- Portada
- Volume 14 (2010)
- numéro spécial 2
- Trehalose as a stress marker of the physiological impact of mixing on yeast production: scale-down reactors and mini-bioreactors investigations
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Trehalose as a stress marker of the physiological impact of mixing on yeast production: scale-down reactors and mini-bioreactors investigations

Résumé
Le tréhalose, un marqueur de stress de l'impact physiologique du mélange sur une production de levure : tests en réacteurs scale-down et mini-bioréacteurs. Le tréhalose est un carbohydrate de réserve produit par Saccharomyces cerevisiae en conditions de stress et utilisé comme cryoprotectant pendant la lyophilisation. Le contenu cellulaire en tréhalose est donc un paramètre important à contrôler dans le contexte industriel. Saccharomyces cerevisiae a été produite dans des réacteurs scale-down qui ont la propriété de mimer les conditions hydrodynamiques rencontrées par les cellules lors d'une production à grande échelle. Trois types de réacteurs scale-down, de géométrie et débit de recirculation différents, ont été testés. Les résultats montrent que les cellules produites dans les réacteurs scale-down produisent moins de tréhalose que celles produites dans le réacteur de référence. Ces résultats sont complétés par l'étude de l'expression du promoteur du gène TPS2 couplé avec une protéine fluorescente (GFP). TPS2 est un gène codant pour une sous-unité du complexe enzymatique synthétisant le tréhalose. Cette souche a été produite en mini-bioréacteurs qui sont des fioles agitées équipées d'une sonde mesurant l'oxygène dissous. Cette approche permet d'utiliser le tréhalose ou des enzymes associées, comme un marqueur cellulaire du stress rencontré par la levure dans un processus industriel.
Abstract
Trehalose is a reserve carbohydrate produced by Saccharomyces cerevisiae under stress conditions and used as a cryoprotectant during freeze drying. So the cellular content of trehalose is an important parameter to control in industrial context. Scale-down reactors have been used to produce Saccharomyces cerevisiae. These reactors permit to mimic the hydrodynamic conditions experienced by cells during large scale production. Three types of scale-down reactors have been tested, differing by the geometry of the non-mixed part and the recirculation flow rate. The results show that cells cultivated in scale-down reactors produced less trehalose compared to the reference reactor. These results are completed by the study of the expression of the TPS2 promoter coupled with a green fluorescent protein (GFP). TPS2 is a gene coding for a subunit of the enzymatic complex responsible of trehalose synthesis. This strain was produced in mini-bioreactors which are shake flasks equipped with a dissolved oxygen probe. This approach allows using trehalose, or related enzymes, as a cellular marker of the stress encountered by yeast in industrial process.
Tabla de contenidos
1. Introduction
1Trehalose is a natural disaccharide composed of two molecules of glucose, connected by a stable link α,α-1,1. It can be found in a wide number of microorganisms: bacteria, fungi, insects, plants and invertebrates. Its applications are food industries, health care, cosmetic industries (Higashiyama, 2002).
2This paper focus on the measurement of intracellular trehalose concentration in different production of Saccharomyces cerevisiae. This metabolite is of great interest at the industrial level because it plays several metabolic roles. First, trehalose is a reserve carbohydrate during the non proliferating periods (Thevelein, 1984) and protects several cytosol components against hard conditions of growth (osmotic and/or thermic chock, deprive of a nutriment). This action permits to the yeast cells with high trehalose content (15-10% of dry matter) to resist to the industrial conditions of production (van Dijck et al., 1995). Thirdly, trehalose seems to be useful during drying or congelation of cells because it protects the membranes of dried cells and increases the thermic stability of proteins (Hounsa et al., 1998). The exogenous trehalose can also have an influence during the conservation treatment by protecting the membranes and the structure of proteins (Diniz-Mendes et al., 1999). For all these reasons, trehalose can be considered as an important metabolite in industrial production of yeast and used as a biomarker of the impact of growth conditions in an industrial production.
3The intracellular trehalose concentration that can be typically reached in a batch reactor is 0.025-0.035 g.g-1 of biomass (dry weight). After a fed-batch culture, this concentration can be 0.13 g.g-1 of biomass (dry weight) (Aranda et al., 2004). The addition of glucose in the reactor induces a reconsumption of trehalose, rapidly followed by a new synthesis, leading to a higher content of trehalose in the cell (Lillie et al., 1980; Thevelein, 1984; Ertugay et al., 1997). So the use of the fed-batch process is the best way to increase the cryotolerance as it increases the trehalose content of cells (Gélinas et al., 1989).
4In this paper, the trehalose content of yeast cells was measured during scale-down experiments. Scale-down reactor (SDR) permits to reproduce the hydrodynamic conditions of large scale reactors at laboratory scale (Kwanmin , 1989; George et al., 1998; Li et al., 2006). This particular reactor is composed in two parts: a mixing and a non mixing part linked by a recirculation pump (Namdev et al., 1992; Neubauer et al., 1995; Delvigne et al., 2006). In a large scale reactor, heterogeneity zones in glucose concentration appear, induced by the poor efficiency of mixing. Microorganisms have to adapt their metabolism to these extracellular fluctuations in glucose concentration (Guillard et al., 1999; Enfors et al., 2001). The scale-up effect is then observed: biomass yield drop, fermentation time and ethanol production increase and viability decreases (Sweere et al., 1988).
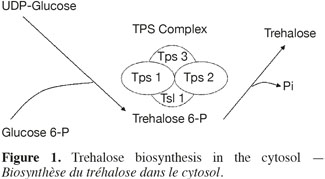
5The trehalose measurement was made using two strategies: a direct method and an indirect one using a fluorescent reporter gene. The direct method is the High Performance Anion Exchange Chromatography coupled with Pulse Amperometric Detection (HPAEC-PAD). It gives more reliable results than the usual techniques based on anthrone or trehalase (Ferreira et al., 1997). For the indirect method, a Saccharomyces cerevisiae strain (YDR074W) was chosen in the Yeast GFP Clones Collection of Invitrogen. In this strain the TPS2 gene was replaced by a gene coding for a green fluorescence protein (GFP). The GFP expression is so under the control of the TPS2 promoter. TPS2 is a gene coding for trehalose-6-P phosphatase, a subunit of the enzymatic complex (TPS complex) responsible of the trehalose synthesis (Figure 1). The TPS complex is located in the cytosol where the biosynthesis of trehalose occurs (Cabib et al., 1958). The regulation of intracellular trehalose concentration is the result of the action of the TPS complex, associated to the action of trehalase, the enzyme responsible of the hydrolysis of trehalose. The expression of the GFP was used as a biomarker, permitting to follow the impact of stress conditions on yeast cells. The cultivation tests were conducted in mini-bioreactors where stress conditions are reproduced.
2. Materials and methods
2.1. Culture of Saccharomyces cerevisiae in scale-down reactors (SDR)
6Saccharomyces cerevisiae (MUCL 43340) was stored at -80°C before use. A 500 ml preculture (dextrose (20 g.l-1), peptone casein (10 g.l-1), yeast extract (10 g.l-1) were prepared before inoculation of the reactor. The culture medium was composed of dextrose (5 g.l-1), peptone casein (10 g.l-1) and yeast extract (10 g.l-1). The mixing part of the scale-down reactors is a 20 l stirred bioreactor (Biolafitte-France) with two turbines TD4-TD4. The regulation of temperature (30°C), pH (5.5) and dissolved oxygen was ensured by using a direct control system (ABB). Dissolved oxygen level was maintained above 30% saturation by the stirrer speed. Aeration was fixed at 1 vvm. The stirred reactor was connected to the appropriate non-mixed part, characterized as follows (Figure 2):
7– SDR type A, glass bulb: diameter = 85 mm, length = 0.25 m, capacity = 1 l;
8– SDR type B, pipe: internal diameter = 8 mm, external diameter = 12 mm, length = 7.5 m, capacity = 0.377 l;
9– SDR type C, pipe: internal diameter = 15 mm, external diameter = 21 mm, length = 5 m, capacity = 0.884 l.
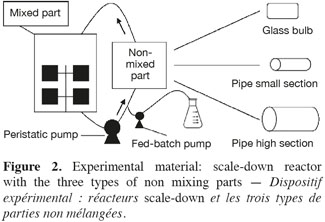
10The recirculation between the two parts of the scale-down reactor was ensured by a peristaltic pump (Watson Marlow 323S/D).
11The fed-batch mode was used in scale-down tests and the dextrose solution was introduced in the non-mixed part. For the reference reactor (with no recirculation), the feed was made at the top of the stirred vessel. After 5 h of batch culture, an exponential fed-batch was used, following the equation: F = 0.0058 x e0.005 x t [F: feed flow rate (ml.min-1), t: culture time (min)]. After the exponential phase, the feeding was fixed at a constant flow rate of 3.5 ml.min-1.
2.2. Culture of Saccharomyces cerevisiae (GFP) in mini-bioreactors
12Mini-bioreactors are shake flasks, equipped with a dissolved oxygen sensor. The tests are made in an orbital incubator (Orbi-Safe, Sanyo). The temperature is maintained at 30°C and the orbital speed at 110 rpm. The dissolved oxygen is recorded using the software “Oxy_4v2”.
13The GFP strain is a Saccharomyces cerevisiae strain (YDR074W) from the Yeast GFP Clones Collection of Invitrogen. The target promoter is TPS2. It codes for the phosphatase subunit of the trehalose-6-phosphate synthase/phosphatase complex, which synthesizes trehalose. Its expression is induced by stress conditions and repressed by the Ras-cAMP pathway (De Virgilio et al., 1993; Winderickx et al., 1996).
14The CFM mineral medium was used. It is composed of (all compounds are expressed in g.l-1): KH2PO4, 6.0; (NH4)2SO4, 12.0; MgSO4, 1.0; EDTA, 0.015; ZnSO4 × 7H2O, 0.0045; MnCl2 × 4H2O, 0.001; CoCl2 × 6H2O, 0.0003; CuSO4 × 5H2O, 0.0003; Na2MoSO4 × 2H2O, 0.004; CaCl2 × 2H2O, 0.0045; FeSO4 × 7H2O, 0.003; H3BO3, 0.001; D-biotin, 0.1; D,L-pantothenic acid, 0.001; nicotinic acid, 0.001; myo-inositol, 0.0025; thiamin, 0.001; pyridoxin, 0.001; para-aminobenzoic acid, 0.0002. Glucose and yeast extract were added at final concentration of 5 g.l-1 each. Fifty ml of this medium were used for the precultures. After 10 h of incubation (30°C, 110 rpm), 200 ml of CFM medium, in mini-bioreactor, were inoculated with the preculture. The fed-batch solution was added after 15 h and was composed of (all compounds are expressed in g.l-1): (NH4)2SO4, 10.0; KH2PO4, 5.0; MgSO4, 0.9; yeast extract, 5.0. Glucose was added at final concentration of 30 g.l-1. The glucose addition was made using a MatLab program. The profile was calculated using older data set. Two feeding strategies were used to estimate the impact of poor mixing conditions on the trehalose production: one leading to glucose excess in the medium and one to no glucose excess in the mini-bioreactor which can be considered as a reference.
2.3. Fermentation follow-up
15During the fermentations, samples were withdrawn at the level of the mixed part of the reactor and different measurements were carried out on these samples. The microbial growth of Saccharomyces cerevisiae was evaluated by optical density measurement (λ = 540 nm, spectrophotometer GENESYS 2). Biomass concentration has been determined by the correlation between optical density and dry matter. Dextrose concentration was determined by enzymatic method (YSI model 2700 Select).
16The analysis of the GFP expression level has been performed with a FACscan (Becton Dickinson) flow cytometer. Samples are taken directly from the reactor and diluted in 900 μl of PBS (Phosphate Buffer Solution) and 100 μl of a cycloheximide solution (1 mg.ml-1) in order to stop protein synthesis. For each measurement, 30,000 cells are analyzed (GFP is excited at 488 nm and emission signals are collected by using filters at 530 nm). The measurements are made with FL1 channel intensities at 530 nm. The results have been analyzed by the CellQuest (Becton Dickinson) software and are subsequently exported to WinMDI and MatLab for further analysis.
2.4. Trehalose analysis
17Trehalose was extracted from the cells after washing samples with water three times. Then the pellet was resuspended in 5 ml of distilled water. Sample tubes were then left 10 min in a boiling water bath. After centrifugation, the supernatant was recovered and analyzed by High Performance Anion Exchange Chromatography coupled with Pulse Amperometric Detection (HPAEC-PAD) on a Dionex DX500 chromatographic system operating at 1 ml.min-1. The volume of samples injected was 25 µl. The stationary phase consisted of a CarboPac PA 100 column (250 x 4 mm) with a pre-column PA 100 (50 x 4 mm) (DIONEX Corp, Sunnyvale, USA). The mobile phase was composed of sodium hydroxide (160 mM) and elution was performed in isocratic mode, followed by a linear gradient with a solution containing both sodium hydroxide (160 mM) and sodium acetate (500 mM). The gradient was completed by washing with sodium hydroxide 500 mM (Ronkart et al., 2006a; 2006b). Before each set of samples measurements, a calibration curve was done with concentration range of 62.5 ppm to 5,000 ppm. The retention time of the trehalose was of 2.83 seconds. This value was very constant for all the measurements realized (data not shown).
3. Results and discussion
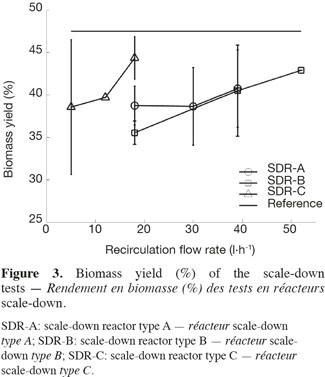
18Three types of scale-down reactors have been used and for each type, three recirculation flow rates have been tested in order to modulate the global mixing efficiency of the scale-down reactors (Figure 2). The biomass yield, ratio between the biomass produced and the glucose consumed during the fermentation, has been calculated for each test. Figure 3 shows the means and the standard deviations that have been calculated for each scale-down type and the horizontal line symbolizes the yield of the reference bioreactor which reaches a value of 47.5%. In the case of scale-down cultures, the biomass yield is lower than in the case of the reference reactor. An effect of the type of SDR and of the recirculation rate is observed (Lejeune et al., 2010). The higher recirculation flow rate gives, for example, a biomass yield similar to the reference reactor.
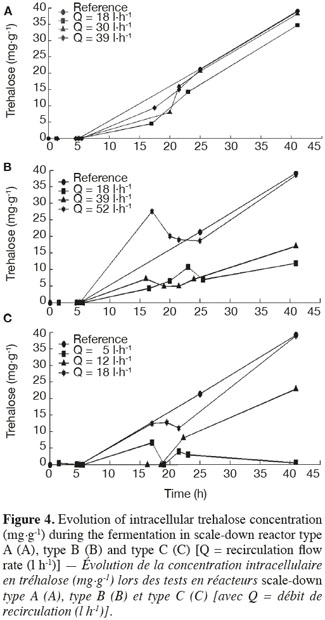
19The results of intracellular trehalose measurement for the three scale-down reactors are presented in figure 4. The trehalose concentration in the reference reactor reached a value close to those found in the literature (Aranda et al., 2004). In all cases, there is an impact of the recirculation flow rate. When it increases, the intracellular trehalose content of cells increases and tends to the curve of the reference reactor. The trehalose content has the same behavior as the biomass yield. It can also be observed that the trehalose content of the cells decreased after the batch period but it re-increases during the fed-batch mode, leading to a higher content of trehalose in the cells. This fact has been related in the literature (Lillie et al., 1980; Thevelein, 1984; Ertugay et al., 1997).
20In an industrial reactor, the mixing efficiency is low, resulting in the appearance of substrate concentration gradients, temperature or pH. In the SDR, the impact of a glucose concentration gradient on yeast growth was tested. The environmental conditions encountered by the cells are constantly changing. Indeed, the cells pass from zones of high glucose concentration to zones of low glucose concentration. These changes disturb the enzymatic cascade of the cells, leading to a biomass yield drop. Here we show that it also causes a lower production of intracellular trehalose. These cells are less protected in case of downstream processing, such as lyophilisation. This fact is observed in yeast produced industrially where the addition of glucose during the growing is unchecked. The measurement of the intracellular trehalose content of the cells confirms that the scale-down reactors that we used can mimic the industrial reactor.
21The use of a GFP reporter strain permits to go further in the explanation. Two feeding strategies have been used for the mini-bioreactor experiments, leading to an excess of glucose in the first test and to a starvation of glucose in the second test. The biomass and glucose concentrations have been measured for the two tests and are presented in figure 5. The glucose excess reaches in the first test a value of 3.5 g.l-1. At this concentration, yeast cells exhibit a fermentative metabolism. In the second experiment, the cells exhibit mainly an oxidative metabolism.
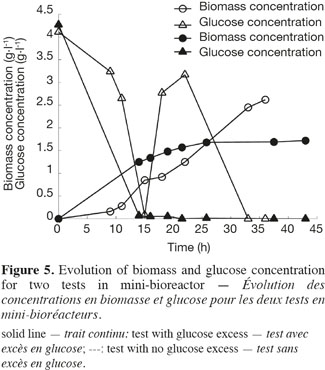
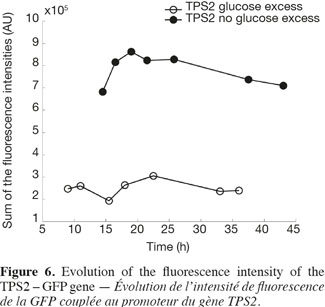
22Figure 6 shows the analysis of the fluorescent intensity of the green fluorescent protein coupled with the TPS2 promoter. The GFP synthesis is more induced in the case of test 2, where there is no glucose excess. The cells are then able to synthesize trehalose in greater quantity. They are therefore able to resist better to conditioning treatments, such as lyophilisation.
4. Conclusion
23Trehalose is an important metabolite involved at the level of yeast metabolism. It plays a role of cryoprotectant and makes the cells able of withstanding conditioning treatment, such as lyophilisation. It is an important parameter to take into account in the industrial production of yeast where the conservation is a main element of the process. In this paper, we studied the trehalose production in scale-down reactors. The results showed that a poor mixing efficiency leads to a lower trehalose production. The mini-bioreactor experiments show that a yeast cell produced with a glucose excess in the medium expresses less the TPS2 gene, allowing the production of trehalose. In front of these results, it can be concluded that the constantly changing environmental conditions experienced by cells in industrial bioreactor have an impact on the enzymatic reactions of the cell and perturb its normal growth.
24Acknowledgements
25We thank the Biological Chemistry Unit of the Univ. Liege-Gembloux Agro-Bio Tech who permits us to realize the trehalose concentration measurements.
Bibliographie
Aranda J.S., Salgado E. & Taillandier P., 2004. Trehalose accumulation in Saccharomyces cerevisiae cells: experimental data and structured modeling. Biochem. Eng. J., 17, 129-140.
Cabib E. & Leloir L.F., 1958. The biosyntesis of trehalose phosphate. J. Biol. Chem., 231, 259-275.
De Virgilio C. et al., 1993. Disruption of TPS2, the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae, causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity. Eur. J. Biochem., 212, 315-323.
Delvigne F., Lejeune A., Destain J. & Thonart P., 2006. Stochastic models to study the impact of mixing on a fed-batch culture of Saccharomyces cerevisiae. Biotechnol. Prog., 22, 259-269.
Diniz-Mendes L. et al., 1999. Preservation of frozen yeast cells by trehalose. Biotechnol. Bioeng., 65(5), 572-578.
Enfors S.O. et al., 2001. Physiological responses to mixing in large scale bioreactors. J. Biotechnol., 85(2), 175-185.
Ertugay N., Hamamci H. & Bayindirli A., 1997. Fed-batch cultivation of baker's yeast: effect of nutrient depletion and heat stress on cell composition. Folia Microbiol., 42, 214-218.
Ferreira J.C., Paschoalin V.M.F., Panek A.D. & Trugo L.C., 1997. Comparison of three different methods for trehalose determination in yeast extracts. Food Chem., 60(2), 251-254.
Gélinas P., Fiset G., LeDuy A. & Goulet J., 1989. Effect of growth conditions and trehalose content on cryotolerance of baker's yeast in frozen doughts. Appl. Environ. Microbiol., 55(10), 2453-2459.
George S., Larsson G., Olsson K. & Enfors S.O., 1998. Comparison of the Baker's yeast performance in laboratory and production scale. Bioprocess Eng., 18, 135-142.
Guillard F. & Trägardh C., 1999. Modeling of the performance of industrial bioreactors with a dynamic microenvironment approach: a critical review. Chem. Eng. J., 22(3), 187-195.
Higashiyama T., 2002. Novel functions and applications of trehalose. Pure Appl. Chem., 74(7), 1263-1269.
Hounsa C.-G. et al., 1998. Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology, 144, 671-680.
Kwanmin J.J., 1989. Scale-down techniques for fermentation. Biopharmacy, 2, 30-39.
Lejeune A., Delvigne F. & Thonart P., 2010. Influence of bioreactor hydraulic characteristics on a Saccharomyces cerevisiae fed-batch culture: hydrodynamic modelling and scale-down investigations. J. Ind. Microbiol. Biotechnol., 37, 225-236.
Li F. et al., 2006. A systematic approach for scale-down model development and characterization of commercial cell culture processes. Biotechnol. Progr., 22, 696-703.
Lillie S.H. & Pringle J.R., 1980. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J. Bacteriol., 143, 1384-1394.
Namdev P.K., Thompson B.G. & Gray M.R., 1992. Effect of feed zone in fed-batch fermentations of Saccharomyces cerevisiae. Biotechnol. Bioeng., 40, 235-246.
Neubauer P., Haggstrom L. & Enfors S.O., 1995. Influence in substrate oscillations on acetate formation and growth yield in E. coli glucose limited fed-batch cultivations. Biotechnol. Bioeng., 47, 139-146.
Ronkart S. et al., 2006. Determination of physical changes of inulin related to sorption isotherms: An X-ray diffraction, modulated differential scanning calorimetry and environmental scanning electron microscopy study. Carbohydr. Polym., 63, 210-217.
Ronkart S. et al., 2006. Determination of total water content in inulin using the volumetric Karl Fischer titration. Talanta, 70, 1006-1010.
Sweere A.P.J., Janse L., Luyben K.C.A.M. & Kossen N.W.F., 1988. Experimental simulation of oxygen profiles and their influence on baker's yeast production: I. One-fermentor system. Biotechnol. Bioeng., 31, 567-578.
Thevelein J. M., 1984. Regulation of trehalose mobilization in fungi. Microbiol. Rev., 48(1), 42-59.
van Dijck P., Colavizza D., Smet P. & Thevelein J.M., 1995. Differential importance of trehalose in stress resistance in fermenting and non fermenting Saccharomyces cerevisiae cells. Appl. Environ. Microbiol., 61(1), 109-115.
Winderickx J. et al., 1996. Regulation of genes encoding subunits of the trehalose synthase complex in Saccharomyces cerevisiae: novel variations of STRE-mediated transcription control? Mol. Gen. Genet., 252(4), 470-482.
Para citar este artículo
Acerca de: Annick Lejeune
Univ. Liège - Gembloux Agro-Bio Tech. Bio-Industries Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: annick.lejeune@ulg.ac.be
Acerca de: Frank Delvigne
Univ. Liège - Gembloux Agro-Bio Tech. Bio-Industries Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Philippe Thonart
Univ. Liège - Gembloux Agro-Bio Tech. Bio-Industries Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






