- Portada
- Volume 14 (2010)
- numéro 2
- Prevalence and sources of Campylobacter spp. contamination in free-range broiler production in the southern part of Belgium
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Prevalence and sources of Campylobacter spp. contamination in free-range broiler production in the southern part of Belgium

Notes de la rédaction
Received on 17 March 2009; accepted on 11 September 2009
Résumé
Prévalence et sources de contamination par Campylobacter spp. des productions de poulets de chair élevés en plein air dans le sud de la Belgique. Une étude épidémiologique d’un an a été menée de février 2005 à février 2006 en région wallonne de Belgique afin d’évaluer la prévalence de Campylobacter dans les productions de poulets de chair élevés en plein air. Trois lots successifs dans six exploitations belges ont été investigués pour la présence de Campylobacter spp. pendant la période d’élevage. À chaque visite, des échantillons ont été prélevés dans le poulailler (litière, matières caecales, lignes d’eau, aliment, sas d’entrée) ainsi que de l’environnement extérieur (parcours). La détection de Campylobacter dans les échantillons a été réalisée selon le standard ISO 10272. L’identification était basée sur la morphologie des colonies, l’examen microscopique et des tests biochimiques. La PCR multiplex a été utilisée pour confirmation génétique. Campylobacter jejuni était la principale espèce isolée de tous les échantillons contaminés. Globalement, les infections mixtes C. jejuni / Campylobacter coli représentaient 40,6 %, tandis que C. jejuni et C. coli représentaient 46,9 % et 12,5 % des isolats de poulets respectivement. Tous les lots (100 %) étaient contaminés dans les six exploitations pendant la période été-automne, alors que seulement 66,7 % et 33,3 % des lots étaient positifs à Campylobacter à la fin de la période d’élevage, au printemps et en hiver respectivement. La moitié des lots contaminés étaient infectés avant que les poulets n’aient accès au parcours extérieur. Différents échantillons environnementaux, plus particulièrement le sol du parcours, ont été détectés positifs à Campylobacter avant l’infection du lot. Les autres sources potentielles de contamination étaient le véhicule de livraison, le sol du sas et les lignes d’eau. La présence d’autres productions animales comme des bovins dans l’exploitation, l’absence de contrôle des rongeurs, l’absence de nettoyage et de désinfection des lignes d’eau entre les lots, le nettoyage sans détergent et la séparation des lots pour l’abattage ont été déterminés comme facteurs de risque. En conclusion, le contact avec l’environnement, plus particulièrement l’accès à un parcours extérieur, apparait comme une source majeure de contamination des poulets par Campylobacter en production de poulets de chair élevés en plein air.
Abstract
A one year epidemiological study was carried out between February 2005 and February 2006 in the southern part of Belgium to assess the Campylobacter prevalence in free-range broiler production. Three successive broiler flocks from six Belgian farms were investigated for the presence of Campylobacter spp. during the rearing period. Each flock was visited four times, before and after the outdoor rearing period. During each visit, samples were taken in the broiler house (litter, cecal droppings, water-lines, feed, anteroom) as well as from the outer rearing environment (open-air range). The Campylobacter detection in all samples was carried out according to the ISO 10272 standard. Identification was based on colonial morphology, microscopic examination, and biochemical tests. PCR multiplex was used for genetic confirmation. Campylobacter jejuni was the main species isolated from all contaminated samples. Overall, mixed infections C. jejuni / Campylobacter coli represented 40.6%, while C. jejuni and C. coli represented 46.9% and 12.5% of chicken samples respectively. A 100% flock contamination was observed in the 6 farms during the summer-autumn period, whereas only 66.7% and 33.3% of the flocks became Campylobacter-positive in spring and winter respectively, at the end of the rearing period. Half of contaminated flocks were infected before chickens have access to the open-air range. Environmental samples, especially the open-air range soil, were found to be Campylobacter-positive before flock infection. The other potential sources of contamination were delivery tray, anteroom floor and water-lines. Other animal productions like cattle on the farm, no applied rodent control, no cleaning and disinfection of water-lines between flocks, no detergent used for cleaning and thinning were recorded as risk factors. In conclusion, the contact with the environment, particularly the access to an open-air range, appeared to be the major way of Campylobacter contamination of chickens in free-range broiler production.
Tabla de contenidos
1. Introduction
1According to several reports from countries all over the world, Campylobacter is now recognized as the most common cause of human bacterial enteritis in developed countries. In 2006, the overall incidence of campylobacteriosis was 46.1 per 100,000 population in the EU-25 (European Food Safety Authority, 2007). More than 95% of registered Campylobacter enteritis is caused by thermotolerant species, i.e. Campylobacter jejuni and Campylobacter coli (Butzler, 2004). C. jejuni and C. coli have been traditionally differentiated by the hippurate hydrolysis test, for which only C. jejuni gives a positive reaction.
2Case control studies has identified consumption of contaminated raw or insufficiently cooked poultry products as the major vehicle for campylobacteriosis (Moore et al., 2005; Zorman et al., 2006), for a variable percentage of cases ranging from 10% in Denmark to more than 70% at a US university (Friedman et al., 2000). Moreover, a quantitative risk assessment carried out by Rosenquist et al. (2003) showed a linear relationship between the poultry flock prevalence of Campylobacter and the incidence of human campylobacteriosis. Therefore, the European Union and governmental agencies are focused on eradicating Campylobacter in live bird and at the processing plant, particularly by improving the control of pathogen in the primary production and by the intensification of epidemiological studies about Campylobacter at national level.
3The development of cost-effective control strategies requires a more precise knowledge of the mechanisms of transmission and epidemiology of Campylobacter spp. in poultry. The transmission routes, the risk factors and sources for flock colonization in poultry production have been identified and quantified in several studies. Horizontal transmission is generally considered the most significant cause of broiler flock colonization (Newell et al., 2003), with the environment of the poultry house being the major reservoir of pathogens. The factors showed to be associated with an increased risk of contamination are the lack of hygiene measures (Kapperud et al., 1993; Evans et al., 2000), the presence of other farm animals on the farm (Kapperud et al., 1993; Van de Giessen et al., 1996; Bouwknegt et al., 2004), several poultry houses on the same farm site (Refrégier-Petton et al., 2001; Bouwknegt et al., 2004), the first disinfection being performed by the farmer instead of a hygiene specialist (Huneau-Salaün et al., 2007), drinking unchlorinated water (Arsenault et al., 2007), as well as rodents, insects and wild birds (Berndtson et al., 1996; Stern et al., 2001; Hansson et al., 2007).
4The potential of the environment as source of Campylobacter has lead to the assumption that characteristics of extensive organic broiler productions, including the access to an open-air range, could be associated with a higher prevalence of Campylobacter than conventional standard production, as mentioned by Huneau-Salaün et al. (2007) and Newell et al. (2003), and confirmed by several studies. Data on the prevalence of Campylobacter in conventional in comparison with non-conventional broiler production actually gave values from 36.7% to 66% and from 89% to 100% respectively (Heuer et al., 2001; Luangtongkum et al., 2006). Furthermore, a seasonal variation in the prevalence of Campylobacter-positive broiler-flocks has also been reported from Norway (Kapperud et al., 1993) as well as from France (Refrégier-Petton et al., 2001) and United Kingdom (Wallace et al., 1997).
5In this context, the aim of the present study was to determine the flock prevalence of Campylobacter from free-range broiler production, a fast-expanding poultry rearing system in the southern part of Belgium. Furthermore, the study aimed to evaluate potential contamination sources at the farm level, including among others food, litter, drinking water or free-open range. In accordance with the request related to Campylobacter national surveillance recorded in the EFSA scientific report, the collected data will help to develop an effective control program to reduce the broiler flocks prevalence of Campylobacter, and than the campylobacteriosis incidence at national level.
2. Materials and methods
2.1. Farm characteristics
6This study was conducted from February 2005 to February 2006 in the southern French-speaking part of Belgium. Six farms, designated farms “A” through “F”, were selected as a convenient sample representing approximatively 10% of Belgian free-range broiler production farms. They were affiliated with three different chicken meat production companies (I, II, III), with two farms/production company. The companies were chosen for inclusion in this study due to their large size and their readiness to collaborate in the project. They supplied the farm locations and subsequent farm selection was based on homogenous geographical distribution, and on diversity regarding broiler house and rearing managements. All contacted farmers accepted free cooperation to the study.
7Two companies were of type organic farmer production system, while the third was of type free-range production system. Characteristics of both broilers production systems are based on the references to free-range chickens according to Commission Regulation (EEC) n°1538/91 introducing detailed rules for implementing Regulation (EEC) n°1906/90 on certain marketing standards for poultry meat.
8Only one of the separate broiler houses on each farm was chosen for the epidemiological study. Three successive broiler flocks coded “a” through “c” were sampled on each broiler house, for the presence of Campylobacter ssp. during the rearing period (Table 1). Flocks a were reared from February to June, flocks b from July to September, and flocks c from October to February. The flock size ranged from 1,200 to 4,800 (mean 3,582) birds at the day of placement. All houses were closed, isolated and had regulated temperature and ventilation. Chickens were raised on floor, with either straw or wood shavings as litter. Slow-growing broiler strains had free access to an open-air range from six weeks of age and were slaughtered at minimum 82 days of age. The chicken densities for organic production in the broiler house (10 birds per m2) and on the open-air range (4 m2 per bird) are however different from for free-range production (11 birds per m2 for the broiler house, and 2 m2 per bird for the open-air range). Cereals in the feed accounted for at least 70% in weight. Between each successive flock, there was a two to four weeks period where the house is cleaned, disinfected and left empty before input of new chickens. The thinning system, i.e. partial flock depopulation at slaughter age, was used for most flocks.

2.2. Sample collection
9Each flock was visited four times, before (1 and 27 days of age) and after (54 and 81 days of age) the outdoor rearing period. The first visit was carried out just before the setting up of the chicks, and samples were taken aseptically in the disinfected broiler house (clean straw litter, water-lines, drinking water, feed, exit trap doors, floor from the anteroom), in the transportation truck (delivery tray liners, floor) as well as in the outer rearing environment (open-air range soil).
10One composite sample of 25 g litter from four areas of the broiler house, one composite sample of 1 l drinking water from one to four drinkers, two composite samples of 250-500 g from fifteen areas of the open-air range and one composite sample of about 130 g feed from the feeders were collected. About 1 l of tap water was sampled in the anteroom to exclude the risk of contamination from the chickens. Samples from the anteroom floor, exit trap doors, paper liners from the chick delivery trays, water drinkers and transport truck floor, were obtained by rubbing a sterile cotton gauze moistened with sterile distilled water over about 10 × 0.0025 m2 of the object’s surface. All the samples were placed in sealed sterile bags or containers.
11For each of the three following visits, samples consisted of four composite samples of cecal droppings taken from the four quarters of the whole house litter and stored in a sterile plastic bag tightly sealed after excluding the air, as well as of swabs from the anteroom and of surface soil of the open-air range, sampled as described for the first visit. All samples were collected on each occasion within 1 to 2 h and transferred in insulated boxes containing ice packs for transport to the laboratory. They were kept at 4°C less than two weeks prior to the microbiological tests.
2.3. Isolation and identification of Campylobacter
12Two reference strains (Campylobacter jejuni LMG 8841 and Campylobacter coli LMG 6440) were used as controls.
13Campylobacter detection and isolation methods were based on the ISO 10272 procedure. Briefly, each swab or 25 g of solid material were inoculated into 100 ml selective enrichment Bolton broth (Oxoid, Belgium) supplemented with 0.5% lysed defibrinated horse blood. Water samples were first filtered through a sterile 0.45 µm membrane filter (Zetapor, CUNO Benelux, Belgium) prior to add the latter to 100 ml of broth. All samples were then subjected to pre-enrichment step at 37°C for 4 h followed by enrichment cultures at 42°C for 44 h in microaerophilic atmosphere. Microaerophilic conditions were generated by using commercial gas generating kits (Anaerocult C, VWR International, Belgium). After enrichment, the samples were streaked onto selective agar media (Karmali agar, Biokar, Belgium; mCCDA agar, Oxoid, Belgium) and the plates were incubated in jars at 42°C for 48 h in microaerophilic atmosphere. From each positive agar plate, several typical Campylobacter colonies were subcultured onto Brucella agar (48 h, 42°C), confirmed as a member of the genus by examination of cellular morphology and motility on a wet mount under phase contrast microscopy and tested for Gram-staining, production of oxidase and catalase, hippurate hydrolysis and antibiotic susceptibility to nalidixic acid and cephalothin.
14A multiplex PCR test was used for the final confirmation. After morphological and biochemical confirmation, three colonies from the mCCDA confirmation plates were picked up and anaerobically sub-cultured into Brucella broth at 42°C for 48 h. The total DNA was extracted from cell pellets, using the Genomic DNA Wizard kit (Promega, WI, USA) according to the salting-out technique. The multiplex PCR reaction was carried out with 16S rDNA primers designed to obtain the specific identification of the genus (Denis et al., 1999) and CeuE and MDmapA primers to discriminate C. coli and C. jejuni species (Nayak et al., 2005). PCR products were visualized on a 1% agarose gel.
2.4. Flock information
15On the first and fourth sampling day, detailed farm and flock management data were collected through the submission of a standardized management questionnaire. The questionnaire was conducted in the form of an in-person interview with the farm owner or manager. A single member of the study team administered the questionnaire on all farms to eliminate inter-observer error. The questionnaire consisted of 111 questions. Thirty-one (28%) of the questions were open-ended (requiring descriptions), 12 (11%) were semi-closed (asking about number of days, animals, or rooms) and 68 (61%) were closed (with Yes/No or preset options for answers). The questionnaire was previously pilot tested by the French Agency for Food Safety (Ploufragan, France), but repeatability of answers was not tested directly. The questionnaire took about 2 h to complete. Collected data, detailed in table 2, concerned sanitary practices, litter, conditions of chick placement, dead-bird management, control of wildlife, house and flock characteristics, house surroundings, water supply and others. At each sampling day, a separate questionnaire was filled out by the person taking the samples, in order to give information about the rodents/insects presence and the sanitary status of the broiler house and surroundings. Farms were considered as Campylobacter-positive when the pathogen was detected in cecal droppings at least at slaughter age.

2.5. Statistical analysis
16Descriptive variables of the flock assessed by questionnaire (in qualitative form) were first selected to eliminate those that generated the same responses for at least 75% of the 18 flocks (Denis et al., 2008). Association between these remaining descriptive variables and explanatory variables were tested using Fisher’s exact test available in SAS software (SAS Institute Inc., NC, USA). Variables generating a response frequency lower than 11% (corresponding to two flocks) were removed. Finally, associations between the remaining descriptive variables and explanatory variables were tested with Fisher’s exact test (P ≤ 0.05). Although risk factors generally result from the combination of several parameters, a multivariate statistical analysis was not performed because of the low number of data.
3. Results
3.1. Flock contamination by C. jejuni and C. coli
17Data for all successive flocks for the six farms are summarized in table 3. From February to June 2005, i.e. in spring, the results of table 3 show that four farms (66.7%) were Campylobacter-positive before slaughter age. The extent of contamination increased in summer until October (flock b), to reach a prevalence of 100%. Furthermore, in winter, only 33.3% of the farms were contaminated by Campylobacter. The species distribution among the Campylobacter-positive flocks shows that C. jejuni, alone or mixed with C. coli, was predominant in broiler chickens from free-range broiler production in Belgium. Overall, mixed infections by C. jejuni/C. coli represented 40.6%, while C. jejuni and C. coli only represented 46.9% and 12.5% of chicken samples respectively, taking into account all days of sampling. At slaughter age, 5 of the 12 contaminated flocks (41.6%) were contaminated by mixed population of C. jejuni/C. coli, four flocks (33.3%) by C. jejuni and three flocks (25%) by C. coli.
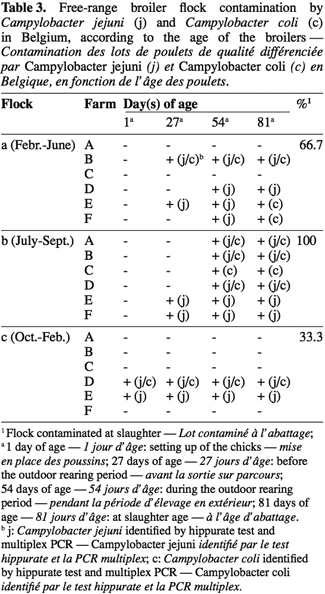
18For the winter period (flocks c), the two Campylobacter-positive flocks were yet contaminated from 1 d of age. In the spring period (flocks a), Campylobacter was detected in two flocks before the exit of the chickens on the free-open range (day 27) and two additional flocks were contaminated after the exit. In the summer time, contamination was detected in flocks Eb and Fb at 27 days of age while three more flocks were Campylobacter-positive at 54 days of age.
19Table 4 gives details of flocks in which environmental samples were found to be Campylobacter-positive before or at the same time that flock infection was detected. In 83.3% of the flocks did positive environmental samples come partly or fully from the open-air range. Moreover, the anteroom floor was detected Campylobacter-positive in 3 of the 13 infected flocks (23%). Other contaminated environmental samples were litter (flock Da) and exit trap door (flock Eb).
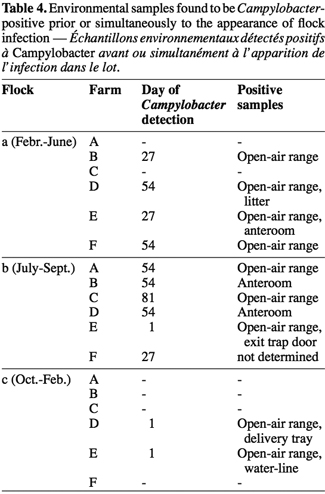
20Moreover, results for the third flock, from October to February, are distinctive. Contamination for the two Campylobacter-positive farms was revealed just before the setting up of the chicks, only from the free-air range soil, the water-line, and the delivery tray. Broilers fecal droppings were infected later, at four to eight weeks of age (day 54).
3.2. Flock characteristics correlated to Campylobacter infection
21Among the 111 items included in the questionnaires submitted to the farmer, seven variables were retained and studied in relation to the Campylobacter infection of flocks. These variables concerned poultry house and flock management (Table 5).
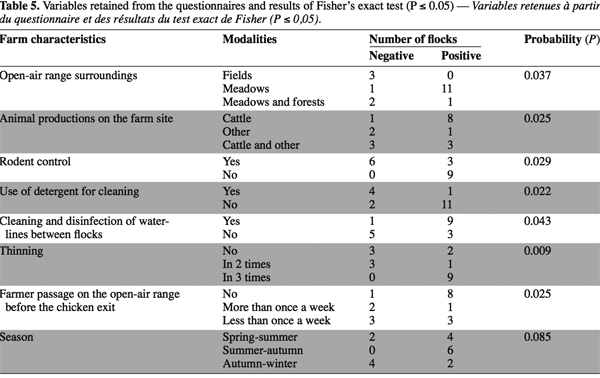
22The kind of other animal production systems in the farm, the mode of cleaning, the disinfection of aprons surrounding the broiler house, the control of rodents, and the environment around the open-air range had significant effect on flock contamination (P ≤ 0.05). On the other hand, the season when sampling was performed did not affect the contamination status of the flock (P > 0.05), although the Campylobacter prevalence decreased from 100% to 33.3% between summer and winter.
4. Discussion
23The Campylobacter prevalence in free-range broiler production of the southern part of Belgium was found very high with 33.3 to 100% of flocks being infected from February 2005 to February 2006. Similar observations were reported in Denmark for 22 organic broiler flocks (Heuer et al., 2001), and in France for 73 flocks from extensive outdoor broiler production (Huneau-Salaün et al., 2007). These values are higher than those observed in conventional broiler production by Herman et al. (2003) and Rasschaert et al. (2006) who reported Campylobacter prevalence at broiler flock level from 39 to 72% in Belgium. Higher Campylobacter prevalence in extensive production in comparison with conventional intensive farms was frequently observed in Denmark (Heuer et al., 2001) and in the USA (Luangtongkum et al., 2006). However, high variations in the proportion of colonized broiler flocks have been observed between countries, from 3% in Finland to > 90% in the United Kingdom (Rasschaert, 2007), which could partly reflect different sampling and isolation methods used.
24A season effect for Campylobacter presence is generally reported in the literature (Refrégier-Petton et al., 2001; Bouwknegt et al., 2004). In our study, the Campylobacter prevalence varied from 33.3% in winter to 100% in summer, the higher number of Campylobacter-positive flocks being found from July to October. The statistical analysis showed no significant effect of the season but only a trend (P = 0.085). The reason for these seasonal variations is still debated but may indicate a possible relationship between temperature and Campylobacter spp. survival and transmission of infection to broilers as stated by Patrick et al. (2004). Insects have been frequently implicated in this seasonal effect of Campylobacter prevalence. Some researches carried out by Hald et al. (2008) showed that insects may be an important source of Campylobacter infection of broiler flocks. Insects that may be present in poultry house are mainly flies identified to the families Muscidae (house fly Musca domestica, little house fly Fannia canicularis,...), but also insects of the families Tenebrionidae (Litter Beetle Alphitobius diaperinus), Dermestidae (Hide Beetle Dermestes maculates), Calliphoridae (Blue bottle fly Calliphora vomitoria), as well as several species of mites, lice and fleas (personal communication). In summer, hundreds of flies passed through the ventilation system into the broiler house and the influx of insects was correlated with the outdoor temperature. The climate impact could be especially important in free-range production for which the birds are in close contact with the environmental conditions. In the future, any epidemiological study should take into account potential correlations between climate factors, like the average temperature or hours of sunlight, and Campylobacter prevalence in poultry flocks.
25The thermotolerant species C. jejuni and C. coli account for most of the human foodborne infections (Zorman et al., 2006). The species distribution among the Campylobacter-positive flocks in this study showed that C. jejuni was predominant in free-range broiler production in Belgium, with a prevalence, varying according to the flock considered, from 42.9% to 50% for C. jejuni alone, and from 80% to 100% when combining C. jejuni and mixed C. jejuni/C. coli isolates. These results are in agreement with most of epidemiological studies where identification to species level has been undertaken (Heuer et al., 2001; Siemer et al., 2005; Denis et al., 2008), while C. coli and even Campylobacter lari had also been isolated as a common species from chickens in some study (Zorman et al., 2006; Kilonzo-Nthenge et al., 2008). Van Looveren et al. (2001) found that among 677 Campylobacter isolates from broiler carcasses and meat from Belgian slaughterhouses, 79% was identified as C. jejuni. It should be mentioned that poultry carcasses could be cross-contaminated by different Campylobacter species during the slaughter processing, leading to different results compared to Campylobacter contamination of the broiler flocks.
26Identification of the sources of flock colonization would enable control measures to be targeted towards the areas posing the greatest risk. In most infected flocks, the first time Campylobacter spp. was found in cecal samples was when broilers were 27-day-old. This is in agreement with other studies where it has been reported that most flocks become infected only two to three weeks after the setting up of chicks into a broiler house (Saleha, 2004; Bull et al., 2006; Hansson et al., 2007). This lag-phase in contamination is a possible protection effect of maternal immunity, as proposed by Sahin et al. (2003). This observation may support the hypothesis that Campylobacter spp. are not transmitted vertically from parents to chicks, as stated by Saleha (2004).
27Moreover, results for the c flocks, from October to February, are distinctive. Contamination for the two Campylobacter-positive farms was revealed just before the setting up of the chicks, from the free-air range soil, the water-line, the delivery tray and the anteroom floor swabs, whereas broilers cecal droppings were infected later, at four to eight weeks of age. This may have been due partly to the residual presence of pathogens either from previous Campylobacter-positive flocks or to the environmental contamination of the house surroundings from which the infection could have arisen. Campylobacter spp. are ubiquitous in the environment and around broiler houses and may be easily transported into the house either in utilities, such as feed, litter and water. In this study, Campylobacter spp. were not isolated from any samples of litter and feed after the setting up of the chick, as observed by Bull et al. (2006) and Hansson et al. (2007). In case of flocks Ec and Fc, water-lines were detected positive from the first day. As Newell et al. (2003) pointed that contamination of the water lines usually follows rather than precedes colonization of the flock, it may be hypothesized that poor disinfection of water-line after flocks Eb and Fb may be responsible for the Campylobacter-detection in the following flocks. As pointed out by Newell et al. (2003), the analysis of risk factors in this study showed that cleaning and disinfection of water-line between flocks may help to reduce the risk of chicken colonization.
28Human activities as entrance of farmers, maintenance staff, veterinarians, and catching crews, may also carry out Campylobacter into the house from the external environment via boots, external clothes and equipment (Newell et al., 2003; Ramabu et al., 2004). The contamination of the anteroom floor may actually suggest the possible infection of flock by the farmer, visitors and equipments. Unsuitable hygiene practices at the farm level, especially poor cleaning and disinfection of the house and not dedicated protective clothing, could then be a major reason of Campylobacter contamination persistence in poultry flocks, as summarized by Allen et al. (2005). Moreover, most samples found to be Campylobacter-positive in this study were from the open-air range which appears to be a major source of Campylobacter contamination. Such conclusion was also reported by Rivoal et al. (2005) who studied genomic diversity and sources of Campylobacter contamination in French free-range broiler farms. The subsequent flock infection may be related to contamination of farmer equipment by the open-air range soil or through contact in the open-air range with wild birds and other animals and with their faeces (Rodenburg et al., 2004). However, the carrying of Campylobacter into the house by human activity and environmental factors has yet to be proven by genotyping confirmation of strains in the environment which subsequently result in flock colonization.
29Other risk factors for Campylobacter contamination have been identified in this study. Efficiency of the rodent control applied at the farm appeared to be important as wild animals like insects and rodent are recognized as vectors of Campylobacter (Stern et al., 2001; Saleha, 2004). The presence of other domestic animals on the farm site is the main risk factor underlined by several authors (Bouwknegt et al., 2004; Saleha, 2004). The risk of spreading Campylobacter from other animals on the farm to the broiler flock, or generally from environmental surroundings, can be reduced by applying strict hygiene measures, like walk-over benches, using boot dips or house dedicated footwear, at least until the access of the birds to the open area.
30Thinning also appeared to be a major risk factor for the introduction of Campylobacter into the broiler house. Thinning or partial depopulation of the flock is a common procedure in many European countries, including Belgium. This practice enables higher productivity and provides the market of birds of different weight. During thinning, the doors of the poultry house are opened and the catching crew and the catching equipment enter the poultry house without any hygiene measures. Ramabu et al. (2004) found that catching equipments like trucks, forklifts, or crates may actually be contaminated by Campylobacter, which represent a major risk of contamination for the remaining birds.
31In summary, the results of this study provided for the first time information about the Campylobacter prevalence in free-range broiler production from the southern part of Belgium and pointed out potential sources of Campylobacter for this kind of rearing system. The high prevalence of enteric Campylobacter in free-range broiler production in Belgium was similar to results from other European states. The Campylobacter prevalence increased in summer-fall. The environment surrounding the broiler house, especially the open-air range, seems to be an important way of contamination of broilers and appears consequently as a burdensome parameter to take into account in further development of Campylobacter control programs.
32Suitable biosecurity measures to exclude Campylobacter spp. from free-range broiler flocks are currently the only intervention available. However, it is generally considered that adequate biosecurity procedures are difficult to sustain in the farm environment (Newell et al., 2003). The development of supplementary on-farm control strategies may be required to achieve predominantly Campylobacter-negative flocks.
33List of abbreviations
34EFSA: European Food Safety Authority
35mCCDA: modified Cefoperazone Charcoal Deoxycholate Agar
36PCR: Polymerase Chain Reaction
37Acknowledgements
38We would like to thank the poultry companies involved and their contract farmers for their participation. The technical assistance of Mrs Pascale Van Hal and the help in epidemiological protocol development from Mrs Valérie Decauwert are gratefully acknowledged. We are also grateful to Jean-Yves Zimmer, from the Department of functional and evolutionary Entomology of the ULg - Gembloux Agro-Bio Tech, for information about insects commonly found in broiler houses. Financial support was provided by the Ministry of the Walloon Region – General Direction of Agriculture, Natural Resources and Environment (DGARNE), Division for Research, Jambes, Belgium.
Bibliographie
Allen V.M. & Newell D.G., 2005. Evidence for the effectiveness of biosecurity to exclude Campylobacter from poultry flocks. Food Standard Agency Report, Commissioned project MS0004, http://www.food.gov.uk/multimedia/pdfs/biocampy.pdf, (04/07/06).
Arsenault J. et al., 2007. Prevalence and risk factors for Salmonella spp. and Campylobacter spp. caecal colonization in broiler chicken and turkey flocks slaughtered in Quebec, Canada. Prev. Vet. Med., 81, 250-264.
Berndtson E., Danielsson-Tham M.-L. & Engvall A., 1996. Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process. Int. J. Food Microbiol., 32, 35-47.
Bouwknegt M. et al., 2004. Risk factors for the presence of Campylobacter spp. in Dutch broiler flocks. Prev. Vet. Med., 62, 35-49.
Bull S.A. et al., 2006. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl. Environ. Microbiol., 71, 645-652.
Butzler J.-P., 2004. Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect., 10, 868-876.
Denis M. et al., 1999. Development of a m-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Lett. Appl. Microbiol., 29(6), 406-410.
Denis M. et al., 2008. Diversity of pulsed-field gel electrophoresis profiles of Campylobacter jejuni and Campylobacter coli from broiler chickens in France. Poult. Sci., 87, 1662-1671.
European Food Safety Authority, 2007. The community summary report on trends and sources of zoonoses, zoonotic agents, antimicrobial resistance and foodborne outbreaks in the European Union in 2006. EFSA J., 130, 1-352.
Evans S.J. & Sayers A.R., 2000. A longitudinal study of campylobacter infection of broiler flocks in Great Britain. Prev. Vet. Med., 46, 209-223.
Friedman C.R., Neimann J., Wegener H.C. & Tauxe R.V., 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I. & Blaser M.J., eds. Campylobacter. 2nd ed. Washington, DC, USA: American Society for Microbiology, 121-138.
Hald B., Skovgård H., Pedersen K. & Bunkenborg H., 2008. Influxed insects as vectors for Campylobacter jejuni and Campylobacter coli in Danish broiler houses. Poult. Sci., 87, 1428-1434.
Hansson I., Vågsholm I., Svensson L. & Engvall E.O., 2007. Correlations between Campylobacter spp. prevalence in the environment and broiler flocks. J. Appl. Microbiol., 103, 640-649.
Herman L. et al., 2003. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol. Infect., 131, 1169-1180.
Heuer O.E., Pedersen K., Andersen J.S. & Madsen M., 2001. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol., 33, 269-274.
Huneau-Salaün A., Denis M., Balaine L. & Salvat G., 2007. Risk factors for Campylobacter spp. colonization in French free-range broiler-chicken flocks at the end of the indoor rearing period. Prev. Vet. Med., 80, 34-48.
Kapperud G. et al., 1993. Epidemiological investigation of risk factors for Campylobacter colonisation in Norwegian broiler flocks. Epidemiol. Infect., 111, 245-255.
Kilonzo-Nthenge A., Nahashon S.N., Chen F. & Adefope N., 2008. Prevalence and antimicrobial resistance of pathogenic bacteria in chicken and guinea fowl. Poult. Sci., 87, 1841-1848.
Luangtongkum T. et al., 2006. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl. Environ. Microbiol., 72, 3600-3607.
Moore J.E. et al., 2005. Campylobacter. Vet. Res., 36, 351-382.
Nayak R., Stewart T.M. & Nawaz M.S., 2005. PCR identification of Campylobacter coli and Campylobacter jejuni by partial sequencing of virulence genes. Mol. Cell. Probes, 19(3), 187-193
Newell D.G. & Fearnley C., 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol., 69, 4343-4351.
Patrick M.E. et al., 2004. Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Appl. Environ. Microbiol., 70, 7474-7480.
Ramabu S.S., Boxall N.S., Madie P. & Fenwick S.G., 2004. Some potential sources for transmission of Campylobacter jejuni to broiler chickens. Lett. Appl. Microbiol., 39, 252-256.
Rasschaert G., 2007. Molecular epidemiology of Salmonella and Campylobacter contamination of poultry during transport and slaughter. PhD thesis: Faculty of Veterinary Medicine, Ghent University (Belgium).
Rasschaert G., Houf K., Van Hende J. & De Zutter L., 2006. Campylobacter contamination during poultry slaughter in Belgium. J. Food Prot., 69, 27-33.
Refrégier-Petton J., Rose N., Denis M. & Salvat G., 2001. Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med., 50, 89-100.
Rivoal K. et al., 2005. Genomic diversity of Campylobacter coli and Campylobacter jejuni isolates recovered from free-range broiler farms and comparison with isolates of various origins. Appl. Environ. Microbiol., 71, 6216-6227.
Rodenburg T.B., Van Der Hulst-Van Arkel M.C. & Kwakkel R.P., 2004. Campylobacter and Salmonella infections on organic farms. Neth. J. Agric. Sci., 52, 101-108.
Rosenquist H. et al., 2003. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol., 83, 87-103.
Sahin O., Luo N., Huang S. & Zhang Q., 2003. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl. Environ. Microbiol., 69, 5372-5379.
Saleha A.A., 2004. Epidemiological study on the colonization of chickens with Campylobacter in broiler farms in Malaysia: possible risk and management factors. Int. J. Poult. Sci., 3, 129-134.
Siemer B.L., Nielsen E.M. & On S.L.W., 2005. Identification and molecular epidemiology of Campylobacter coli isolates from human gastroenteritis, food, and animal sources by amplified fragment length polymorphism analysis and Penner serotyping. Appl. Environ. Microbiol., 71, 1953-1958.
Stern N.J. et al., 2001. Distribution of Campylobacter spp. in selected U.S. poultry production and processing operations. J. Food Prot., 64, 1705-1710.
Van de Giessen A.W., Bloemberg B.P., Ritmeester W.S. & Tilburg J.J., 1996. Epidemiological study on risk factors and risk reducing measures for campylobacter infections in Dutch broiler flocks. Epidemiol. Infect., 117, 245-250.
Van Looveren M. et al., 2001. Antimicrobial susceptibilities of Campylobacter strains isolated from food animals in Belgium. J. Antimicrob. Chemother., 48, 235-240.
Wallace J. et al., 1997. Seasonality of thermophilic Campylobacter populations in chickens. J. Appl. Microbiol., 82, 219-224.
Zorman T., Heyndrickx M., Uzunović-Kamberović S. & Smole Možina S., 2006. Genotyping of Campylobacter coli from retail chicken meat and humans with campylobacteriosis in Slovenia and Bosnia and Herzegovina. Int. J. Food Microbiol., 110, 24-33.
Para citar este artículo
Acerca de: Sabrina Vandeplas
Univ. Liege - Gembloux Agro-Bio Tech. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: svandeplas@ulg.ac.be
Acerca de: Robin Dubois-Dauphin
Univ. Liege - Gembloux Agro-Bio Tech. Bio-industry Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Rodolphe Palm
Univ. Liege - Gembloux Agro-Bio Tech. Applied Statistics, Computer Science and Mathematics Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Yves Beckers
Univ. Liege - Gembloux Agro-Bio Tech. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Philippe Thonart
Univ. Liege - Gembloux Agro-Bio Tech. Bio-industry Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: André Théwis
Univ. Liege - Gembloux Agro-Bio Tech. Animal Science Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






