- Accueil
- Volume 14 (2010)
- numéro 4
- Phenotypic traits variation among native diploid, native tetraploid and invasive tetraploid Senecio inaequidens DC. (Asteraceae)
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Phenotypic traits variation among native diploid, native tetraploid and invasive tetraploid Senecio inaequidens DC. (Asteraceae)

Notes de la rédaction
Received on February 1, 2010; accepted on June 24, 2010
Résumé
Variation des traits d’histoire de vie entre formes diploïdes indigènes, tétraploïdes indigènes et tétraploïdes invasives de Senecio inaequidens DC. (Asteraceae). Senecio inaequidens DC. est une plante invasive à travers l’Europe. Dans son aire d’indigénat, deux cytotypes coexistent : diploïde et tétraploïde. Seuls des individus tétraploïdes sont recensés en Europe, mais le cytotype diploïde est invasif dans d’autres parties du monde. Nous avons comparé des populations diploïdes indigènes et des populations tétraploïdes indigènes et invasives dans un jardin commun en Europe, pour plusieurs traits d’histoire de vie. Les plants diploïdes se sont développés, ont montré des capacités de production de biomasse importantes et ont produit plus de capitules que les tétraploïdes. Néanmoins, les diploïdes n’ont pas survécu aux conditions hivernales. Le taux de survie hivernal fut faible pour les populations tétraploïdes natives, mais atteignit 40 % pour les populations invasives. Ceci suggère que les diploïdes tendent vers un cycle de vie annuel en Europe, avec une floraison précoce et abondante. Par contre, les tétraploïdes sont pérennes, ce qui semble favoriser leur potentiel invasif.
Abstract
Senecio inaequidens DC. is a rapidly spreading plant invader in Europe. In its native range, it occurs at two co-existing diploid and tetraploid cytotypes. To date, only tetraploids are reported in Europe, even though invasive diploids were recorded in other parts of the world. We compared native diploid and both native and invasive tetraploid populations in common gardens in Europe for a suite of life history traits. Diploids were able to develop, showed high biomass production and produced more flower heads than tetraploids. In contrast, winter survival was null for diploids. It was low for native tetraploids, but reached 40% in invasive tetraploids. Results suggested that diploid cytotype tends to an annual life form when grown in Western Europe, with earlier and more abundant flowering. In contrast, the tetraploid cytotype was mainly perennial which may enhance its invasiveness.
Table des matières
1. Introduction
1Polyploidy, the possession of more than two sets of chromosomes, often leads to novel physiological and life history characteristics not present in diploid ancestors (Levin, 1983). A growing number of studies indicate that polyploidy may contribute to invasive behavior and spread of alien plant species (Vilà et al., 1998; Ainouche et al., 2004; Soltis et al., 2004; Pandit et al., 2006). Benett et al. (1998) found that the proportion of polyploids were higher in weedy species than in other plant groups. However, to date, no general relationship between polyploidy and colonizing success has been demonstrated (Barrett et al., 1986).
2Senecio inaequidens DC. is a dwarf shrub from the family Asteraceae, native to southern Africa. It belongs to a sympatric species complex including Senecio madagascariensis Poir. and Senecio harveianus MacOwan (e.g. Hilliard, 1977; Scott et al., 1998; Radford et al., 2000b). However, molecular studies suggested that the three taxa were the same species (Scott et al., 1998; Lafuma et al., 2003). Lafuma et al. (2003) showed that this complex occurred as two co-existing cytotypes in its southern African native range and mapped populations of diploid and tetraploid cytotypes. Species of the complex are presently invasive in Europe, Australia and South America. In Australia, Argentina and Mexico, only diploids are reported and generally referred to as S. madagascariensis (Michael, 1981; Verona et al., 1982; Scott et al., 1998; Radford et al., 2000b; Lafuma et al., 2003). In Europe, the species (now only referred to as S. inaequidens) was accidentally introduced as a wool contaminant in several wool-processing centres during the late 19th-early 20th century (Ernst, 1998). Notably, the species invaded southern France from the city of Mazamet, where it was initially recorded in 1936 (Guillerm et al., 1990; Monty et al., 2010), and Belgium from Verviers where it was initially recorded in 1892 (Verloove, 2006). To date, Belgian and French invaded areas are still relatively unconnected (Monty A., unpublished data) and genetic pools introduced may differ between the two European regions (Bossdorf et al., 2005). It is not known whether both cytotypes were introduced to Europe, but only tetraploids have been recorded in Europe to date (Chichiricco et al., 1979; Lafuma et al., 2003). The absence of diploids due to non-introduction is rather unlikely, because of the relative sympatry with tetraploids in the native range (Lafuma et al., 2003). Another possibility is that both ploidy levels were introduced but diploids failed to establish whereas tetraploids became successful invaders. In the present study we compared functional life history traits between diploid populations, tetraploid populations from the native range and tetraploid populations from two invaded ranges in Europe, i.e. Belgium and France.
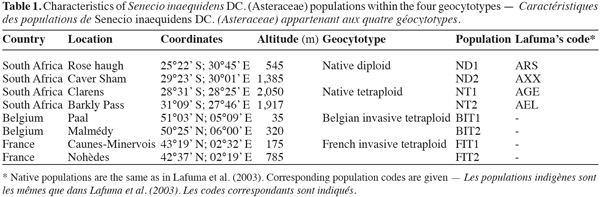
2. Materials and methods
2.1. Seed collection
3In February 2001 and 2002, achenes (further referred to as "seeds") were collected in two diploid and two tetraploid S. inaequidens populations in the mountainous regions of southern Africa (Table 1). The ploidy level of these populations was known from flow cytometry analysis (Lafuma et al., 2003). In November 2005 and November 2006, seed collections were made in two Belgian and two French invasive tetraploid populations (Table 1). In each population, a total of 20 maternal plants were sampled. Seeds were stored at 4°C in paper bags. Per parent individual (totaling 160), ten seeds without anomaly were sorted using a dissecting microscope, in order to reduce potential maternal carry-over effects (Monty et al., 2009a). Flat, whitish, damaged and empty seeds were considered abnormal and discarded.
2.2. Life history traits measurements
4In spring 2006, a randomized ten-block common garden experiment was established on an open field in Gembloux (Belgium). We used seeds from ten parent individuals per population in this experiment. On March 17th and 18th, the ten sorted seeds per parent individual were collectively sown in pots containing a mixture of 2.5 l of sand, 2.5 l of compost and 0.5 l of hydro-granulates. Each pot was placed in a 0.5 m x 0.8 m area for plant development without competition. Seedlings were counted every 2-3 days. The first emerged seedling in each pot was marked with a short rod for subsequent measurements. Other seedlings were removed after counting. Parent individuals were thus represented each by ten seeds for germination study, then by one offspring. Germination was considered complete when no additional seedling was counted for 10 days and germination rate was defined for each pot as the percentage of emerged seedlings. During summer drought in July and August, plants were watered five times with 1.5 l per plant. Time from sowing to germination (days) and flowering age (days since germination) were recorded, based on daily observations. When flowering began, the number of flowering heads per plant was determined every 17-20 d, which is approximately the period between flower opening and fruit maturation in Belgium. Every flower head produced was thus on average counted one time and the sum of counted flower heads is an accurate quantification of total flower head production. Mid-December 2006, final height (cm) was recorded and aboveground biomass (g) was measured on plants harvested at ground level and oven-dried for 48 h at 60°C after.
2.3. Winter survival analysis
5In spring 2007, a similar experiment was set out at the same site, with seeds from the ten other parent individuals sampled per population. Sowing was carried out on April 17th 2007, and the plants were grown for more than one year. Winter survival was monitored in late spring (May-June) 2008, so that all surviving individuals had had time to bud and to start growing. Individuals were considered surviving if shoots had sprouted.
2.4. Data analysis
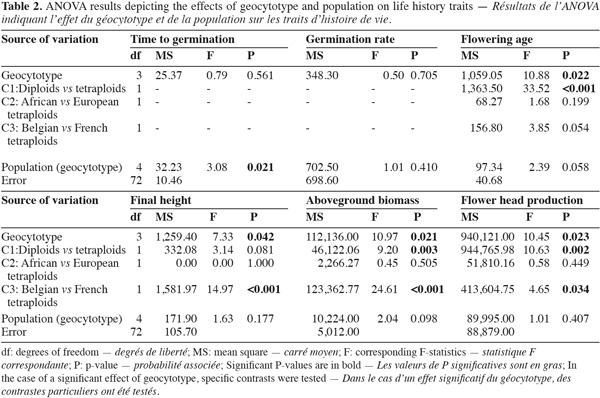
6Ploidy levels (diploid and tetraploid) and geographic origins (native range, Belgian and French invaded ranges) were combined in four geocytotypes: "native diploid" (ND); "native tetraploid" (NT); "Belgian invasive tetraploid" (BIT) and "French invasive tetraploid" (FIT) (Table 1). Each geocytotype was represented by two populations. A two-way ANOVA was performed on the measured life history traits. Populations (random) were nested within geocytotypes (fixed). When a significant (P < 0.05) geocytotype effect was detected, ANOVA contrasts were further used to test for three specific biological hypotheses. First (contrast C1), an effect of the ploidy level was tested for, by contrasting plant traits of ND vs the three tetraploid geocytotypes (NT, FIT and BIT). Second (C2), life history traits differences between African and European tetraploids were tested for by contrasting traits of NT vs European geocytotypes (FIT and BIT). Third (C3), differences among European geocytotypes were tested for (FIT vs BIT). Winter survival was not a continuous variable but a qualitative frequency within populations. ANOVA could therefore not be performed on these data. We looked for a dependence of winter survival on geocytotype by performing a Chi-square test of independence with the four geocytotypes as rows and the two modalities of winter survival (dead or surviving) as columns. Analyses were performed using Minitab software version 14.20 (Minitab Inc. 2000). Aboveground biomass data were log-transformed to reach the assumptions of statistical analyses.
3. Results
3.1. Life history measurements
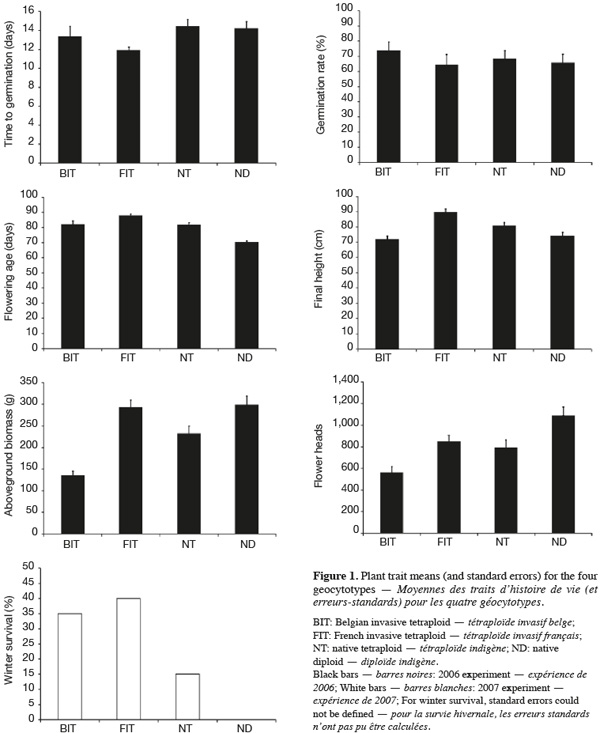
7Geocytoypes significantly differed in flowering age, final height, above-ground biomass and flower head production, as revealed by the analysis of variance (Table 2, Figure 1). Contrasts among ploidy levels (C1) revealed that diploids flowered earlier, produced more biomass and more flower heads than tetraploids (Table 2). The contrast comparing African and European tetraploids (C2) was not significant. French tetraploids were taller and produced more biomass and flower heads than Belgian ones (C3). Within geocytotypes, population differed in time to germination and flowering age (Table 2).
3.2. Winter survival
8Winter survival frequencies greatly varied among geocytotypes. It was null for ND, equal to 15% for NT and reached 35% and 40 % for BIT and FIT, respectively (Figure 1). Chi-square test of independence revealed a global dependence of winter survival on plant geocytotype (Pearson Chi-Square test = 10.963; DF = 3; P = 0.012).
4. Discussion
9We compared native diploid populations and both native and invasive tetraploid populations under north-western European climate for a suite of functional life history traits. Germination traits did not differ according to ploidy level or geographic origin, whereas growth, flowering age and winter survival appeared to significantly depend on ploidy level. The two European regions of introduction and invasion were also found to differ in growth and reproduction traits.
4.1. Ploidy level influence on life history
10Polyploidy in S. inaequidens did not lead to better performance, during the experiment under north-western European climate. Diploids were able to germinate, develop and produce more biomass and flower heads than tetraploids. Similarly, a recent study showed that polyploidy hardly enhanced growth of the invasive Lythrum salicaria (Kubátová et al., 2008), a species that also occurs at different ploidy levels in its native range, but only tetraploids are invasive. In contrast, monitoring winter survival in S. inaequidens showed that tetraploids were more resistant to harsh climatic conditions in central Belgium, which is consistent with the higher resistance of polyploids to extreme environmental conditions (Levin, 1983; Bretagnolle et al., 1998). Diploids are described as short perennial in invaded areas (Radford et al., 2000a; Rzedowski et al., 2003; López et al., 2008). The fact that they do not survive winter in Europe can much reduce their reproductive potential or can have prevented establishment in this self-incompatible species.
4.2. Differences in tetraploids across continents
11The native and invasive tetraploid types did not differ in most measured traits. However, winter survival varied among continents, with better performances for the invasive than native tetraploids. These results are consistent with a recent study by Bossdorf et al. (2008), who suggested that among the panel of genotypes found in Africa, only a subset of pre-adapted ones managed to become successful invaders in Eastern Europe. During naturalization in Western Europe (Belgium, France), a subset of winter resistant genotypes may have been selected.
4.3. Differences between French and Belgian tetraploids
12In the common garden, French tetraploids were taller and produced more biomass and flower heads than Belgian ones. This suggests that present genetic pools differ between invaded areas. Such a difference may have arisen from the introduction of separated genetic pools, i.e. seeds brought to Belgium and to France came from different native populations or groups of native populations. Another, non-exclusive possibility is that evolutionary processes, namely founder effect, genetic drift and/or selection, led to contrasting life history traits in the two invaded areas (Monty et al., 2009b). As experiments were carried out in Belgium, it is surprising that Belgian populations showed lower flower head and biomass production, since these traits are proxies of fitness. A stronger founder effect and/or genetic drift, i.e. a lower genetic variation introduced to Belgium, was already suggested by Bossdorf et al. (2005). This could be responsible for the pattern observed. It has to be noted that as we grew plants for only one season, our measurements may not reflect the lifetime fitness. Through high winter survival, possibly coupled with longer lifespan, Belgian populations may still exhibit a high lifetime flower head production. Longer-term studies are needed to assess the actual fitness differences among invasive tetraploids.
5. Conclusion
13This study brought evidence of variation in life history traits among geocytotypes in the weedy alien plant S. inaequidens. Diploids performed very well during the first growing season under north-western European climate. However, this cytotype showed no winter survival, which potentially results in a lower invasive potential than tetraploids. Among tetraploids, invasive populations showed higher winter survival. This suggests that winter resistance may be an important trait that promotes invasiveness in this species.
14Acknowledgements
15This research was supported by the project FRFC 2.4605.06 from the Belgian “Fonds National de la Recherche Scientifique”. Arnaud Monty holds a postdoctoral position at the Fonds National de la Recherche Scientifique. We thank Guy Buchet for his technical assistance.
Bibliographie
Ainouche M.L., Baumel A. & Salmon A., 2004. Spartina anglica C.E.Hubbard: a natural model system for analysing early evolutionary changes that affect allopolyploid genomes. Biol. J. Linn. Soc., 82, 475-484.
Barrett S.C.H. & Richardson B.J., 1986. Genetic attributes of invading species. In: Groves R.H. & Burdon J.J., eds. Ecology of biological invasions: an Australian perspective. Canberra: Australian Academic Science, 21-33.
Bennett M.D., Leitch I. & Hanson L., 1998. DNA amounts in two samples of angiosperm weeds. Ann. Bot., 82(Supplement A), 121-134.
Bossdorf O. et al., 2005. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia, 144(1), 1-11.
Bossdorf O., Lipowsky A. & Prati D., 2008. Selection of preadapted populations allowed Senecio inaequidens to invade Central Europe. Diversity Distrib., 14, 676-685.
Bretagnolle F., Felber F., Calame F.G. & Küpfer P., 1998. La polyploïdie chez les plantes. Bot. Helv., 108, 5-37.
Chichiricco G., Frizzi G. & Tammaro F., 1979. Numeri cromosomici per la Flore Italiana. Informatore Bot. Ital., 11, 659-660.
Ernst W.H.O., 1998. Invasion, dispersal and ecology of the South African neophyte Senecio inaequidens in the Netherlands: from wool alien to railway and road alien. Acta Bot. Neerl., 47, 131-151.
Guillerm J.L., Floc'h E.l., Maillet J. & Boulet C., 1990. The invading weeds within the Mediterranean Basin. In: di Castri F., Hansen A.J. & Debussche M., eds. Biological invasions in Europe and the Mediterranean Basin. Dordrecht, The Netherlands: Kluwer Academic Publishers, 61-84.
Hilliard O.M., 1977. Compositae in Natal. Pietermaritzburg, South Africa: University of Natal Press.
Kubátová B. et al., 2008. DNA ploidy-level variation in native and invasive populations of Lythrum salicaria at a large geographical scale. J. Biogeogr., 35, 167-176.
Lafuma L. et al., 2003. Ploidy level and origin of the European invasive weed Senecio inaequidens (Asteraceae). Plant Syst. Evol., 243, 59-72.
Levin D.A., 1983. Polyploidy and novelty in flowering plants. Am. Nat., 122, 1-25.
López M.G., Wulff A.F., Poggio L. & Xifreda C.C., 2008. South African fireweed Senecio madagascariensis (Asteraceae) in Argentina: relevance of chromosome studies to its systematics. Bot. J. Linn. Soc., 158(4), 613-620.
Michael P.W., 1981. Alien plants. In: Groves R.H., ed. Australian vegetation. Cambridge, UK: Cambridge University Press, 57-83.
Monty A., Lebeau J., Meerts P. & Mahy G., 2009a. An explicit test for the contribution of environmental maternal effects to rapid clinal differentiation in an invasive plant. J. Evol. Biol., 22(5), 917-926.
Monty A. & Mahy G., 2009b. Clinal differentiation during invasion: Senecio inaequidens along altitudinal gradients in Europe. Oecologia, 159, 305-315.
Monty A. & Mahy G., 2010. Evolution of dispersal traits along an invasion route in the wind-dispersed Senecio inaequidens (Asteraceae). Oikos, 119, 1563-1570.
Pandit M.K., Tan H.T.M. & Bisht M.S., 2006. Polyploidy in invasive plant species of Singapore. Bot. J. Linn. Soc., 151, 395-403.
Radford I.J. & Cousens R.D., 2000a. Invasiveness and comparative life-history traits of exotic and indigenous Senecio species in Australia. Oecologia, 125(4), 531-542.
Radford I.J., Muller P., Fiffer S. & Michael P.W., 2000b. Genetic relationships between Australian fireweed and South African and Madagascan populations of Senecio madagascariensis Poir. and closely related Senecio species. Aust. Syst. Bot., 13, 409-423.
Rzedowski J., Vibrans H. & Calderón de Rzedowski G., 2003. Senecio inaequidens DC. (Compositae, Senecioneae), a harmful weed introduced into Mexico. Acta Bot. Mex., 63, 83-96.
Scott L.J., Congdon B.C. & Playford J., 1998. Molecular evidence that fireweed (Senecio madagascariensis, Asteraceae) is of South African origin. Plant Syst. Evol., 213, 251-257.
Soltis D.E. et al., 2004. Recent and recurrent polyploidy in Tragopogon (Asteraceae): cytogenetic, genomic and genetic comparisons. Biol. J. Linn. Soc., 82, 485-501.
Verloove F., 2006. Catalogue of neophytes in Belgium. Vol. 39, Scripta Botanica Belgica. Meise, Belgium: National Botanic Garden of Belgium.
Verona C.A., Fernandez O.N., Montes L. & Alonso S.I., 1982. Agroecological and biological aspects of Senecio madagascariensis Poiret (Compositae). Ecol. Argent., 7, 17-30.
Vilà M. & D'Antonio C., 1998. Fruit choice and seed dispersal of invasive vs non-invasive Carpobrotus (Aizoaceae) in coastal California. Ecology, 79, 1053-1060.
Pour citer cet article
A propos de : Arnaud Monty
Univ. Liege - Gembloux Agro-Bio Tech. Unité Biodiversité et Paysage. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail : Arnaud.Monty@ulg.ac.be
A propos de : Sandrine Maurice
Université de Montpellier II. Institut des Sciences de l’Évolution. UMR 5554. F-34095 Montpellier (France).
A propos de : Grégory Mahy
Univ. Liege - Gembloux Agro-Bio Tech. Unité Biodiversité et Paysage. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






