Lectins, lectin genes, and their role in plant defense. Plant Cell, 3, 1-9.
Chrispeels M.J., Grossi-de-Sa M.F. & Higgins T.J.V., 1998. Genetic engineering with alpha-amylase inhibitors makes seeds resistant to bruchids. Seed Sci. Res., 8, 257-263.
Christou P. et al., 2006. Recent developments and future prospects in insect pest control in transgenic crops. Trends Plant Sci., 11, 302-308.
Cloutier C. et al., 2000. Adult Colorado potato beetles, Leptinotarsa decemlineata compensate for nutritional stress on oryzacystatin I-transgenic potato plants by hypertrophic behavior and over-production of insensitive proteases. Arch. Insect Biochem. Physiol., 44, 69-81.
Cole R.A., 1994. Isolation of a chitin-binding lectin with insecticidal activity in chemically-defined synthetic diets from two wild Brassica species with resistance to cabbage aphid Brevicoryne brassicae. Entomol. Exp. Appl., 72, 181-187.
Conner A.J., Glare T.R. & Nap J.P., 2003. The release of genetically modified crops into the environment-Part II. Overview of ecological risk assessment. Plant J., 33(1), 19-46.
Couty A. et al., 2001. Effects of artificial diet containing GNA and GNA-expressing potatoes on the development of the aphid parasitoid, Aphidius ervi Haliday (Hymenoptera: Aphidiidae). J. Insect Physiol., 47(12), 1357-1366.
Czapla T.H. & Lang B.A., 1990. Effect of plant lectins on the larval development of European corn borer (Lepidoptera: Pyralidae) and southern corn rootworm (Coleoptera: Crysomelidae). J. Econ. Entomol., 83, 2480-2485.
Da Silva M.C. et al., 2000. Analysis of structural and physico-chemical parameters involved in the specificity of binding between alpha-amylases and their inhibitors. Protein Eng., 13(3), 167-177.
De Leo F. et al., 1998. Opposite effects on Spodoptera littoralis larvae of high expression level of a trypsin proteinase inhibitor in transgenic plants. Plant Physiol., 118(3), 997-1004.
De Leo F., Bonade-Bottino M., Ceci L.R. & Jouanin L., 2001. Effects of a mustard trypsin inhibitor expressed in different plants on three lepidopteran pests. Insect Biochem. Mol. Biol., 31, 593-602.
De Leo F. & Gallerani R., 2002. The mustard trypsin inhibitor 2 affects the fertility of Spodoptera littoralis larvae fed on transgenic plants. Insect Biochem. Mol. Biol., 32(5), 489-496.
Desoignies N., 2008. Étude des interactions virus-vecteur: transmission par Myzus persicae d'un virus sur le mode non-persistant (PVY) et d'un virus sur le mode persistant (PLRV). Mémoire DEA : Université Catholique de Louvain (Belgique).
Dimitrov D.S., 2004. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol., 2(2), 109-122.
Dixon H.B.F., 1981. Defining a lectin. Letter to Nature. Nature, 292(1981), 192.
Down R.E., Gatehouse A.M.R., Hamilton W.D.O. & Gatehouse J.A., 1996. Snowdrop lectin inhibits development and decreases fecundity of the glasshouse potato aphid (Aulacorthum solani) when administered in vitro and via transgenic plants both in laboratory and glasshouse trial. J. Insect Physiol., 42(11), 1035-1045.
Down R.E. et al., 2001. Influence of plant development and environment on transgene expression in potato and consequences for insect resistance. Transgenic Res., 10, 223-236.
Elden T.C., 2000. Influence of a cysteine proteinase inhibitor on alfafa weevil (Coleptera: Curculionidae) growth and development over successive generations. J. Entomol. Sci., 35, 70-76.
Endo Y. & Tsurugi K., 1987. RNA N-glycosidase activity of ricin Achain: mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem., 263, 8735-8739.
Etzler M.E., 1986. Distribution and function of plant lectins. In: Liener I.E., Sharon N. & Goldstein I.J., eds. The lectins. San Diego, CA, USA: Academic Press, 371-435.
Federici B.A., 2005. Insecticidal bacteria: an overwhelming success for invertebrate pathology. J. Invert. Pathol., 89(1), 30-38.
Ferrari C., Barbieri L. & Stirpe F., 1991. Effects of plant ribosome inactivating proteins on ribosomes from Musca domestica. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol., 100(2), 223-227.
Ferre J. & Rie J.V., 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Ann. Rev. Entomol., 47, 501-533.
Ferry N. et al., 2006. Transgenic plants for insect pest control: a forward looking scientific perspective. Transgenic Res., 15, 13-19.
Fitches E., Gatehouse A.M.R. & Gatehouse J.A., 1997. Effects of snowdrop lectin (GNA) delivered via artificial diet and transgenic plant on the development of tomato moth (Lacanobia oleracea) larvae in laboratory and glasshouse trials. J. Insect Physiol., 43(8), 727-739.
Fitches E. & Gatehouse J.A., 1998. A comparison of the short and long term effects of insecticidal lectins on the activities of soluble and brush border enzymes of tomato moth larvae (Laconobia oleracea). J. Insect Physiol., 44(12), 1213-1224.
Fitches E., Woodhouse S.D., Edwards J.P. & Gatehouse J.A., 2001a. In vitro and in vivo binding of snowdrop (Galanthus nivalis agglutinin; GNA) and jackbean (Canavalia ensiformis; ConA) lectins within tomato moth (Lacanobia oleracea) larvae; mechanisms of insecticidal action. J. Insect Physiol., 47(7), 777-787.
Fitches E. et al., 2001b. The effects of Phaseolus vulgaris erythro- and leucoagglutinating isolectins (PHA-E and PHA-L) delivered via artificial diet and transgenic plants on the growth and development of tomato moth (Lacanobia oleracea) larvae; lectin binding to gut glycoproteins in vitro and in vivo. J. Insect Physiol., 47(12), 1389-1398.
Foissac X. et al., 2000. Resistance to green leafhopper (Nephotettix virescens) and brown planthopper (Nilaparvata lugens) in transgenic rice expressing snowdrop lectin (Galanthus nivalis agglutinin; GNA). J. Insect Physiol., 46(4), 573-583.
Francis F. et al., 2003. Fungal lectin, XCL, is internalized via clathrin-dependent endocytosis and facilitates uptake of other molecules. Eur. J. Cell Biol., 82(10), 515-522.
Garret A., Kerlan C. & Thomas D., 1993. The intestine is a site of passage for potato leafroll virus from the gut lumen into the haemocoel in the aphid vector, Myzus persicae Sulz. Arch. Virol., 131(3), 377-392.
Gatehouse A.M.R. et al., 1984. Effect of seed lectins from Phaseolus vulgaris on the development of larvae of Callosobruchus maculatus; mechanism of toxicity. J. Sci. Food Agric., 35(4), 373-380.
Gatehouse A.M.R., Barbieri L., Stirpe F. & Croy R.R.D., 1990. Effects of ribosome inactivating proteins on insect development differences between Lepidoptera and Coleoptera. Entomol. Exp. Appl., 54(1), 43-51.
Gatehouse A.M.R. et al., 1991. Biochemical basis of insect resistance in winged bean (Psophocarpus tetragonolobus) seeds. J. Sci. Food Agric., 55, 63-74.
Gatehouse A.M.R. et al., 1993. Approaches to insect resistance using transgenic plants. Philos. Trans. R. Soc. London. Biol. Sci., 342(1301), 279-286.
Gatehouse A.M.R. et al., 1995. Insecticidal properties of plant lectins: their potential in plant protection. In: Pusztai A. & Bardocz S., eds. Lectins: biomedical perspectives. London: Taylor & Francis, 35-58.
Gatehouse A.M.R. et al., 1996. Transgenic potato plants with enhanced resistance to the peach-potato aphid, Mizus persicae. Entomol. Exp. Appl., 79(3), 295-307.
Gatehouse A.M.R. et al., 1997. Transgenic potato plants with enhanced resistance to the tomato moth, Lacanobia oleracea: growth room trials. Mol. Breed., 3(1), 49-63.
Gatehouse A.M.R. & Gatehouse J.A., 1998. Identifying proteins with insecticidal activity: use of encoding genes to produce insect-resistant transgenic crops. Pest Sci., 52(2), 165-175.
Gatehouse A.M.R. et al., 1999. Concanavalin A inhibits development of tomato moth (Lacanobia oleracea) and peach-potato aphid (Myzus persicae) when expressed in transgenic potato plants. Mol. Breed., 5(2), 153-165.
Gildow F.E., 1993. The aphid salivary gland basal lamina as a selective barrier associated with vector-specific transmission of barley yellow dwarf luteoviruses. Phytopathology, 83(12), 1293-1302.
Goldstein I.J. & Poretz R.D., 1986. Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins. In: Liener I.E., Sharon N. & Goldstein I.J., eds. The lectins. Orlando, FL, USA: Academic Press, 33-247.
Gray S.M., 1996. Plant virus proteins involved in natural vector transmission. Trends Microbiol., 4(7), 259-264.
Gray S.M. & Banerjee N., 1999. Mechanisms of arthropod transmission of plant and animal viruses. Microbiol. Mol. Biol. Rev., 63(1), 128-148.
Gray S.M. & Gildow F.E., 2003. Luteovirus-aphid interactions. Annu. Rev. Phytopathol., 41, 539-566.
Green T.R. & Ryan C.A., 1972. Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science, 175, 776-777.
Gruden K. et al. 1998. The cysteine protease activity of Colorado potato beetle (Leptinotarsa decemlineata Say) guts, which is insensitive to potato protease inhibitors, is inhibited by thyroglobulin type-1 domain inhibitors. Insect Biochem. Mol. Biol., 28, 549-560.
Gurjar M.M. et al., 2000. Growth inhibition and total loss of reproductive potential in Tribolium castaneum by Artocarpus hirsut lectin. Invertebr. Reprod. Dev., 38, 95-98.
Habibi J.E., Backus E.A. & Czapla T.M., 1993. Plant lectins affect survival of the potato leaf-hopper (Homoptera: Cicadellidae). J. Econ. Entomol., 86(3), 945-951.
Habibi J.E., Backus E.A. & Czapla T.H., 1998. Subcellular effects and localization of binding sites of phytohemagglutinin in the potato leafhopper, Empoasca fabae (Insecta: Homoptera: Cicadellidae). Cell Tissue Res., 294(3), 561-571.
Habibi J.E., Backus E.A. & Huesing J.E., 2000. Effects of phytohemagglutinin (PHA) on the structure of midgut epithelial cells and localization of its binding sites in western tarnished plant bug Lygus hesperus Knight. J. Insect Physiol., 46(5), 611-619.
Halitschke R. et al., 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol., 125(4), 711-717.
Haruta M. et al., 2001. A Kunitz trypsin inhibitor gene family from trembling aspen (Populus tremuloides Michx.): cloning, functional expression, and induction by wounding and herbivory. Plant Mol. Biol., 46(3), 347-359.
Hilder V.A. et al., 1987. A novel mechanism for insect resistance engineered into tobacco. Nature, 330, 160-163.
Hilder V.A. et al., 1995. Expression of snowdrop lectin in transgenic tobacco results in added protection against aphids. Transgenic Res., 4(1), 18-25.
Hilder V.A. & Boulter D., 1999. Genetic engineering of crop plants for insect resistance: a critical review. Crop Prot., 18(3), 177-191.
Hogenhout S.A., Ammar E.D., Whitfield A.E. & Redinbaugh M.G., 2008. Insect vector interactions with persistently transmitted viruses. Ann. Rev. Phytopathol., 46, 327-359.
Ishimoto M. & Kitamura K., 1989. Growth inhibitory effects of an a-amylase inhibitor from kidney bean, Phaseolus vulgaris (L.) on three species of bruchids (Coleoptera: Bruchidae). Appl. Entomol. Zool., 24, 281-286.
Janmaat A.F. & Myers J., 2003. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni. Proc. R. Soc. London Ser. B Biol. Sci., 270, 2263-2270.
Jongsma M.A. & Bolter C., 1997. The adaptation of insects to plant protease inhibitors. J. Insect Physiol., 43, 885-895.
Kanrar S., Venkateswari J., Kirti P.B. & Chopra V.L., 2002. Transgenic Indian mustard (Brassica juncea) with resistance to the mustard aphid (Lipaphis erysimi Kalt.). Plant Cell Rep., 20, 976-981.
Karimi J. et al., 2006. Use of artificial diet system to assess the potential bio-insecticide effect of a fungal lectin from Xerocomus chrysenteron (XCL) on Myzus persicae. Comm. Appl. Biol. Sci. Ghent Univ., 71(2b), 497-505.
Karimi J. et al., 2007. Effect of a fungal lectin from Xerocomus chrysenteron (XCL) on the biological parameters of aphids. Comm. Appl. Biol. Sci. Ghent Univ., 72(3), 629-638.
Kitch L.W. & Murdock L.L., 1986. Partial characterization of a major gut thiol proteinase from larvae of Callosobruchus maculatus. Arch. Insect Biochem. Physiol., 3, 561-575.
Kluh I. et al., 2005. Inhibitory specificity and insecticidal selectivity of a-amylase inhibitor from Phaseolus vulgaris. Phytochemistry, 66, 31-39.
Koiwa H., Bressan R.A. & Hasegawa P.M., 1997. Regulation of proteinase inhibitors and plant defense. Trends Plant Sci., 2, 379-384.
Koiwa H. et al., 1998. Phage display selection can differentiate insecticidal activity of soybean cystatins. Plant J., 14, 371-379.
Kumar M.A. et al., 1993. Characterization of the lectin from the bulbs of Eranthis hyemalis (winter aconite) as an inhibitor of protein synthesis. J. Biol. Chem., 268(33), 25176-25183.
Kuroda M. et al., 1996. Oryzacystatins exhibit growth-inhibitory and lethal effects on different species of bean insect pests Callosobruchus chinensis (Coleoptera) and Riptortus clavatus (Hemiptera). Biosci. Biotechnol. Biochem., 60(2), 209-212.
Laskowski M. & Kato I., 1980. Protein inhibitors of proteinases. Annu. Rev. Biochem., 49, 593-626.
Lecardonnel A. et al., 1999. Effects of rice cystatin I expression in transgenic potato on Colorado potato beetle larvae. Plant Sci., 140, 71-79.
Leple J.C. et al., 1995. Toxicity to Chrysomela tremulae (Coleoptera: Crysomelidae) of transgenic poplars expressing a cysteine proteinase inhibitor. Mol. Breed., 1, 319-328.
Lis H. & Sharon N., 1998. Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev., 98, 637-674.
Loc N.T. et al., 2002. Linear transgene constructs lacking vector backbone sequences generate transgenic rice plants which accumulate higher levels of proteins conferring insect resistance against a range of different rice pest. Mol. Breed., 9, 231-244.
Lu X.F., Xia Y.X. & Pei Y., 1998. Roles of plant proteinase inhibitors in the resistance of plant against insects and pathogens. Prog. Biochem. Biophys., 25, 328-333.
Macedo M.L.R., Freire M.G.M., Novello J.C. & Marangoni S., 2002 Talisia esculenta lectin and larval development of Callosobruchus maculatus and Zabrotes subfasciatus (Coleoptera: Bruchidae). Biochim. Biophys. Acta, 1571, 83-88.
Macedo M.L.R. et al., 2003. Purification and characterization of an N-acetylglucosamine-binding lectin from Koelreuteria paniculata seeds and its effect on the larval development of Callosobruchus maculatus (Coleoptera: Bruchidae) and Anagasta kuehniella (Lepidoptera: Pyralidae). J. Agric. Food Chem., 51, 2980-2986.
Macedo M.L.R., Freire M.G.M., Silva M.B.R. & Coelho L.C.B.B., 2006. Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculatus (Coleoptera: Bruchidae). Comp. Biochem. Physiol., 146, 486-498.
Machuka J., Van Damme E.J.M., Peumans W.J. & Jackai L.E.N., 1999. Effect of plant lectins on survival development of the pod borer Maruca vitrata. Entomol. Exp. Appl., 93, 179-187.
Machuka J.S., Okeola O.G., Chrispeels M.J. & Jackai L.E.N., 2000. The African yam bean seed lectin affects the development of the cowpea weevil but does not affect the development of larvae of the legume pod borer. Phytochemistry, 53, 667-674.
Maddock S.E. et al., 1991. Expression in maize plants of wheat germ agglutinin, a novel source of insect resistance. In: Third International Congress in Plant Molecular Biology, Tucson, Arizona, USA.
Majumder P., Mondal H.A. & Das S., 2005. Insecticidal activity of Arum maculatum tuber lectin and its binding to the glycosylated insect gut receptors. J. Agric. Food Chem., 53(17), 6725-6729.
Maqbool S.B. et al., 2001. Expression of multiple insecticidal genes confers broad resistance against a range of different rice pests. Mol. Breed., 7, 85-93.
Marsh M. & Helenius A., 2006. Virus entry: open sesame. Cell, 124(4), 729-740.
McGaughey W.H. & Whalon M.E., 1992. Managing insect resistance to Bacillus thuringiensis toxins. Science, 258(5087), 1451-1455.
Mosolov V.V., Grigor'eva L.I. & Valueva T.A., 2001. Involvement of proteolytic enzymes and their inhibitors in plant protection (review). Appl. Biochem. Microbiol., 37(2), 115-123.
Murdock L.L. & Shade R.E., 2002. Lectins and protease inhibitors as plant defense against insects. J. Agric. Food Chem., 50(22), 6605-6611.
Murdock L.L. et al., 1987. Cysteine digestive proteinases in coleoptera. Comp. Biochem. Physiol. B. Comp. Biochem., 87, 783-787.
Murdock L.L., Shade R.E. & Pomeroy M.A., 1988. Effects of E-64, a cysteine proteinase-inhibitor, on cowpea weevil growth, development, and fecundity. Environ. Entomol., 17, 467-469.
Naidu R.A., Ingle C.J., Deom C.M. & Sherwood J.L., 2004. The two envelope membrane glycoproteins of tomato spotted wilt virus show differences in lectin-binding properties and sensitivities to glycosidases. Virology, 319, 107-117.
Nelson D. & Cox M., 2005. Lehninger principles of biochemistry. 4th ed. Freeman.
Ng T.B., 2004. Peptides and proteins from fungi. Peptides, 25(6), 1055-1073.
Ng T.B., Chan W.Y. & Yeung H.W., 1992. Proteins with abortifacient, ribosome inactivating, immunomodulatory, antitumor and anti-AIDS activities from Cucurbitaceae plants. Gen. Pharmacol., 23(4), 575-590.
Nielsen K. & Boston R.S., 2001. Ribosome-inactivating proteins: a plant perspective. Ann. Rev. Plant Physiol. Plant Mol. Biol., 52, 785-816.
Okeola O.G. & Machuka J., 2001. Biological effects of African yam bean lectins on Clavigralla tomentosicollis (Hemiptera: Coreidae). J. Econ. Entomol., 94, 724-729.
Paulillo L.C.M.S. et al., 2000. Changes in midgut-endopeptidases activity of Spodoptera frugiperda (Lepidoptera: Noctuidae) are responsible for adaptation to soybean proteinase inhibitors. J. Econ. Entomol., 93, 892-896.
Pereira E.M.A. et al., 2008. Lectins and/or xyloglucans/alginate layers as supports for immobilization of dengue virus particles. Colloids Surf. B Biointerfaces, 66(1), 45-52.
Pernas M. et al., 1999. Antifungal activity of a plant cystatin. Mol. Plant-Microbe Interact., 12, 624-627.
Peumans W.J. & Van Damme E.J.M., 1995. Lectins as plant defense proteins. Plant Physiol., 109(2), 347-352.
Peumans W.J., Hao Q. & Van Damme E.J.M., 2001. Ribosome-inactivating proteins from plants: more than RNA N-glycosidases. FASEB J., 15(9), 1493-1506.
Powell K.S., 2001. Antimetabolic effects of plant lectins towards nymphal stages of the planthoppers Tarophagous proserpina and Nilaparvata lugens. Entomol. Exp. Appl., 99(1), 71-77.
Powell K.S., Gatehouse A.M.R., Hilder V.A. & Gatehouse J.A., 1993. Antimetabolic effects of plants lectins and fungal enzymes on the nymphal stages of two important rice pests, Nilaparvata lugens and Nephotettix cinciteps. Entomol. Exp. Appl., 66(2), 119-126.
Powell K.S. et al., 1995. Different antimetabolic effects of related lectins towards nymphal stages of Nilaparvata lugens. Entomol. Exp. Appl., 75(1), 61-65.
Powell K.S. et al., 1998. Immunohistochemical and developmental studies to elucidate the mechanism of action of the snowdrop lectin on the rice brown planthopper Nilaparvata lugens (Stal). J. Insect Physiol., 44(7), 529-539.
Rahbé Y. & Febvay G., 1993. Protein toxicity to aphid: an in vitro test on Acyrthosiphon pisum. Entomol. Exp. Appl., 67(2), 149-160.
Rahbé Y. et al., 1995. Toxicity of lectins and processing of ingested proteins in the pea aphid Acyrthosiphon pisum. Entomol. Exp. Appl., 76, 143-155.
Rahbé Y. et al., 2003a. Effects of the cysteine protease inhibitor oryzacystatin (OC-I) on different aphids and reduced performance of Myzus persicae on OC-I expression transgenic oilseed rape. Plant Sci., 164(4), 441-450.
Rahbé Y., Ferrasson E., Rabesona H. & Quillien L., 2003b. Toxicity to the pea aphid Acyrthosiphon pisum of anti-chymotrypsin isoforms and fragments of Bowman-Birk protease inhibitors from pea seeds. Insect Biochem. Mol. Biol., 33, 299-306.
Ramos V.S., Freire M.G.M., Parra J.R.P. & Macedo M.L.R., 2009. Regulatory effects of an inhibitor from Plathymenia foliolosa seeds on the larval development of Anagasta kuehniella (Lepidoptera). Comp. Biochem. Physiol., 152, 255-261.
Ranjekar P.K. et al., 2003. Genetic engineering of crop plants for insect resistance. Curr. Sci., 84, 321-329.
Rao K.V. et al., 1998. Expression of snowdrop lectin (GNA) in transgenic rice plants confers resistance to rice brown planthopper. Plant J., 15(4), 469-477.
Richardson M., 1977. The proteinase inhibitors of plants and microorganisms. Phytochemistry, 16, 159-169.
Roy A., Banerjee S., Majumder P. & Das S., 2002. Efficiency of mannose-binding plant lectins in controlling a homopteran insect, the red cotton bug. J. Agric. Food Chem., 50, 6775-6779.
Rudiger H. & Gabius H.J., 2001. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconjugate J., 18(8), 589-613.
Ryan C.A., 1990. Proteinase inhibitors in plants: genes for improving defenses against insects and pathogens. Ann. Rev. Phytopathol., 28, 425-449.
Sauvion N. et al., 1996. Effects of GNA and other mannose binding lectins on development and fecundity of the potato-peach aphid Myzus persicae. Entomol. Exp. Appl., 79, 285-293.
Sauvion N. et al., 2004. Binding of the insecticidal lectin Concanavalin A in pea aphid, Acyrthosiphon pisum (Harris) and induced effects on the structure of midgut epithelial cells. J. Insect Physiol., 50(12), 1137-1150.
Schnepf H.E. & Whiteley H.R., 1981. Cloning and expression of the Bacillus thuringiensis crystal protein gene in Escherichia coli. Proc. Natl Acad. Sci. USA, 78(5), 2893-2897.
Schuler T.H., Poppy G.M., Kerry B.R. & Denholm I., 1998. Insect resistant transgenic plants. Trends Biotechnol., 16, 168-175.
Scott J.G. & Wen Z.M., 2001. Cytochromes P450 of insects: the tip of the iceberg. Pest Manage. Sci., 57(10), 958-967.
Setamou M. et al., 2002. Evaluation of lectin-expressing transgenic sugarcane against stalkborers (Lepidoptera: Pyralidae): effects on life history parameters. J. Econ. Entomol., 95(2), 469-477.
Shahidi-Noghabi S., Van Damme E.J.M. & Smagghe G., 2008. Carbohydrate-binding activity of the type-2 ribosome-inactivating protein SNA-I from elderberry (Sambucus nigra) is a determining factor for its insecticidal activity. Phytochemistry, 69(17), 2972-2978.
Shahidi-Noghabi S., Van Damme E.J.M. & Smagghe G., 2009. Expression of Sambucus nigra agglutinin (SNA-I0) from elderberry bark in transgenic tobacco plants results in enhanced resistance to different insect species. Transgenic Res., 18, 249-259.
Sharma N. et al., 2004. Isolation and characterization of an RIP (Ribosome-Inactivating Protein)-like protein from tobacco with dual enzymatic activity. Plant Physiol., 134(1), 171-181.
Smith C.M. & Boyko L.V., 2006. The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomol. Exp. Appl., 122(1), 1-16.
Solomon M. et al., 1999. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell, 11, 431-444.
Srinivasan A. et al., 2005. A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts anti-metabolic effect on podborer (Helicoverpa armigera) larvae. Plant Mol. Biol., 57, 359-374.
Stillmark H., 1888. Ueber Ricin, ein giftiges Ferment aus dem Samen von Ricinus communis L. und einigen anderen Euphorbiaceen. Arb. Pharmak. Inst. Dorpat., 3, 59-151.
Stirpe F. & Battelli M.G., 2006. Ribosome-inactivating proteins: progress and problems. Cell Mol. Life Sci., 63(16), 1850-1866.
Stoger E. et al., 1999. Expression of the insecticidal lectin from snowdrop (Galanthus nivalis agglutinin, GNA) in transgenic wheat plants: effects predation by the grain aphids Sitobion avenae. Mol. Breed., 5(1), 65-73.
Sun X., Wu A. & Tang K., 2002. Transgenic rice lines with enhanced resistance to the small brown planthopper. Crop Prot., 21(6), 511-514.
Tabashnik B.E., Cushing N.L., Finson N. & Johnson M.W., 1990. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol., 83, 1671-1676.
Tang K. et al., 2001. Production of transgenic rice homozygous lines with enhanced resistance to the rice brown planthopper. Acta Biotechnol., 21, 117-128.
Terra W.R. & Ferreira C., 1994. Insect digestive enzymes—properties, compartmentalization and function. Com. Biochem. Physiol. Part B. Biochem. Mol. Biol., 109, 1-62.
Thielens N.M., Tacnet-Delorme E.P. & Arlaud G.J., 2002. Interaction of C1q and mannan-binding lectin with viruses. Immunobiology, 205, 563-574.
Tinjuangjun P. et al., 2000. Enhanced insect resistance in Thai rice varieties generated by particle bombardment. Mol. Breed., 6, 391-399.
Toledo A.L. et al., 2007. Purification by expanded bed adsorption and characterization of an α-amylase FORILASE NTLR from Aspergillus niger. J. Chromatogr., 846(1), 51-56.
Tomov B.W. & Bernal J.S., 2003. Effects of GNA transgenic sugarcane on life history parameters of Parallorhogas pyralophagus (Marsh) (Hymenoptera: Braconidae), a parasitoid of Mexican rice borer. J. Econ. Entomol., 96, 570-576.
Trigueros V. et al., 2003. Xerocomus chrysenteron lectin: identification of a new pesticidal protein. Entomol. Exp. Appl., 1621(3), 292-298.
Urwin P.E., Atkinson H.J. & McPherson M.J., 1995. Involvement of the NH2-terminal region of oryzacystatin-I in cysteine proteinase inhibition. Protein Eng., 8, 1303-1307.
Urwin P.E., McPherson M.J. & Atkinson H.J., 1998. Enhanced transgenic plant resistance to nematodes by dual proteinase inhibitor constructs. Planta, 204, 472-479.
Ussuf K.K., Laxmi N.H. & Mitra R., 2001. Proteinase inhibitors: plant-derived genes of insecticidal protein for developing insect-resistant transgenic plants. Curr. Sci., 80(7), 847-853.
Valencia A., Bustillo A.E., Ossa G.E. & Chrispeels M.J., 2000. α-Amylases of the coffee berry borer (Hypothenemus hampei) and their inhibition by two plant amylase inhibitors. Insect Biochem. Mol. Biol., 30(3), 207-213.
Van Damme E.J.M., Peumans W.J., Pusztai A. & Bardocz S., 1998. Handbook of plant lectins: properties and biomedical applications. Bognor Regis, UK: John Wiley and Sons.
Van Damme E.J.M. et al., 2001. Ribosome-inactivating protein: a family of plant proteins that do more than inactivate ribosome. Crit Rev. Plant Sci., 20, 395-465.
Vasconcelos I.M. & Oliveira J.T.A., 2004. Antinutritional properties of plant lectins. Toxicon, 44(4), 385-403.
Vervecken W., Kleff S., Pfuller U. & Bussing A., 2000. Induction of apoptosis by mistletoe lectin I and its subunits. No evidence for cytotoxic effects caused by isolated A and B-chains. Int. J. Biochem. Cell. Biol., 32, 317-326.
Volpicella M. et al., 2003. Properties of purified gut trysin from Helicover zea, adapted to proteinases inhibitors. Eur. J. Biochem., 270, 10-19.
Wang G. et al., 2006. Engineered Bacillus thuringiensis G033A with broad insecticidal activity against lepidopteran and coleopteran pests. Appl. Microbiol. Biotechnol., 72(5), 924-930.
Wang H., Ng T.B. & Liu Q., 2003. A novel lectin from the wild mushroom Polyporus adusta. Biochem. Biophys. Res. Commun., 307, 535-539.
Wang H.X, Ng T.B. & Ooi V.E.C., 1998. Lectins from mushroom. Mycol. Res., 102, 897-906.
Wang M. et al., 2002. Proteins as active compounds involved in insecticidal activity of mushroom fruitbodies. J. Econ. Entomol., 95, 603-617.
Wang Z.B. & Guo S.D., 1999. Expression of two insect resistant genes cryIA (bandc) GNA in transgenic tobacco plants results in added protection against both cotton bollworm and aphids. Chin. Sci. Bull., 44, 2051-2058.
Wei G.Q., Liu R.S., Wang Q. & Liu W.Y., 2004. Toxicity of two type II ribosome-inactivating proteins (cinnamomin and ricin) to domestic silkworm larve. Arch. Insect Biochem. Physiol., 57, 160-165.
Wei T. et al., 2007. Entry of rice dwarf virus into cultured cells of its insect vector involves clathrin-mediated endocytosis. J. Virol., 81(14), 7811-7815.
Wu A., Sun X., Pang Y. & Tang K., 2002. Homozygous transgenic rice lines expressing GNA with enhanced resistance to the rice sap-sucking pest Laodelphax striatellus. Plant Breed., 121, 93-95.
Yamada T., Hattori K. & Ishimoto M., 2001. Purification and characterization of two α-amylase inhibitors from seeds of tepary bean (Phaseolus acutifolius A.gray). Phytochemistry, 58(1), 59-66.
Zhao J.Z. et al., 2003. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat. Biotechnol., 21, 1493-1497.
Zhu K. et al., 1996. An insecticidal N-acetylglucosamine specific lectin gene from Griffonia simplicifolia (Leguminosae). Plant Physiol., 110, 195-202.
Zhu-Salzman K. et al., 1998. Carbohydrate binding and resistance to proteolysis control insecticidal activity of Griffonia simplicifolia lectin II. Proc. Natl Acad. Sci. USA, 95, 15123-15128.
Zhu-Salzman K. & Salzman R., 2001. A functional mechanic of the plant defensive Griffonia simplicifolia lectin II: resistance to proteolysis is independent of glycoconjugate binding in the insect gut. J. Econ. Entomol., 94, 1280-1284.
Zhu-Salzman K. et al., 2003. Cowpea bruchid Callosobruchus maculatus uses a threecomponent strategy to overcome a plant defensive cysteine protease inhibitor. Insect Mol. Biol., 12, 135-145.
To cite this article
About: Karimi Jaber
ULg - Gembloux Agro-Bio Tech. Functional & Evolutionary Entomology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: karimi_jaber@yahoo.com – Shahed University. Plant Protection Department. Opposite Holy Shrine of Imam Khomeini. Khalig Fars Express Way. P.O.Box 18155/159. Teheran (Iran).
About: Éric Haubruge
ULg - Gembloux Agro-Bio Tech. Functional & Evolutionary Entomology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
About: Frédéric Francis
ULg - Gembloux Agro-Bio Tech. Functional & Evolutionary Entomology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






