- Home
- Volume 14 (2010)
- numéro 3
- Plant-RNA viroid relationship: a complex host pathogen interaction
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Plant-RNA viroid relationship: a complex host pathogen interaction

Editor's Notes
Received on September 2, 2009; accepted on December 11, 2009
Résumé
Les relations plante-ARN viroïdal : une interaction hôte-pathogène complexe. Les viroïdes sont des petits ARNs pathogènes de nombreuses cultures herbacées ou ligneuses non encapsidés et ne codant pour aucune protéine. La façon dont les viroïdes induisent des maladies est encore très peu connue. Cependant, des études récentes ont montré que les viroïdes étaient capables de modifier l’expression des gènes de leur plante hôte, de modifier la sensibilité de certaines protéines à la phosphorylation, d’interagir avec des protéines de l’hôte impliquées dans le mouvement des ARN et dans la phosphorylation des protéines. De plus, les végétaux possèdent un mécanisme de défense vis-à-vis d’ARN exogène : l’extinction de gène. Ce mécanisme, stimulé lors d’infections viroïdales, est en outre capable de réguler l’expression des gènes endogènes et serait impliqué dans l’expression des symptômes dus aux viroïdes. Depuis quelques années, des études sont réalisées afin de comprendre les relations hôte-pathogène s’établissant entre les viroïdes et leurs hôtes.
Abstract
Viroids are non encapsidated small RNA plant pathogens unable to produce any protein. They are able to infect dramatically a broad range of plants including herbaceous and tree crops. The ways by which viroids are able to induce diseases are actually unknown. However, recent studies have shown that viroids are able to regulate the gene expression of their hosts, they can modify the host-protein phosphorylation sensibility and they interact with host-protein implicated RNA trafficking and protein phosphorylation. Moreover during their evolution plants have developed a mechanism able to regulate their gene expression and to degrade exogenous RNAs like viroids: the gene silencing. Unfortunately, this pathway seems, now, also highly implicated in the symptoms development. This review describes studies that are realized since a few years to increase the knowledge about the plant-viroid relationship.
Table of content
1. Introduction
1Viroids are considered to belong to a group of non coding RNAs that are able to regulate the host gene expression through means other than encoding proteins for specific functions (Qi et al., 2003).
2Viroids are the smallest plant pathogens known so far. They only infect plants and cause diseases on economically important herbaceous and woody plants including some ornamentals (Tessitori et al., 2007). Morphological and cytological changes associated with viroid infections are well documented. Typical symptoms are intensified by high temperature and from them, leaf epinasty, chlorosis and stunting (accompanied by a reduction of the root mass) are the most frequent. At the cellular level, the distortion of the cell walls and the plasma membranes are the most visible symptoms (Itaya et al., 2002; Tessitori et al., 2007)
3Viroids are studied since the 1970s (Diener, 1971) but most studies focused on the primary and the secondary structures of these pathogens, or on the interaction between viroids and plant proteins. These studies have led to a better knowledge about the structure, the conformation, the replication and the pathogenicity of viroids. Unfortunately there is actually a lack of understanding concerning the host-pathogen relationship. This includes the molecular mechanisms of such a relationship, as well as the interactions between viroids and host plant species (Tessitori et al., 2007). However since a few years some studies are undertaken on this research field. A recent review has been published on the interactions between viroids and their hosts and is focused on the replication mechanisms, the structure and the trafficking of the viroids in plants (Ding, 2009). Our review will examine the molecular aspects of the viroid infections in their hosts and particularly the viroid pathogenicity and the plant defense mechanisms.
2. Viroids: two families of small plant pathogenic RNAs
4Viroids are single stranded small RNA molecules [246-401 nucleotides (nt)]. They are not encapsidated and do not code for any protein (Diener, 1971; Daros et al., 2006). Those molecules possess all the information to complete their life cycle by interacting with host proteins without the need of a helper virus. Viroids can be classified into two families based on biochemical and structural characteristics: the Avsunviroidae and the Pospiviroidae. The type species of the Pospiviroidae family is Potato spindle tuber viroid (PSTVd). The Pospiviroidae are soluble in LiCl 2M, replicate in the nucleus and some of their functional domains are already identified in their sequence such as a Central Conserved Region (CCR), a pathogenicity domain (P), a Terminal Conserved Region (TCR) and a Terminal Conserved Hairpin (TCH) (Figure 1) (Flores et al., 2004). The sequence of the CCR and the presence or the absence of the TCR and TCH permit to classify the 26 members of this family into five genera (Flores et al., 2004). The PSTVd and by extension the other members of its family adopt in vitro (and most-likely in vivo) a rod-like secondary structure characterized by alternating double-stranded and single-stranded regions. Mutations, deletions or repetitions observed in the sequence preserve the rod-like secondary structure. This structure has been divided into five structural/functional domains: Central (C), Pathogenic (P), Variable (V), Terminal right (TR) and Terminal left (TL). The CCR is localized into the C domain, the TCR and the TCH are within the TL domain (Keese et al., 1985; Flores et al., 2004). Functions have been associated with some of these structural domains: the C domain (and particularly the upper strand of the CCR) is involved in the cleavage and ligation of the multimeric PSTVd RNA intermediates during the rolling circle replication. The P domain is involved in the pathogenicity of the Pospiviroidae, probably in interactions with the TR, TL et V domains (Gora-Sochacka, 2004).
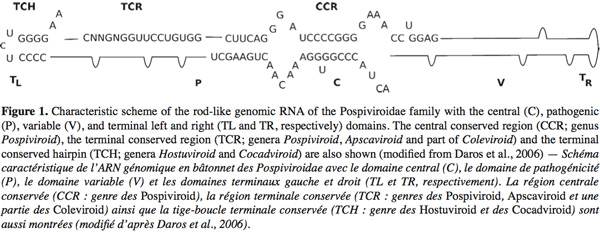
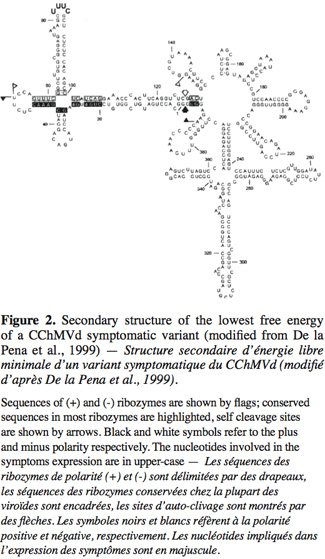
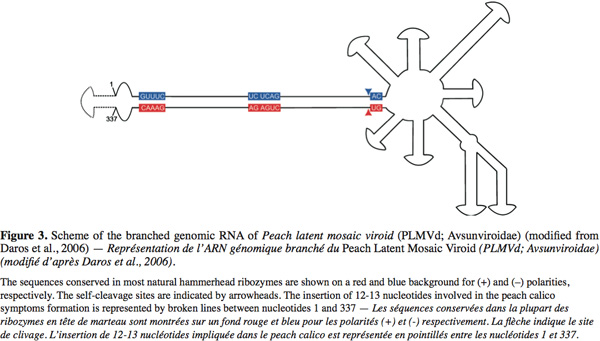
5The Avsunviroidae family, whose type species is Avocado sunblotch viroid (ASBVd), regroups the four viroids which precipitate in LiCl 2M, replicate in the chloroplast and possess a ribozyme catalytic domain but no Central Conserved Region (CCR) (Flores et al., 2000; Daros et al., 2006). Those viroids have a branched secondary structure formed by hairpins, except the ASBVd that folds in a quasi rod-like secondary structure (Bussière et al., 1996; Flores et al., 2004). To our knowledge, no functional domains (except the ribozyme) have already been identified in their structure. However, De La Pena et al. (1999) have highlighted the importance of four nucleotides in the pathogenicity of the Chrysanthemum chlorotic mottle viroid (CChMVd) and Malfitano et al. (2003) have studied the role of a 12-13 nt insertion in the peach calico symptomatology induced by certain Peach latent mosaic viroid (PLMVd) variants. The study of CChMVd symptomatic and non-symptomatic variants has shown a loop formed by the nucleotides 82-85 (in the ribozyme region) implicated in the pathogenicity (Figure 2) (De La Pena et al., 1999; 2002). A substitution of UUUC82-85 by GAAA82-85 induces a change in the pathogenicity from symptomatic to a non-symptomatic variant. This mutation does not affect the quantity of viroids in the plant tissues which confirms its specific implication on the symptoms expression (De La Pena et al., 1999; 2002). In the case of the PLMVd, isolates inducing an extreme chlorosis (also known as peach calico) possess an hairpin insertion of 12-13nt capped by a U-rich loop at the left of the ribozyme hairpin (Figure 3) (Rodio et al., 2006; 2007). As for the CChMVd, mutations from U to A in the loop of the hairpin induced a lost of pathogenicity. Only PLMVd variants with this insertion show an important pathogenicity by affecting the plastid transcription and translation (Rodio et al., 2007). Furthermore, the elimination of this hairpin leads to totally non-symptomatic variants without affecting the replication of the PLMVd (Malfitano et al., 2003). These studies are the only ones showing an implication of the sequence/structure of two Avsunviroidae in their pathogenicity.
3. Molecular host-viroid relationships
6For ten years, several studies have been carried out to characterize the pathogenicity mechanisms involved during a viroid infection. These studies concern essentially the Potato spindle tuber viroid (PSTVd) in tomato plants (Hammond et al., 2000; Itaya et al., 2002; Qi et al., 2003; Owens, 2007). A recent study on the differential gene expression induced by the Citrus viroid III (another Pospiviroidae) has shown that this viroid modified the expression of genes involved in plant stress/defense responses, signal transduction, amino acids transport, cell wall structure and RNA silencing suppression. Unfortunately it remains unclear how these non coding small RNA molecules are able to regulate gene expression in their host and how they induce symptoms (Tessitori et al., 2007).
3.1. Regulated genes during a viroid infection
7Most of the studies are actually made on tomatoes infected by PSTVd due to the easiness of manipulations and the wide knowledge on these two genomes. The PSTVd is certainly the best known viroid concerning the links existing between the sequence, the structure and the pathogenicity. The characterization of domains involved in the replication, the movement and the pathogenicity of this viroid has allowed the study of these mechanisms with a high degree of complexity (Gora-Sochacka, 2004).
8Infections by PSTVd can regulate a broad range of genes. Such genes are specifically regulated by the viroid infection while others are also regulated during an infection by the Tobacco Mosaic Virus (TMV) (Itaya et al., 2002). The study has shown that two PSTVd strains (a severe and a mild strain) are associated with the regulation of the expression of some genes in the infected tomato. An over-expression of different intensity was observed for the two strains: infection by the severe strain affects strongly the gene expression (Itaya et al., 2002) compared to the mild strain. The table 1 shows the regulated genes during a severe PSTVd infection. The highlighted tomato genes are involved in defense/stress response (like chitinase and PR genes) as well as in cell wall structure (Cell wall protein), chloroplast function (Cab genes), protein metabolism (like ubiquitin and heat shock protein) and other diverse functions (such as sucrose transporter and ADP/ATP translocator) (Itaya et al., 2002).
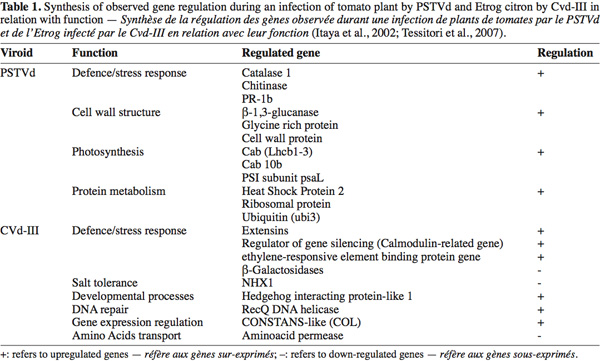
9Surprisingly, some genes belonging to a family with similar function have shown a different altered expression depending on PSTVd or TMV infection. For example, the Cab (chlorophyll a/b binding protein) gene was induced by both pathogens, while Cab 10b and Cab 9 were only induced by PSTVd (Itaya et al., 2002).
10Due to the difficulties for the growing and the maintenance of woody plant species in greenhouse conditions, fewer studies were carried out on these models. Among them one concerned the differential gene expression analysis on citrus infected by Citrus Viroid-III (CVd-III) (Tessitori et al., 2007). This study has led to the identification of some genes with modified expression during the viroid infection. The upregulated identified genes are also known to be involved in environmental stress response (extensin genes), plant defense (ethylene-responsive element binding protein gene), regulation of the gene silencing (Calmodulin-related protein gene), gene expression regulation, developmental processes (Hedgehog interacting protein-like 1) and DNA repair (RecQ DNA helicase gene). The down-regulated genes are also involved in several pathways such as plant defense, salt tolerance and amino acids transport (Tessitori et al., 2007). Table 1 summarizes the observed gene expression regulation during infection by the studied viroids.
11Both studies demonstrate that the host response during a viroid infection can be specific at the level of the gene expression. Moreover, the study of Itaya et al. (2002) demonstrated that different viroid strains with subtle nucleotide differences (the mild and severe strains of PSTVd only differed for three nucleotides) and different pathogenicity can induce or suppress the expression of common and unique genes in their host but the little differences between the used strains cannot explain, in the present state of knowledge, the major differences in the host gene expression (Itaya et al., 2002). Furthermore, plant responses to PSTVd and TMV infection may share some common mechanisms in addition to their unique features (Itaya et al., 2002). As for the PSTVd study, the CVd-III regulates the expression of genes implicated in several physiological processes but no information are actually available concerning the mechanisms involved in the regulation of the expression of these genes. It has been hypothesized that viroids could interfere with mRNA splicing or with RNA export from the nucleus but the mechanisms involved remain unclear (Tessitori et al., 2007). Finally, the induction of genes encoding for elongation factors, ribosomal proteins, ubiquitins, ubiquitin extension proteins and heat shock proteins during a viroid infection suggests that there are an active synthesis and degradation of proteins as for viral and fungal infections (Itaya et al., 2002). Unfortunately we do not actually know how viroids are able to enhance this protein turnover.
3.2 How viroids regulate genes: the gene silencing hypothesis
12The gene silencing is a cytoplasmic mechanism to regulate gene expression of eucaryote organisms. This phenomenon was first discovered in transgenic plants and named co-suppression or Post-Transcriptional Gene Silencing (PTGS) (Baulcombe, 1996; Jana et al., 2004). It became rapidly clear that PTGS can act against pathogens like viruses or viroids (Angell et al., 1997; Jana et al., 2004).
13The dsRNA plays an important role uniting the silencing pathway either as a trigger or an intermediate. The gene silencing is characterized by the implication of small RNA molecules (ranging size 21-25 nt long) in the regulation of the gene expression. These small RNAs can be endogenous or exogenous RNAs. The first ones are called micro-RNAs (miRNAs) and are implicated in the normal gene expression regulation during the development. At the opposite, the second ones are called small interfering RNAs (siRNAs) and involved in the plant response against exogenous (pathogenic or not) RNAs. This second pathway will be analyzed.
14Exogenous RNAs can induce two mechanisms leading to the silencing of a gene (or to the degradation of its mRNA): Transcriptional Gene Silencing (TGS) and PTGS (Jana et al., 2004).
15During PTGS an exogenous double-stranded RNA molecule triggered the mechanism. This molecule is cleaved by the Dicer, a ribonuclease of the RNAse III family, into small (21 and 25 nt long) interfering RNA (siRNA) of both polarities. These RNA species are double stranded and possess two nucleotides long 3' overhangs and 5' phosphates that are the hallmarks of Dicer cleavage products. These two siRNAs classes are produced by two distinct Dicers (Landry et al., 2004) and at least four homologue Dicers were detected in plants. The produced siRNAs are incorporated to the RNA Induced Silencing Complex (RISC) where one of the strands is lost. This new complex targets and cleaves an exogenous (or an endogenous) single stranded (m)RNA showing a good complementarity with these small incorporated RNAs. In plants and other organisms siRNAs can serve as primers (by hybridization on a complementary sequence) for the synthesis of dsRNA by the RNA-dependant RNA polymerase (RdRP). This leads to the creation of more siRNAs, thus amplifying the gene silencing mechanism (Figure 4) (Landry et al., 2004).
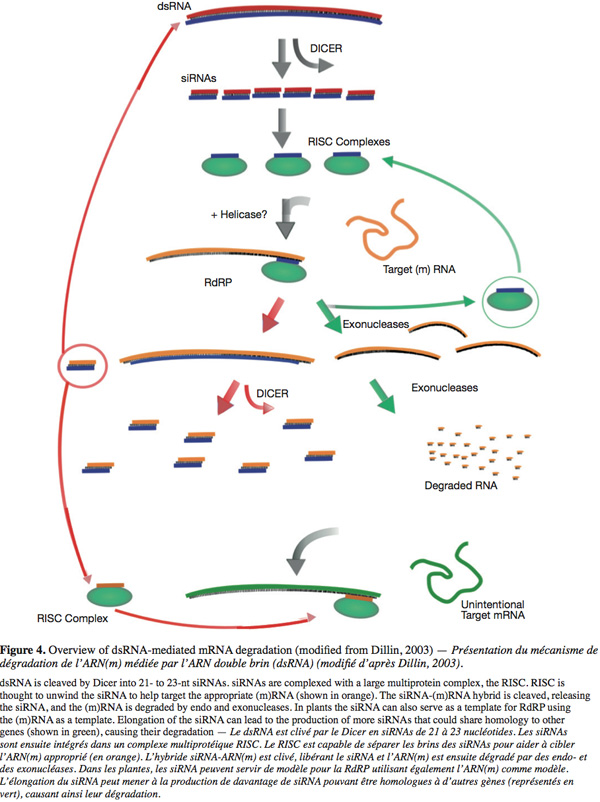
16The TGS phenomenon is characterized by the RNA Directed DNA methylation (RdDM) of the promoter sequences. During TGS a dsRNA molecule triggers a dense methylation of the promoter region (leading to the inactivation of this promoter) due to the sequence homology existing between this RNA and the promoter. The length of the target can be as small as 30 nucleotides giving a link between PTGS and TGS. In other terms, the exogenous dsRNA can be degraded on siRNA in the cytoplasm and then induce the methylation of the promoter region by transfer of a signal molecule into the nucleus (Papaefthimiou et al., 2001). The gene silencing (especially PTGS) seems to be common during viral and viroidal infection and is considered as an antiviral immunity in plants (Ding et al., 2004). This is supported by three arguments for infections by plant viruses.
17First, viral infections trigger RNA silencing in infected plants that target the viral RNA and all homologous RNA molecules. Detection of viral siRNA of both polarities are effectively common during plant infection (Hamilton et al., 1999; Papaefthimiou et al., 2001).
18Secondly, plant viruses encode proteins that are able to block the silencing machinery (Li et al., 2001). Three types of viral suppressors were identified: HC-Pro, Cmv2b and p25. The first one (encoded by Potyviruses) induces a decrease of the 25-nt siRNA quantity but does not block the silencing signal. The Cmv2b (encoded by Cucumber mosaic virus, CMV) cannot suppress the silencing when it is established indicating that this suppressor acts at an earlier stage than HC-Pro. It was found that Cmv2b blocks the silencing signal or inhibits the signal-mediated de novo induction of RNA silencing (Li et al., 2001). Furthermore, a study has demonstrated that Cmv2b encodes a functional nuclear localization signal. This indicates that the suppression of RNA silencing induced by Cmv2b may occur in the nucleus or that nuclear trafficking is essential for the suppressor activity (Li et al., 2001). Finally, p25 (the 25K protein of Potato virus X, PVX) suppresses the systemic silencing signal but not the local silencing pathway (Li et al., 2001).
19Finally plants defective in RNA silencing are often most susceptible to viral infections (or at least as susceptible) than the wild type plants (Ding et al., 2004). This was shown with Arabidopsis thaliana Heynh., ecotype Wassilewskijia, and tobacco plants defective or compromise in RNA silencing. The RNA silencing-defective A. Thaliana was hypersensitive to CMV, but was susceptible as the wild type plant to five other tested viruses (Ding et al., 2004). Tobacco plants were most susceptible to Tobacco mosaic virus when deficient in RNA silencing (Ding et al., 2004).
20Together these three arguments show that the gene silencing is an antiviral immunity mechanism against plant virus.
21The situation should be nuanced during viroid infections. As during viral infections, viroids trigger the RNA silencing pathway in infected plants. Detection of viroidal siRNA of both polarities is also common during plant infections (Gomez et al., 2008). However this immunity mechanism can be bypassed by the viroids without encoding any protein. The explanation of this phenomenon is actually unclear. Several hypothesises were emitted. Viroids should escape the silencing machinery through:
22– their cellular localization. The silencing machinery is able to degrade RNA in the cytoplasm but viroids replicate in the nucleus (Pospiviroidae) or in the chloroplast (Avsunviroidae) so they can escape the silencing mechanism during the critical step of their life cycle;
23– the secondary structure of viroid and especially the highly branched and compact secondary structure of the Avsunviroidae could protect these viroids against the silencing enzymes. Furthermore the presence of mismatches in hairpins of the viroid structure restricts the cleavage by the Dicer which needs, preferentially, 19 bp of contiguous dsRNA.
24– their possible interactions with host proteins leading them inaccessible for the silencing enzymes could play a role on the protection against the silencing machinery (Wang et al., 2004; Landry et al., 2004). Furthermore the gene silencing is not only a defense mechanism against viroids as it seems to be also implicated in the pathogenicity of these disease agents (Wang et al., 2004). Tomato plants expressing an hairpin RNA structure (hpRNA) with a partial PSTVd sequence produce siRNA homologous to the hpRNA and present viroid-like symptoms. However these symptoms are less important than during a PSTVd infection (Wang et al., 2004). Moreover Gomez et al. (2008) has recently demonstrated that Hop stunt viroid-infected Nicotinia benthamiana plants defective for the RNA-dependent RNA polymerase 6 (RDR6) activity (which is an essential enzyme for the PTGS) shows a non-symptomatic infection compared to the wild type plants infected by the same viroid strain. This is the first study which clearly links the gene silencing and the symptoms expression for a viroid.
25These results could explain how viroids induce symptoms and regulate the host gene expression. Viroids degradation by the host plant defence mechanisms could induce plant damage when viroid siRNAs are homologous to host mRNA or to promoter regions. The plant genes could be regulated and induce typical viroid symptoms. Furthermore it is clear that viroids are also able to induce TGS which can cause symptoms but this pathway was less studied with viroids contrary to the PTGS. However it seems that gene silencing cannot explain all the induced symptoms. Other interactions should be involved in the gene regulation and/or in the symptoms expression.
3.3. Other interactions: protein-viroid interactions
26Alone, the gene silencing cannot explain how viroids regulate the gene expression of their host because genes involved in several biological processes appeared over-expressed during an infection (Owens, 2007). Some studies have shown that viroids can interact with host protein but the knowledge on this topic is poor. Only few interactions between PSTVd and tomato plant proteins have been highlighted due to the difficulties to study viroids-proteins interactions. These can be specific like the Dicer-mediated cleavage of the viroid or totally non-specific to the viroid infection like their transport through the vascular system by the phloem lectin PP2 (involved in the long distance transport of RNA molecule through the plant) (Owens, 2007).
27The most studied protein-viroid interaction concerns the VIRP1 tomato protein whose binding site is in the right terminal domain of the viroid structure. Martinez de Alba et al. (2003) have demonstrated (by immunoprecipitation of the viroid-protein complex) that PSTVd interacts strongly in vivo with VIRP1. This protein possesses a bromodomain which can be implicated in several cellular processes. However the presence of a bromodomain can be considered as a marker of the nuclear localization (at the level of the dynamic chromatin) of the corresponding protein (Martinez de Alba et al., 2003). Moreover some bromodomain-containing proteins have a developmental role in different organisms and viroids, and especially the PSTVd, induce developmental disorders (Martinez de Alba et al., 2003). It is consequently possible that the interaction between the PSTVd and VIRP1 is involved in the symptoms formation (Martinez de Alba et al., 2003). Furthermore, the RNA-directed DNA methylation plays an important role as regulatory or defense mechanisms in plants (Martinez de Alba et al., 2003). The interaction with a bromodomain-containing protein is interesting in this context. PSTVd is able to initiate the methylation of homologous nuclear DNA sequences. It is possible that VIRP1 may play a role in the transmitting of the RNA-directed methylation on all the viroid-homologous sequences. This methylation blocks the synthesis of the corresponding mRNA and could induce the symptoms. The VIRP1 could also have possible roles in the viroid life cycle:
28– this protein could play a role into the viroid trafficking processes into and from the nucleus but also in the whole plant;
29– VIRP1 could be linked with the Pol II transcription of the PSTVd (Martinez de Alba et al., 2003; Ding et al., 2007).
30A second protein viroid interaction has been characterized between the PP2 (Phloem protein 2) and Hop Stunt Viroid (HSVd). It was found (by an immunoprecipitation assay carried out in a phloem exudates of an HSVd-infected cucumber plant) that the phloem protein 2 of Cucumis sativus (CsPP2) forms a ribonucleicprotein complex with HSVd RNA in vitro and in vivo (Gomez et al., 2004). This interaction permits a long distance transport of the HSVd through the phloem of its host plant (Gomez et al., 2004; Owens, 2007). Up to now, VIRP1 and CsPP2 are the two best characterized proteins implicated in the viroid translocation.
31Finally, there are some evidences that viroids, especially PSTVd and Citrus exocortis viroid (CEVd) are able to regulate the protein phosphorylation. It was demonstrated that PSTVd could stimulate the phosphorylation of a tomato protein associated with double-stranded RNA-stimulated protein kinase activity (Hiddinga et al., 1988; Langland et al., 1995). Incubations of its mammalian homologue with PSTVd strains of various pathogenicity leads to a differential activation (Diener et al., 1993) supporting an implication in the viroid pathogenicity (Langland et al., 1995). More recently Hammond et al. (2000) have characterized a new protein kinase (the PKV protein) whose transcription is up-regulated during a PSTVd infection in tomato. They have found that the level of transcription was higher in the plant infected by a severe or an intermediate PSTVd strain than by a mild strain or in a healthy plant. PKV is similar to cyclic nucleotide-dependent kinase of mammalian implying involvement in the transduction of extracellular signals (Hammond et al., 2000). The modification of the transcription of this gene could have a great influence on the symptoms development. Vera et al. (1990) have shown that CEVd is also able to induce and to reduce the in vitro phosphorylation of diverse proteins during the infection (after the symptoms appearance). They also noted that those modifications were higher in presence of Mn2+ showing the importance of the Mn2+-dependent protein kinase in the phosphorylation modifications of the host protein. It is actually unclear how viroids can change the protein phosphorylation but we can afford that these modifications might have a critical incidence on several biological pathways.
4. Conclusion
32The study of the host-pathogen relationship is very important to develop control methods. Viroids seem able to encounter the plant defense. This shows the importance to have a comprehensive knowledge of the host-pathogen relationship to act efficacy against viroids.
33The main studies carried out on viroids concern essentially their structure and their replication. Studies carried out on their interactions with host plants are more recent. Actually viroids are shown to affect the transcription level of genes involved in various functions including defense against pathogen as well as DNA repair, development, stress response and probably other actually not highlighted functions. The same assessment can be made about interactions between viroids and plant proteins with the particularity that there are fewer interactions described than for the gene expression modification (probably due to the chloroplastic localization of the Avsunviroidae and the subsequent difficulties for protein isolation limiting the field of these studies to the Pospiviroidae). However these studies have given some hypothesises to explain the viroid trafficking through the plant and the symptoms enhancement by the way of the gene silencing or the protein phosphorylation. More efforts should be made in this research field.
34The study of these interactions will help to better understand how these non coding RNA molecules can be so pathogenic and to develop control methods against viroids.
35Finally, these small pathogens are probably the best molecules to study the RNA translocation in the plant cells and also to study the plant RNA pathogen evolution. Researches on viroids and viroid-host interactions will support a better understanding of the RNA world.
Bibliographie
Angell S.M. & Baulcombe B., 1997. Consistent gene silencing in transgenic plants expressing a replicating potato virus X RNA. EMBO J., 16(12), 3675-3684.
Baulcombe D.C., 1996. RNA as a target and an initiator of post-transcriptional gene silencing in transgenic plants. Plant Mol. Biol., 32, 79-88.
Bussière F., Lafontaine D. & Perreault J.P., 1996. Compilation and analysis of viroid and viroid-likeRNA sequences. Nucleic Acids Res., 24(10), 1793-1798.
Daros J.A., Elena S.F. & Flores R., 2006. Viroids: an Ariadne's thread into the RNA labyrinth. EMBO J., 7(6), 593-598.
De La Pena M., Navarro B. & Flores R., 1999. Mapping the molecular determinant of pathogenicity in a hammerhead viroid: a tetraloop within the in vivo branched RNA conformation. Proc. Natl Acad. Sci. USA, 96(17), 9960-9965.
De la Pena M. & Flores R., 2002. Chrysanthemum Chlorotic Mottle Viroid RNA: dissection of the pathogenicity determinant and comparative fitness of symptomatic and non-symptomatic variants. J. Mol. Biol., 321, 411-421.
Diener T.O, 1971. Potato spindle tuber “virus”. IV. A replicating, low molecular weight RNA. Virology, 45(2), 411-428.
Diener T.O., Hammond R.W., Black T. & Katze M.G., 1993. Mechanism of viroid pathogenesis: differential activation of the interferon-induced, double-stranded RNA-activated, M(r) 68,000 protein kinase by viroid strains of varying pathogenicity. Biochimie, 75, 533-538.
Dillin A, 2003. The specifics of small interfering RNA specificity. Proc. Natl Acad. Sci. USA, 100(11), 6289-6291.
Ding B., 2009. The biology of viroid-host interactions. Annu. Rev. Phytopathol., 47, 105-131.
Ding S. et al., 2004. RNA silencing: a conserved antiviral immunity of plants and animals. Virus Res., 102(1), 109-115.
Ding B. & Itaya A., 2007. Viroid: a useful model for studying the basic principles of infection and RNA biology. Mol. Plant-Microbe Interact., 20(1), 7-20.
Flores R., Randless J.W., Bar-Joseph M. & Diener T.O., 2000. Subviral agents: viroids. In: van Regenmortel M.H.V. et al. Virus Taxonomy. 7th report of the International Committee on Taxonomy of Viruses. San Diego, CA, USA: Academic Press, 1009-1024.
Flores R. et al., 2004. Viroids: the minimal non-coding RNAs with autonomous replication. FEBS Lett., 567, 42-48.
Gomez G. & Pallas V., 2004. A long-distance translocatable phloem protein from cucumber forms a ribonucleoprotein complex in vivo with hop stunt viroid RNA. J. Virol., 78(10), 10104-10110.
Gomez G., Martinez G. & Pallas V., 2008. Viroid-induced symptoms in Nicotinia Benthamiana plants are dependent on RDR6 activity. Plant Physiol., 148, 414-423.
Gora-Sochacka A., 2004. Viroids: unusual small pathogenic RNAs. Acta Biochim. Pol., 51(3), 587-607.
Hamilton A.J. & Baulcombe D.C., 1999. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science, 286, 950-952.
Hammond R.W. & Zhao Y., 2000. Characterization of a tomato protein kinase gene induced by infection by Potato spindle tuber viroid. Mol. Plant-Microbe Interact., 13(9), 903-910.
Hiddinga H.J., Crum C.J., Hu J. & Roth D.A., 1988. Viroid-induced phosphorylation of a host protein related to a dsRNA-dependent protein kinase. Science, 241, 451-453.
Itaya A. et al., 2002. Potato spindle tuber viroid strains of different pathogenicity induces and suppresses expression of common and unique genes in infected tomato. Mol. Plant-Microbe Interact., 15(10), 990-999.
Jana S., Chakraborty C. & Nandi S., 2004. Mechanisms and roles of the RNA-based gene silencing. Electron. J. Biotechnol., 7(3), 324-335.
Keese P. & Symons R.H., 1985. Domains in viroids: evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc. Natl Acad. Sci. USA, 82(14), 4582-4586.
Landry P., Thompson D. & Perreault J., 2004. The role of viroids in gene silencing: the model case of Peach Latent Mosaic viroid. Can. J. Plant Pathol., 26, 1-8.
Langland J.O., Jin S., Jacobs B.L. & Roth D.A., 1995. Identification of a plant-encoded analog of PKR, the mammalian double stranded RNA-dependent protein kinase. Plant Physiol., 108, 1259-1267.
Li W.X. & Ding S.H., 2001. Viral suppressor of RNA silencing. Curr. Opin. Biotechnol., 12, 150-154.
Malfitano M. et al., 2003. Peach Latent Mosaic Viroid variants inducing peach calico (extreme chlorosis) contain a characteristic insertion that is responsible for this symptomatology. Virology, 313, 492-501.
Martinez de Alba A.E., Sägesser R., Tabler M. & Tsagris M., 2003. A bromodomain-containing protein from tomato specifically binds Potato Spindle Tuber Viroid RNA in vitro and in vivo. J. Virol., 77(17), 9685-9694.
Owens R.A., 2007. Potato Spindle Tuber Viroid: the simplicity paradox resolved. Mol. Plant Pathol., 8(5), 549-560.
Papaefthimiou I. et al., 2001. Replicating potato spindle tuber viroid RNA is accompagnied by short RNA fragments that are characteristic of post-transcriptional gene silencing. Nucleic Acids Res., 29(11), 2395-2400.
Qi Y. & Ding B., 2003. Inhibition of cell growth and shoot development by a specific nucleotide sequence in a noncoding viroid RNA. Plant Cell, 15, 1360-1374.
Rodio M.E., Delgado S., Flores R. & Di Serio F., 2006. Variants of peach latent mosaic viroid inducing peach calico: uneven distribution in infected plant and requirements of the insertion containing the pathogenicity determinant. J. Virol., 87, 231-240.
Rodio M.E. et al., 2007. A viroid RNA with specific structural motif inhibits chloroplast development. Plant Cell, 19, 3610-3626.
Tessitori M. et al., 2007. Differential display analysis of gene expression in Ertog citron leaves infected by Citrus Viroid III. Biochim. Biophys. Acta, 1769, 228-235.
Vera P. & Conejero V., 1990. Citrus Exocortis Viroid infection alters the in vitro pattern of protein phosphorylation of tomato leaf proteins. Mol. Plant-Microbe Interact., 3(1), 28-32.
Wang M. et al., 2004. On the role of RNA silencing in the pathogenicity and evolution of viroids and viral satellites. Proc. Natl Acad. Sci. USA, 101(9), 3275-3280.
To cite this article
About: Olivier Parisi
Univ. Liege - Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: olivier.parisi@ulg.ac.be
About: Philippe Lepoivre
Univ. Liege - Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
About: M. Haissam Jijakli
Univ. Liege - Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






