- Accueil
- Volume 14 (2010)
- numéro 3
- The lipoxygenase metabolic pathway in plants: potential for industrial production of natural green leaf volatiles
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
The lipoxygenase metabolic pathway in plants: potential for industrial production of natural green leaf volatiles

Notes de la rédaction
Received on June 9, 2009; accepted on December 1, 2009
Résumé
La voie métabolique de la lipoxygénase chez les plantes : un potentiel pour la production d'arômes naturels à notes vertes. Voie métabolique incontournable du règne végétal, la voie enzymatique de la lipoxygénase est très largement décrite dans la littérature. Au sein de celle-ci, les actions combinées de trois enzymes, lipase, lipoxygénase (LOX) et hydroperoxyde lyase (HPL), convertissent un substrat lipidique (acides C18:2 et C18:3) en molécules volatiles à courte chaine. Ces réactions stimulées par l'endommagement des cellules membranaires produisent des composés communément appelés Molécules à Note Verte (MNV) dénominant des aldéhydes et des alcools en C6 ou en C9. Ces MNVs sont des composés aromatiques largement utilisés pour conférer une impression de fraicheur et d'authenticité aux produits alimentaires. Par conséquent, des systèmes de production compétitifs ont été développés afin de subvenir à la haute demande en molécules aromatiques naturelles. Des huiles végétales, choisies selon leur profil en acides gras, sont converties par la LOX de soja et par l'HPL en MNVs naturelles. Cependant, la seconde étape de cette bioconversion présente des rendements faibles causés par l'instabilité de l'HPL et par son inhibition à son propre substrat. Cet article scientifique va brièvement décrire les différentes enzymes impliquées dans cette bioconversion selon leurs propriétés chimiques et enzymatiques. Des techniques biotechnologiques d'amélioration de leur potentiel de production seront ensuite exposées dans le cadre d'un processus complet de bioconversion, du substrat lipidique aux arômes correspondants.
Abstract
Lipoxygenase enzymatic pathway is a widely studied mechanism in the plant kingdom. Combined actions of three enzymes: lipase, lipoxygenase (LOX) and hydroperoxide lyase (HPL) convert lipidic substrates such as C18:2 and C18:3 fatty acids into short chain volatiles. These reactions, triggered by cell membrane disruptions, produce compounds known as Green Leaf Volatiles (GLVs) which are C6 or C9-aldehydes and alcohols. These GLVs are commonly used as flavors to confer a fresh green odor of vegetable to food products. Therefore, competitive biocatalytic productions have been developed to meet the high demand in these natural flavors. Vegetable oils, chosen for their lipidic acid profile, are converted by soybean LOX and plant HPL into natural GLVs. However this second step of the bioconversion presents low yield due to the HPL instability and the inhibition by its substrate. This paper will shortly describe the different enzymes involved in this bioconversion with regards to their chemical and enzymatic properties. Biotechnological techniques to enhance their production potentialities will be discussed along with their implication in a complete bioprocess, from the lipid substrate to the corresponding aldehydic or alcoholic flavors.
Table des matières
1. Introduction
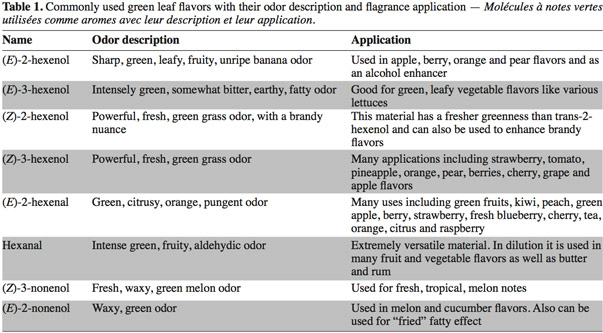
1Green Leaf Volatiles (GLVs) synthesized through the lipoxygenase (LOX) enzymatic pathway are involved in plant aromatic reactions. GLVs are metabolized from C18-polyunsaturated fatty acids (including linoleic and linolenic acids) producing C6- and C9-aldehydes (Matsui et al., 2006). The first C6-GLV compound synthesized by the LOX pathway is (Z)-3-hexenal which is formed after tissue disruption (Matsui et al., 2000a) and is then converted to other GLVs such as (E)-2-hexenal (leaf aldehyde), (Z)-3-hexenol (leaf alcohol) and (Z)-3-hexenyl acetate (leaf ester) (Shiojiri et al., 2006). These C6-volatiles compounds are important components contributing to the aroma and flavor of fruits and vegetables and are associated with the green note odor (Hatanaka, 1993). For this reason, these molecules are widely used in food and beverage industry (Fukushige et al., 2005) (Table 1). These last years, the global market for these products was USD 30 billion per year including USD 18 billion for the natural flavor class (Schrader et al., 2004; Whitehead et al., 1995). Due to their anti-microbial activities some C6 and C9-GLVs, such as hexanal and (E)-2-Hexenal have also some industrial use in food storage (Hubert et al., 2008).
2Chemical synthesis is the easiest way to produce large amounts of stable C6- or C9- aldehydes and alcohols. However, for food application, consumers have a strong preference for naturally synthesized additives and aromas. Given the high demand for such natural flavors, an efficient biocatalytic process has to be developed for large scale production. Vegetal oils containing C18:2 and C18:3 fatty acids are converted by lipoxygenase (LOX) and hydroperoxide lyase (HPL) into hexanal and (E)-2- or (Z)-3-hexenal. Large-scale conversion of fatty acids into hydroperoxides (HPOs) by LOX has been successfully accomplished (Drouet et al., 1994; Fauconnier et al., 1996). However, the conversion of hydroperoxy-fatty acids by HPL is more difficult to handle since the HPL enzyme present in plant extracts is very unstable and conversion yield remains very low (Almosnino et al., 1996; Cass et al., 2000). This paper will expose the approaches and techniques developed to produce GLVs from vegetal oil with regards to the catalytic activities of the different enzymes.
2. Green leaf volatiles synthesis pathway
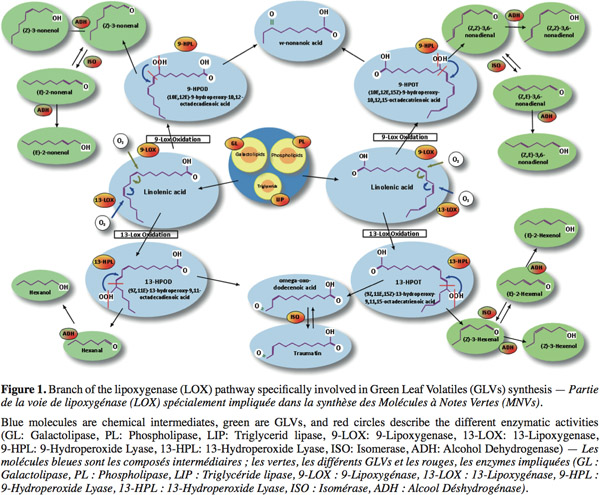
3GLVs commonly denominate all aldehydes and alcohols produced by the HPL. Thus, GLVs are produced under the LOX pathway through a complex metabolism in the leaves (Figure 1). First, lipids are hydrolysed in free fatty acids by different types of lipases. Afterwards, LOX catalyses the stereospecific oxidation of unsaturated free fatty acids. (9Z,11E,15Z)-13-hydroperoxy-9,11,15-octadecatrienoic acid (13-HPOT) is produced from linolenic acid and is further metabolized by HPL to form 12-oxo-(Z)-9-dodecenoic acid (a precursor of the traumatin) and (Z)-3-hexenal (Grechkin, 1998). These products can be isomerized, enzymatically or not, to form 12-oxo-(E)-9-dodecenoic acid and (E)-2-hexenal. Another type of LOX can also synthesize (10E,12E,15Z)-9-hydroperoxy-10,12,15-octadecatrienoic acid (9-HPOT) from the linolenic acid. These products can also be transformed by HPL in C9-oxo-acids and C9-aldehydes. Linoleic acid is the second substrate (Figure 1) of LOX and is oxydated in 9-HPOD and 13-HPOD, precursors of several aldehydes: such as hexanal and nonenal isomers. All these aldehydes can finally be further transformed by alcohol dehydrogenase to form the corresponding C6- or C9-alcohols. This mixture of volatile compounds leads to a characteristic odor for each plant called “the green note” (Hatanaka, 1993). These molecules have an increasingly significant place within the world market of flavors, conferring freshness and authenticity to a product.
2.1. Lipase activity
4Lipase is an important class of lipid acyl hydrolase (E.C 3.1.1.3), widely present in living organisms. Several studies have shown that the preferential substrate for GLVs production is galactolipids (Geimel, 1987). If the activity is extremely high phospholipids and finally mono- di- and triglycerids can also be metabolized (Ishiguro et al., 2001). Galactolipids are present in thilakoid membranes and contain large amounts of trienoic acids, such as C18:3 and C18:2 while phospholipids constitute the major fraction of the plant cell membrane. Generally, galactolipase (GL) and other lipase activity levels are naturally very low in plants. The quick release of free fatty acid from galactolipids, such as monogalactosyldiacylglycerol (MGDG), could be related to some inducible defense system. This is the first step of a cascade of reactions leading to apoptose programmed cell death following intracellular signal (Cacas et al., 2005). Lipase enzymes, especially those from plants, work in mild conditions: neutral pH and room temperature, but are usually low concentrated. Under industrial purpose, replacement of the native lipase in plant by a microbial immobilized enzyme leads to larger amounts of free fatty acids produced. Each year, more than 1 million kg of free fatty acids are produced through enzymatic processes using microbial lipases (Gargouri et al., 2008).
2.2. Lipoxygenase activity
5LOX (EC 1.13.11.12) catalyses the second stage of the GLVs synthesis. Several isoenzymes from the LOX catalytic group have been identified in several plants (LOX-1 to LOX-6 in Glycine max) (Matsui et al., 1998), all of them containing an iron atom in their active site and being about 100 kDa in size. Free fatty acids are the main LOX substrates, but glycerolipids and phospholipids were also reported as substrates for oxygenation (Baysal et al., 2007). The different LOX iso-enzymes can be classified in two types according to their stereospecificity, the 9-LOX, oxygenating the ninth carbon of the fatty acid and the 13-LOX, the thirteenth. Most of the time, one of the LOX isoenzyme is more abundant, thereby orienting the complete LOX pathway of the plant (9-LOX in cucumber, 13-LOX in watermelon, etc.). LOX activity is temperature and pH dependent, leading to different rates of position isomer production. For example, LOX reactions performed at temperatures below 10°C lead to specific reaction with more than 90% of 13-HPO isomers. As a matter of fact, LOX always synthesizes high levels of S diastereoisomer (95%), by contrast with spontaneous auto-oxidation, which is not stereospecific and produces racemic (R, S) mixture of isomers (Coffa et al., 2005).
6Nowadays, LOX have been found in a lot of varieties of plants, such as tomato leaves (Fauconnier et al., 1997), soybean seeds (Axerold et al., 1981), dry fruits (walnuts, almonds, etc.) (Buranasompob et al., 2007) or olive fruit (Lorenzi et al., 2006). LOX is distributed in plant organs according to the type of environmental conditions, and the age of the plant (Kato et al., 1992). Several plants, such as soybean seeds, have been reported to contain sufficiently high and stable LOX activity for an industrial application (Fauconnier et al., 1996; Rabetafika et al., 2008). LOX is generating HPOs which are potentially cytotoxic for cell membrane and would not therefore be expected to accumulate in plant tissues. These HPOs act as reactions hubs, they are quickly metabolized in chemical compounds involved in signaling, plant defense and apoptose. LOX could also lead to off-flavor synthesis in several food products (Laine et al., 2006). Therefore, LOXs can be thermally inactivated above 60°C and under a pH of 4 with a resulting improvement in the shelf life of foods (Wang et al., 1991).
2.3. Hydroperoxide lyase activity
7Hydroperoxide lyase (HPL) catalyses the chain cleavage of an HPO between the hydroperoxide acid group and the neighbouring carbon group. 9-HPO are converted in C9 oxo-acid and C9 aldehydes, and 13-HPO in C12 oxo-acid and C6 aldehydes. HPLs contain an heme prosthetic group called iron-porphyrin, conferring special folding to the protein. The conformation of the enzyme leads to the formation of a hydrophobic corridor within which the HPO takes place. This molecule is successively metabolized in an epoxyallylic radical, a vinyl ether radical and an hemiacetal before being cleaved in aldehyde and oxo-acid (Grechkin et al., 2006). This enzymatic reaction is really fast, producing 6,000 turn-overs in a second. But, the reaction velocity is gradually decreased to zero before all the substrate is used. This is a typical behavior of an enzyme under suicide inactivation, phenomenon particularly found in P450 enzymatic family. This is a form of irreversible inhibition that occurs when HPL binds a substrate analogue, such as alkyl or alkoxyl radicals and forms an irreversible complex (Santiago-Gómez et al., 2007).
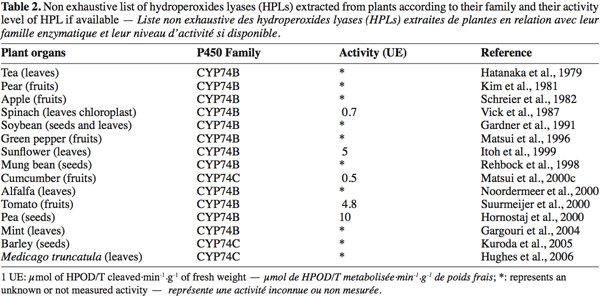
8Most of the HPL are membrane-bound enzymes except for watermelon HPL (Fukushige et al., 2005) and mainly located in chloroplast membranes (Blee et al., 1996). HPL is constituted by three or four monomers of 55 kDa each. The optimum pH is usually between 6 and 9.5 and the optimum temperature close to 30°C, HPLs are very susceptible to freezing and need cryoprotectants to be conserved (Rodrigo et al., 2007). HPL activity is associated with an isomerase activity, transforming Z into the E-isomer. This phenomenon leads to a large variety of different aldehydes and oxo-acids products. According to their particular structure and their specific activity, HPLs have been widely studied in several plants (Table 2). HPLs have important homology levels with allene oxide synthase (AOS) (Family CYP74A), and belong to the P450 enzyme family. These enzymes could be separated in two families CYP74B being specific for 13-HPOD/T and CYP74C specific for 9-HPOD/T.
2.4. Alcohol dehydrogenase activity
9ADH (alcohol-NAD-oxidoreductase; E.C. 1.1.1.1) is a well studied enzyme, not really abundant in plants. It was first detected in tea leaves and in soybean seeds but with an instable and low activity. ADH metabolizes aldehydes from the LOX pathway in their corresponding alcohols, conferring higher stability to the molecules (Fauconnier et al., 1999). Short chain alcohols are quite often detected in plant leaves, which suggests that the enzyme activity is sporadic and essentially specific to C6-aldehydes. This enzyme is approximately 150 kDa in size and is NAD dependent, which can be limiting for industrial application due the cost of this cofactor.
3. Modeling of an industrial biosynthesis of GLVs
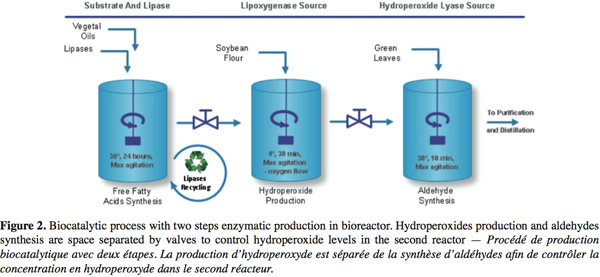
10Nowadays, natural C6- and C9-aldehydes are produced through an enzymatic industrial process (Figure 2) using plant extracts or cell cultures as sources of enzymes. In these processes, free fatty acids with high level of unsaturated forms are obtained from vegetal oil (Almosnino et al., 1996; Cass et al., 2000). Soybean flour (with a high level of LOX-1 isoenzyme, a stereospecific enzyme genereting high level of 13-HPO) is added under high oxygen flow and low temperature to produce HPOs (yield 80-90%). Finally, aldehydes are produced on diluted HPOs substrate in a short time reaction with plants extracts or cell cultures containing high HPL activity. Optional further transformation of aldehydes into alcohols can be realized with ADH usually obtained from yeast. Large scale conversion of fatty acids into HPOs has already been successfully developed (Drouet et al., 1994; Fauconnier et al., 1996) but the subsequent conversion into aldehydes is more problematic. HPLs extracted from plants are at low concentration and rather unstable and thus yields are lower than 15% for the molecular conversion of free fatty acids into GLVs.
3.1. Hydroperoxide synthesis from triglycerids
11As specified before, the choice of the vegetal oil will determine the class of HPO produced. Generally, olive oil or sunflower oil is hydrolyzed for HPOD production, and linseed oil for HPOT synthesis by commercial lipases. Biotransformation of free fatty acids into HPOs must be performed under high agitation level to increase the solubility of the substrates in aqueous solution and the level of oxygen available. In these conditions, LOX reaction is fast and completed within one hour. Fauconnier et al. (1996) have described HPOs synthesis in 10 l vessel with a yield ranging from 60 to 94%, depending on substrate concentrations. Alternatively, biphasic reactors were also used to combine lipase and LOX reactions. In these systems, lipase reaction is performed at the oil/water interface and free fatty acids produced remain in the organic phase. This phase acts as tank substrate for the LOX while HPOs are stored in the aqueous phase (Kaewthong et al., 2005). Furthermore, several immobilization assays have been performed successfully in a micellar system conferring higher resistance to temperature and high recovery of the LOX enzyme (Kermasha et al., 2002).
3.2. GLVs production from HPOs
12Production of specific GLV compounds is directed by the selection of adequate substrates and enzymes. Many plants organs with HPL activities were tested for GLVs production and obviously several retain some potential to enter in industrial process (Table 2). Several authors have developed systems for production of high concentrations of GLVs. They can increase more than hundred times the natural level of GLV through their production process (Rehbock et al., 1998; Tijet et al., 2001; Schade et al., 2003; Nemeth et al., 2004; Rabetafika et al., 2008). However these levels are still low for industrial purpose and in many cases stability and specificity of the enzyme have to be optimized to improve the productivity.
13The low level of HPO transformation is first due to the difficulty of HPL extraction. Specific chloroplasts extraction could be performed to obtain highly concentrated extracts of HPL (Husson et al., 2002). However the use of chemical detergents often reduces enzyme stability and it must be performed under controlled condition to prevent total enzymatic degradation. As alternative, the use of HPL in its native form from the grounded plant materials is possible without extraction. This method is cheaper but presents low yields because only a few enzymatic sites are accessible.
14The second main problem for HPOs transformation into GLV compounds is the abundance of hydroperoxide metabolizing enzymes other than HPL. Divinyl Ether Synthase (DES) and Peroxygenase (POX) are not abundant in plant leaves (Blee, 1998), but AOS is highly concentrated in some plants organs (Froehlich et al., 2001). Furthermore, AOS has a very close structure compared to HPL, and could only be specifically inhibited by highly expensive chemical compound synthesized from imidazole (Oh et al., 2006). A solution is to study enzymes concentrations and evolutions to perform synthesis when the ratio HPL/AOS is the most favorable. Also, using specific culture methods to favor one pathway instead of another may lead to higher HPL activities (De Domenico et al., 2007). Mutagenesis of wild type plant into non expressive AOS mutants is also possible. Indeed, modification of genomic determinant domains of the CYP74A and CYP74B enzymes reduces AOS activity and transforms this catalase reaction into HPL activity (Toporkova et al., 2008).
3.3. Choice and management of HPL sources
15Natural HPL from plants. HPL is widely distributed in vegetal kingdom. Tomato leaves and fruits, mint leaves, pea seeds, cucumbers, bell pepper fruits, almonds, soybean, tomato fruits, are examples of available sources of enzyme (Table 2). Plant sources must be selected for industrial transformation capabilities such as optimal reaction conditions (pH, temperature), availability, specificity and concentration. Until now, industrial interest for HPL utilization led to several patents. Goers et al. (1989) and Kanisawa et al. (1988) have established methods to produce green aroma compounds with soybean seeds and strawberry leaves respectively. More recently, Brunerie (1989) developed a complete production from unsaturated fatty acids to GLVs with different plant leaves as HPL source. These authors showed that acetylsalicylic acid, chlorophyll B and catalase improve the GLV synthesis. Other production processes using 9-HPL from muskmelon (Brash et al., 2001) or watermelon (Holtz et al., 2001) have been also developed.
16Recombinant HPL. Due to their low stability and difficulty to purify, HPL is the limiting component for the conversion of fatty acids into food flavor. Thereby considerable efforts have been made to clone and produce this enzyme with enhanced stability and activity. HPLs from tomato fruit (Matsui et al., 2000b), alfalfa (Noordermeer et al., 2000), tomato leaves (Atwal et al., 2005) and green bell pepper (Bourel et al., 2004) have been cloned successfully. These enzymes can be produced in Pichia pastoris, Yarrowia lipolitica or Escherichia coli leading to different yields of purified enzymes. Cloning in E. coli allows to obtain high activities from 5,000 to 8,000 U.l-1, but extraction from the cell membrane remains fastidious (Delcarte et al., 2003; Noordermeer et al., 2000). So, HPL secretion was developed by adding a secretory sequence to the HPL gene and by cloning it in different yeasts. This technique avoids certain laboratory manipulations but best yield achieved was only 1,000 U.l-1 in Y. lipolitica along with hexanal/(E)-2-hexenal production of 300 mg.l-1 (Bourel et al., 2004). But globally, recombinant sources of enzyme have two advantages compared to plant natural sources. Firstly, they do not depend on plant culture and are available all year long. Secondly, they do not contain AOS, DES or POX activity that would lead to undesired by-product and loss of substrate.
17Enzyme purification and stability. The best result for HPL purification was obtained with tomato fruit (Suurmeijer et al., 2000) and involves successively filtration, concentration by ultra filtration, purification on a G100 sephadex column and finally chromatography on a DEAE column. With this system, enzyme extract is purified more than 400 fold, and can be stored for months at -80°C or at 4°C with additives like Triton X-100. Another purification method was developed by Fauconnier (1997), involving selective precipitation with different PEG6000 amounts, ultracentrifugation and chromatography on DEAE column. This purification permits to obtain a 120 fold concentrated pure extract. Furthermore, enzyme extraction and storage is another solution to provide HPL during all the year, but techniques must be optimized to prevent loss of activity. Stabilization of the biocatalyst could be achieved by addition of various chemical compounds, including salt, sugars and polyols. The stabilization effects have been attributed to the ability of these compounds to counteract forces leading to enzyme inactivation. Several authors have already used chemicals such as dithiothreitol (Hornostaj et al., 2000), glycerol (Salas et al., 2005) or detergent (Matsui et al., 2000c) to prevent HPL degradation during extraction. Also Hall et al. (2007) have shown that KCl prevent enzyme destabilization during long-term storage or lyophilisation, and that glycine is one of the best additives to keep high enzyme activity and to improve stability. But recently, new and reliable protocols have been developed to produce high amount of recombinant CYP74 proteins, and patent-protected procedures have also been established for the stabilization (Hughes et al., 2009). It appears that presence of detergent during freeze-drying is extremely detrimental to the storage and stability of the proteins, denaturating the haem conformation. So, detergent free HPL extracts conserve stability during long-term storage.
3.4. Reduction reaction upon ADH activity
18Hexanol, (Z)-3-Hexenol, and other GLV alcohols are widely used in food industry because of their higher stability compared to the corresponding aldehydes. Although these alcohols are found in larger amounts in plants organs compared to aldehydes, they are still not extractible from natural source at industrial scale. Transformation in alcohols requires ADH activity which is naturally low in plant. Therefore, the use of ADH from different yeast is recommended to achieve high conversion of aldehydes. Fauconnier et al. (1999) have shown that this reduction reaction is best performed between 30°C and 50°C and under pH between 5 and 7. Pischia anamola is an efficient microorganism for such bioconversion. And no inhibitory effects of the Z-3-Hexenal substrate have been detected. Fauconnier et al. (1999) also showed an efficient combination of aldehydes production by HPL and alcoholic reduction by ADH without significant loss of alcoholic transformation yield.
4. Conclusion
19Actually, GLVs are the most valuable flavor class commonly sold in natural food industry but its large scale synthesis remains a great challenge for producers. Bioconversions of vegetal oil are usually encountered to achieve high GLVs levels from low cost substrates. HPL is at the centre of these processes, being the limiting factor for flavor production. Scientifics are developing biotechnological tools to improve this critical step. Application of stimuli enhancing activity, production of recombinant enzymes and GLVs synthesis stabilization by chemical compounds are ways to explore to raise HPL levels.
20Abbreviations
21GLV Green Leaf Volatile
22LOX Lipoxygenase (enzyme or enzymatic pathway)
23AOS Allene Oxide Synthase
24DES Divinyl Ether Synthase
25ADH Alcohol Dehydrogenase
26POX Peroxygenase
27GL Galactolipase
28MGDG Monogalactosyldiacylglycerol
29HPO Hydroperoxy-acids (general term)
309-HPOD (10E,12E)-9-hydroperoxy-10,12-octadecadienoic acid
319-HPOT (10E,12E,15Z)-9-hydroperoxy-10,12,15-octadecatrienoic acid
3213-HPOD (9Z,11E)-13-hydroperoxy-9,11-octadecadienoic acid
3313-HPOT (9Z,11E,15Z)-13-hydroperoxy-9,11,15-octadecatrienoic acid
34Acknowledgement
35The work in laboratory was financed by the Ministry of the Walloon Region. Cédric Gigot is a recipient of FRIA grant and Marc Ongena is a research associate at the F.R.S.-FNRS in Belgium.
Bibliographie
Almosnino A.M., Bensoussan M. & Belin J.M., 1996. Unsaturated fatty acid bioconversion by apple pomace enzyme system. Factors influencing the production of aroma compounds. Food Chem., 55(4), 327-332.
Atwal A.S. et al., 2005. Cloning and secretion of tomato hydroperoxide lyase in Pichia pastoris. Process Biochem., 40(1), 95-102.
Axerold B., Chesbrough T.M. & Laakso S., 1981. Lipoxygenase from soybean. Methods enzymology. New York, NY, USA: Academic Press, 441-451.
Baysal T. & Demirdoven A., 2007. Lipoxygenase in fruits and vegetables: a review. Enzyme Microb. Technol., 40(4), 491-496.
Blee E., 1998. Biosynthesis of phytooxylipins: the peroxygenase pathway. Fett, 100(4-5), 121-127.
Blee E. & Joyard J., 1996. Envelope membranes from spinach chloroplasts are a site of metabolism of fatty acid hydroperoxides. Plant Physiol., 110(2), 445-454.
Bourel G. et al., 2004. Fatty acid hydroperoxide lyase of green bell pepper: cloning in Yarrowia lipolytica and biogenesis of volatile aldehydes. Enzyme Microb. Technol., 35(4), 293-299.
Brash A., Tijet N. & Whitehead I.M., 2001. Muskmelon (Cucumis melo) hydroperoxide lyase and uses thereof. US Patent 7037693. 02/05/06.
Brunerie P., 1989. Procédé de synthèse du cis-3-hexène-1-ol à partir d'acide gras insaturé. Brevet français 12 89. 16/12/90.
Buranasompob A. et al., 2007. Lipoxygenase activity in walnuts and almonds. Lwt-Food Sci. Technol., 40(5), 893-899.
Cacas J.L. et al., 2005. The combined action of 9 lipoxygenase and galactolipase is sufficient to bring about programmed cell death during tobacco hypersensitive response. Plant Cell Environ., 28(11), 1367-1378.
Cass B.J. et al., 2000. Production of tomato flavor volatiles from a crude enzyme preparation using a hollow-fiber reactor. Biotechnol. Bioeng., 67(3), 372-377.
Coffa G., Schneider C. & Brash A.R., 2005. A comprehensive model of positional and stereo control in lipoxygenases. Biochem. Biophys. Res. Commun., 338(1), 87-92.
De Domenico S. et al., 2007. Subcellular localisation of Medicago truncatula 9/13-hydroperoxide lyase reveals a new localisation pattern and activation mechanism for cyp74c enzymes. BMC Plant Biol., 7(58), 1-13.
Delcarte J. et al., 2003. Optimisation of expression and immobilized metal ion affinity chromatographic purification of recombinant (his)(6)-tagged cytochrome p450 hydroperoxide lyase in Escherichia coli. J. Chromatogr. B., 786(1-2), 229-236.
Drouet P., Thomas D. & Legoy M.D., 1994. Production of 13 (s)-hydroperoxy-9 (z), 11 (e)-octadecadienoic acid using soybean lipoxygenase 1 in a biphasic octane-water system. Tetrahedron Lett., 35(23), 3923-3926.
Fauconnier M.-L., 1997. Contribution à l'étude de la production du (e)-hex-2-ènal naturel par synthèse enzymatique. Thèse de doctorat : Faculté universitaire des Sciences agronomiques de Gembloux (Belgique).
Fauconnier M.-L. & Marlier M., 1996. An efficient procedure for the production of fatty acid hydroperoxides from hydrolyzed flax seed oil and soybean lipoxygenase. Biotechnol. Techn., 10(11), 839-844.
Fauconnier M.-L. & Marlier M., 1997. Fatty acid hydroperoxides pathways in plants. A review. Grasas Aceites, 48(1), 30-37.
Fauconnier M.-L. et al., 1999. Conversion of green note aldehydes into alcohols by yeast alcohol dehydrogenase. Biotechnol. Lett., 21(7), 629-633.
Froehlich J.E., Itoh A. & Howe G.A., 2001. Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome p450s involved in oxylipin metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol., 125(1), 306-317.
Fukushige H. & Hildebrand D.F., 2005. Watermelon (Citrullus lanatus) hydroperoxide lyase greatly increases C6 aldehyde formation in transgenic leaves. J. Agric. Food Chem., 53(6), 2046-2051.
Gardner H.W., Weisleder D. & Plattner R.D., 1991. Hydroperoxide lyase and other hydroperoxide-metabolizing activity in tissues of soybean, glycine max. Plant Physiol., 97(3), 1059-1072.
Gargouri M., Drouet P. & Legoy M.D., 2004. Hydroperoxide-lyase activity in mint leaves volatile C6-aldehyde production from hydroperoxy-fatty acids. J. Biotechnol., 111(1), 59-65.
Gargouri M., Akacha N.B., Kotti F. & Rejeb I.B., 2008. Voie de la lipoxygénase : valorisation d'huiles végétales et biosynthèse de flaveurs. Biotechnol. Agron. Soc. Environ., 12, 185-202.
Geimel F.F.A., 1987. Comparison of galactolipase activity and free fatty acid levels in chloroplasts of chill-sensitive and chill-resistant plants. Eur. J. Biochem., 166, 233.
Goers S.K., Ghossi P., Patterson J.T. & Young C.L., 1989. Process for producing a green leaf essence. US Patent 4806379. 02/21/89.
Grechkin A., 1998. Recent developments in biochemistry of the plant lipoxygenase pathway. Prog. Lipid Res., 37(5), 317-352.
Grechkin A.N. et al., 2006. Hydroperoxide lyases (cyp74c and cyp74b) catalyze the homolytic isomerization of fatty acid hydroperoxides into hemiacetals. Biochim. Biophys. Acta Mol. Cell Biol. Lipids, 1761(12), 1419-1428.
Hall C.E., Karboune S., Husson F. & Kermasha S., 2007. Stabilization of an enzymatic extract from Penicillium camemberti containing lipoxygenase and hydroperoxide lyase activities. Process Biochem., 43, 258-264.
Hatanaka A., 1993. The biogeneration of green odor by green leaves. Phytochemistry, 34(5), 1201-1218.
Hatanaka A., Kajiwara T., Sekiya J. & Fujimura K., 1979. Participation of 13-hydroperoxide in the formation of n-hexanal from linoleic acid in tea chloroplasts. Agric. Biol. Chem., 43, 175-176.
Holtz R.B. et al., 2001. Method for providing green note compounds. US Patent 6274358. 08/14/01.
Hornostaj A.R. & Robinson D.S., 2000. Purification of hydroperoxide lyase from pea seeds. Food Chem., 71(2), 241-247.
Hubert J. et al., 2008. Acaricidal effects of natural six-carbon and nine-carbon aldehydes on stored-product mites. Exp. Appl. Acarology, 44(4), 315-321.
Hughes R.K. et al., 2006. Characterisation of Medicago truncatula hydroperoxide lyase (cyp74c3), a water-soluble detergent-free cytochrome p450 monomer whose biological activity is defined by monomer-micelle association. Biochem. J., 395, 641-652.
Hughes R.K., Domenico S.D. & Santino A., 2009. Plant cytochrome cyp74 family: biochemical features, endocellular localisation, activation mechanism in plant defence and improvements for industrial applications. ChemBioChem, 10(7), 1122-1133.
Husson F. & Belin J.M., 2002. Purification of hydroperoxide lyase from green bell pepper (Capsicum annuum L.) fruits for the generation of C6-aldehydes in vitro. J. Agric. Food Chem., 50(7), 1991-1995.
Ishiguro S. et al., 2001. The defective in anther dehiscence1 gene encodes a novel phospholipase a1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell, 13(10), 2191-2209.
Itoh A. & Vick B.A., 1999. The purification and characterization of fatty acid hydroperoxide lyase in sunflower. Biochim. Biophys. Acta Mol. Cell Biol. Lipids, 1436(3), 531-540.
Kaewthong W., Sirisansaneeyakul S., Prasertsan P. & H-Kittikun A., 2005. Continuous production of monoacylglycerols by glycerolysis of palm olein with immobilized lipase. Process Biochem., 40,1525-1530.
Kanisawa T. & Itoh H., 1988. Method for preparing green aroma compounds. US Patent 4769243. 09/06/88.
Kato T. et al., 1992. Lipoxygenase activity increment in infected tomato leaves and oxidation product of linolenic acid by its in vitro enzyme reaction. Biosci. Biotechnol. Biochem., 56(3), 373-375.
Kermasha S., Dioum N., Bisakowski B. & Vega M., 2002. Biocatalysis by immobilized lipoxygenase in a ternary micellar system. J. Mol. Catal. B: Enzym., 19, 305-317.
Kim I.S. & Grosch W., 1981. Partial purification and properties of a hydroperoxide lyase from fruits of pear. J. Agric. Food Chem., 29(6), 1220-1225.
Kuroda H., Kojima H., Kaneda H. & Takashio M., 2005. Characterization of 9-fatty acid hydroperoxide lyase-like activity in germinating barley seeds that transforms 9 (s)-hydroperoxy-10 (e), 12 (z)-octadecadienoic acid into 2 (e)-nonenal. Biosci. Biotechnol. Biochem., 69(9), 1661-1668.
Laine G. et al., 2006. Study of precursors responsible for off-flavor formation during storage of potato flakes. J. Agric. Food Chem., 54(15), 5445-5452.
Lorenzi V., Maury J., Casanova J. & Berti L., 2006. Purification, product characterization and kinetic properties of lipoxygenase from olive fruit (Olea europaea L.). Plant Physiol. Biochem., 44(7-9), 450-454.
Matsui K., Shibutani M., Hase T. & Kajiwara T., 1996. Bell pepper fruit fatty acid hydroperoxide lyase is a cytochrome p450 (cyp74b). FEBS Lett., 394(1), 21-24.
Matsui K. et al., 1998. Cucumber root lipoxygenase can act on acyl groups in phosphatidylcholine. Biochim. Biophys. Acta, Lipids Lipid Metab., 1390(1), 8-20.
Matsui K., Kurishita S., Hisamitsu A. & Kajiwara T., 2000a. A lipid-hydrolysing activity involved in hexenal formation. Biochem. Soc. Trans., 28, 857-860.
Matsui K. et al., 2000b. Fatty acid hydroperoxide lyase in tomato fruits: cloning and properties of a recombinant enzyme expressed in Escherichia coli. Biosci. Biotechnol. Biochem., 64(6), 1189-1196.
Matsui K. et al., 2000c. Fatty acid 9-and 13-hydroperoxide lyases from cucumber. FEBS Lett., 481(2), 183-188.
Matsui K. et al., 2006. Biosynthesis of fatty acid derived aldehydes is induced upon mechanical wounding and its products show fungicidal activities in cucumber. Phytochemistry, 67(7), 649-657.
Nemeth A.S. et al., 2004. Biocatalytic production of 2(e)-hexenal from hydrolysed linseed oil. Enzyme Microb. Technol., 34(7), 667-672.
Noordermeer M.A. et al., 2000. Characterization of three cloned and expressed 13-hydroperoxide lyase isoenzymes from alfalfa with unusual n-terminal sequences and different enzyme kinetics. FEBS Lett., 267(9), 2473-2482.
Oh K. et al., 2006. Characterization of novel imidazole derivative, jm-8686, a potent inhibitor of allene oxide synthase. FEBS Lett., 580(24), 5791-5796.
Rabetafika H.N. et al., 2008. Sugar beet leaves as new source of hydroperoxide lyase in a bioprocess producing green-note aldehydes. Biotechnol Lett., 30(6), 1115-1119.
Rehbock B., Ganszer D. & Berger R.G., 1998. Efficient generation of 2e-hexenal by a hydroperoxide lyase from mung bean seedlings. Food Chem., 63(2), 161-165.
Rodrigo D., Jolie R., Van Loey A. & Hendrickx M., 2007. Thermal and high pressure stability of tomato lipoxygenase and hydroperoxide lyase. J. Food Eng., 79(2), 423-429.
Salas J.J., Sanchez C., Garcia-Gonzalez D.L. & Aparicio R., 2005. Impact of the suppression of lipoxygenase and hydroperoxide lyase on the quality of the green odor in green leaves. J. Agric. Food Chem., 53(5), 1648-1655.
Santiago-Gómez M.P. et al., 2007. Characterization of purified green bell pepper hydroperoxide lyase expressed by Yarrowia lipolytica: radicals detection during catalysis. Enzyme Microb. Technol., 41(1-2), 13-18.
Schade F., Thompson J.E. & Legge R.L., 2003. Use of a plant-derived enzyme template for the production of the green-note volatile hexanal. Biotechnol. Bioeng., 84(3), 265-273.
Schrader J. et al., 2004. Applied biocatalysis for the synthesis of natural flavour compounds: current industrial processes and future prospects. Biotechnol. Lett., 26(6), 463-472.
Schreier P. & Lorenz G., 1982. Separation, partial purification and characterization of a fatty acid hydroperoxide cleaving enzyme from apple and tomato fruits. Z. Naturforsch., 37c, 165-173.
Shiojiri K. et al., 2006. Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J. Chem. Ecol., 32(5), 969-979.
Suurmeijer C. et al., 2000. Purification, stabilization and characterization of tomato fatty acid hydroperoxide lyase. Phytochemistry, 53(2), 177-185.
Tijet N., Schneider C., Muller B.L. & Brash A.R., 2001. Biogenesis of volatile aldehydes from fatty acid hydroperoxides: molecular cloning of a hydroperoxide lyase (cyp74c) with specificity for both the 9-and 13-hydroperoxides of linoleic and linolenic acids. Arch. Biochem. Biophys., 386(2), 281-289.
Toporkova Y.Y., Gogolev Y.V., Mukhtarova L.S. & Grechkin A.N., 2008. Determinants governing the cyp74 catalysis: conversion of allene oxide synthase into hydroperoxide lyase by site-directed mutagenesis. FEBS Lett., 582, 3423-3428.
Vick B.A. & Zimmerman D.C., 1987. Pathways of fatty acid hydroperoxide metabolism in spinach leaf chloroplasts. Plant Physiol., 85(4), 1073-1078.
Wang Y.-J., Miller L. & Addis P., 1991. Effect of heat inactivation of lipoxygenase on lipid oxidation in lake herring (Coregonus artedii). J. Am. Oil Chem. Soc., 68(10), 752-757.
Whitehead I.M., Muller B.L. & Dean C., 1995. Industrial use of soybean lipoxygenase for the production of natural green note flavor compounds. Cereal Foods World, 40(4), 193-197.
Pour citer cet article
A propos de : Cédric Gigot
Univ. Liege. Walloon Center of Industrial Biology. Passage des Déportés, 2. B- 5030 Gembloux (Belgium). E-mail: c.gigot@ulg.ac.be
A propos de : Marc Ongena
Univ. Liege. Walloon Center of Industrial Biology. Passage des Déportés, 2. B- 5030 Gembloux (Belgium).
A propos de : Marie-Laure Fauconnier
Univ. Liege - Gembloux Agro-Bio Tech. Plant Biology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Jean-Paul Wathelet
Univ. Liege - Gembloux Agro-Bio Tech. General and Organic Chemistry Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Patrick du Jardin
Univ. Liege - Gembloux Agro-Bio Tech. Plant Biology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Philippe Thonart
Univ. Liege. Walloon Center of Industrial Biology. Passage des Déportés, 2. B- 5030 Gembloux (Belgium).






