- Startpagina tijdschrift
- Volume 14 (2010)
- numéro 4
- The effect of Cu and Cu-humic acids on the adsorption of imazethapyr herbicide by montmorillonite clay
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
The effect of Cu and Cu-humic acids on the adsorption of imazethapyr herbicide by montmorillonite clay

Nota's van de redactie
Received on October 8, 2009; accepted on June 1, 2010
Résumé
L'effet du Cu et Cu-acides humiques sur l'adsorption de l'herbicide imazéthapyr sur une argile de type montmorillonite. L'adsorption de l'imazéthapyr, un herbicide de la famille des imidazolinones, a été mesurée à partir des suspensions diluées sur la montmorillonite et des complexes de montmorillonite avec le cuivre et un acide humique. L'adsorption a été trouvée plus grande pour les complexes montmorillonite-Cu et montmorillonite-Cu-acide humique que pour la montmorillonite seule. La forme des isothermes était semblable pour tous les supports étudiés. L'adsorption de l'imazéthapyr varie avec le pH pour la montmorillonite et pour le complexe montmorillonite-Cu-acide humique. Le pH n'a pas d'effet apparent sur l'adsorption de l'imazéthapyr sur le complexe montmorillonite-Cu. Cela peut être dû à la rétention à pH élevé de l'imazéthapyr par des ions Cu liés à l'argile. Les cinétiques d'adsorption de l'imazéthapyr ont été plus rapides pour les complexes montmorillonite-Cu (15 min) et montmorillonite-Cu-acide humique (30 min) que pour la montmorillonite seule (240 min). Les complexes montmorillonite-Cu et/ou acide humique avec la montmorillonite pourraient être utilisés pour assainir l'eau contaminée par imazéthapyr.
Abstract
Adsorption of imazethapyr, an imidazolinone herbicide, was measured from dilute suspensions onto montmorillonite clay and complexes of montmorillonite with Cu and humic acid. Adsorption of imazethapyr was found to be greater for the Cu-montmorillonite and Cu-humic acid-montmorillonite complexes than by montmorillonite alone. Isotherm shape was similar for all supports studied. Adsorption of imazethapyr varied with pH for montmorillonite and for Cu-humic acid-montmorillonite complex. The pH had no apparent effect on imazethapyr adsorption to the Cu-montmorillonite complex. This might be due to imazethapyr retention at high pH by Cu ions linked to the clay. Imazethapyr adsorption kinetics was faster for Cu-montmorillonite (15 min) and Cu-humic acid-montmorillonite (30 min) than for montmorillonite (240 min). The copper-humic acid complexes with montmorillonite might be used to detoxify water contaminated with imazethapyr.
Inhoudstafel
1. Introduction
1Agricultural, industrial and urban development has caused damage to human health and to the environment due to the high pollution rates, especially in water. Elimination of contaminants from polluted waters is an objective of increasing importance in a variety of environmental settings. The extent of pesticide contamination of water has recently raised much concern due to the potential health hazards associated with the entry of these compounds into the food chain of humans and animals (Pal, 2001).
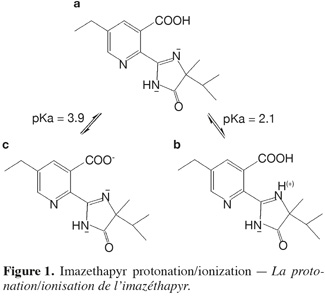
2Imazethapyr [5-ethyl-2-(4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl) nicotinic acid] belongs to a class of chemicals known as imidazolinones. This class has a very specific mode of action inhibiting certain plant systems, but does not interact in animals (Decision Document E 94-03, 1994). Imazethapyr is an effective herbicide for the control of various broad-leaved weeds and grass weed in soybeans and leguminous crops (Gennari el al., 1998; Stougaard et al., 1990; Nègre et al., 2001). It has both soil and foliar activity (Stougaard et al., 1990) and is absorbed through roots and foliage and translocated in both xylem and phloem and, thereby, accumulated in plants at growing points (Decision Document E 94-03, 1994). Imazethapyr is an amphoteric herbicide, having a carboxylic acid and a basic pyridine functional group (Stougaard et al., 1990). Its structure is shown in figure 1a. It can be noted that figure 1b and figure 1c are respectively the protonated and deprotonated forms of imazethapyr.
3Adsorption of pesticides on soil is one of the most important processes affecting their biological activity as well as environmental fate. The clay fraction and associated organic matter have been shown to be the primary adsorbents for pesticides in soil (Koskinen et al., 1990) as well as with other chemical species such as cations and anions (Liu et al., 2002).
4Copper is an essential element for plants and animals. Its behavior in soil is very important from an agricultural and environmental point of view. However, an increase of its level in soils can, in some cases, inhibit organic matter biodegradation as well as its subsequent nitrification (Maqueda et al., 1998).
5Several studies have been published on the adsorption of pesticides (Satrallah et al., 2002) and heavy metals (Houng et al., 1998) on soil constituents independently, but little attention has been given to adsorption when both are present together in soil or in solution.
6The pH effect on the adsorption of imazethapyr by different adsorbents has been reported by several researchers (Stougaard et al., 1990; Gennari et al., 1998). However, to our knowledge, no studies have been done on the complexes Cu-montmorillonite (Cu-M) and Cu-humic acid-montmorillonite (Cu-HA-M).
7The purpose of this study was to determine the effect of copper, extracted humic acid and pH on the adsorption of imazethapyr on montmorillonite (M) and to investigate the adsorption isotherms for each adsorbent.
8Adsorption of imazethapyr on soils, clays and humic acids has been investigated in the past by a number of scientists (Gennari et al., 1998; Stougaard et al., 1990) but, except of our previous paper on the retention of imazethapyr by Moroccan soils (El Madani et al., 2003), no kinetic studies have been carried out till now. Therefore, the adsorption kinetics of imazethapyr on M, Cu-M and Cu-HA-M were studied.
2. Experimental part
2.1. Chemicals
9Imazethapyr, Purity > 99%, was purchased from Riedel-de-Haen, Germany, and used without further purification. Ultra pure water was produced with a MilliQ system (Millipore, Billerica, MA, USA). All other chemicals were analytical grade.
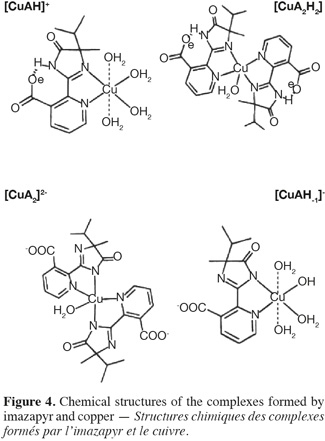
2.2. Sorbents
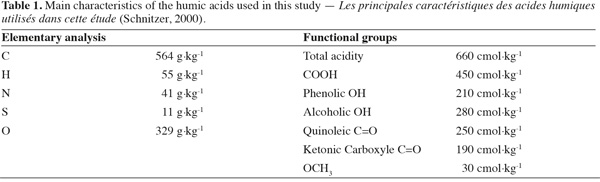
10The clay used in this study was montmorillonite (K10 with a fraction < 2 µm) purchased from Fluka Chemie, Switzerland. Humic acids are complex compounds of the soil. They play an important role in the interaction with pesticides. In this study we use a well characterized humic acid by Schnitzer (2000) which was kindly donated by Dr S.U. Khan, Professor, in George Mason University, USA. Their characteristics are summarized in table 1 (Schnitzer, 2000).
11The method used for the synthesis of the complex Cu-montmorillonite (Cu-M) was described by Cox et al. (1998). Fifteen grams of montmorillonite were shaken in 200 ml of 0.1M CuCl2 for 24 h. The mixture was then kept at 4°C for 16 h to facilitate decantation. The solid obtained was washed three times with distilled water to eliminate the non complexed clay and chloride and finally placed on a steam bath at 80°C for one night for water evaporation. The complex Cu-M obtained was carefully conserved to prevent any contamination.
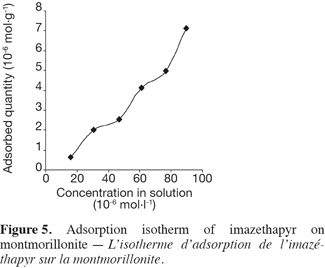
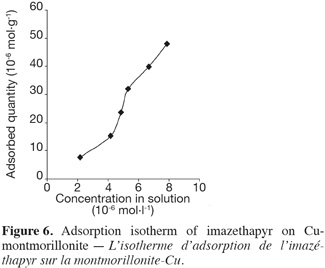
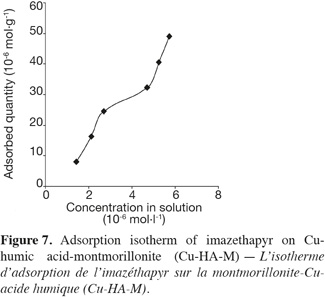
12The complex Cu-HA-M was prepared according to the method described by Schnitzer (2000). Five grams of montmorillonite K10 were shaken in 200 ml of a 0.1 M CuCl2 solution for 24 h at room temperature. The mixture was kept at 4°C for 16 h to facilitate decantation. The solid obtained was shaken for 24 h in a solution of (0.5 g.l-1) humic acid previously solubilized in 0.01 M NaCl solution. The solid obtained was then washed five times with distilled water and kept in a stream bath at 80°C for one night to evaporate and remove water.
2.3. Sorption
13All tests were carried out using 25 ml centrifuged tubes. The tubes were closed with specially designed PTFE caps covered with aluminum foil to prevent losses by adsorption to the caps. Samples without clays showed no decreases in imazethapyr solution concentration under the experimental conditions. Therefore, degradation, volatilization or adsorption to the flasks were negligible. The supernatant was separated from sorbents by centrifugation and then by filtration using glass fiber filters.
14Interaction studies at different time intervals were performed using a batch equilibrium method. Twenty milligrams of clay were added to 20 ml of imazethapyr solution (34.6 μmol.l-1 in 0.01 N CaCl2). The pH of the samples was adjusted with H2SO4 or KOH to obtain pH 5 (± 0.2). The different concentrations used include the working doses expected in the field.
15Interaction studies at different pH were carried out using the same procedure. The pH of the samples was adjusted with H2SO4 or KOH to obtain pH levels of 4, 5, 6 and 7 (± 0.2).
16Adsorption isotherms were determined at room temperature and at pH 5 (± 0.2). Twenty milligrams of clay samples were added to 20 ml of imazethapyr solutions of concentrations: 17.3, 34.6, 51.8, 69.1, 85.4 and 103.7 µmol.l-1. The mixtures were shaken for 24 h before their analysis. Each experiment was done in three replicates.
2.4. Analysis
17HPLC analysis was performed using a Perkin-Elmer Series 200 equipped with a diode array detector 235°C (Wellesley, MA, USA). A LiChrospher100 RP-18 column (250 × 4 mm id, 5 µm) was used for the imazethapyr adsorption studies and the flow rate of isocratic elution (60% H2O acidified to pH 2 = 0 with H3PO4 and 40% CH3CN) was 1.0 ml.min-1. The injection volume was 50 µl and data were collected and quantified at 255 nm.
3. Results and discussion
3.1. Kinetic studies
18Figure 2 shows that adsorption of imazethapyr by montmorillonite clay was completed after 240 min. It was very slow compared to the results obtained for the adsorption of this pesticide on Moroccan soils (El Madani et al., 2003). Moreover, we could also observe no decrease of imazethapyr concentration after reaching equilibrium.
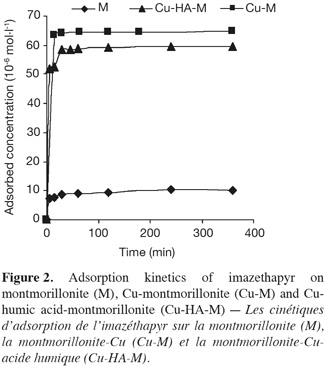
19We observed that the kinetics of imazethapyr adsorption by the Cu-M complex was faster than that obtained for montmorillonite (Figure 2). The adsorption was completed after 15 min and no decrease of imazethapyr concentration was observed after reaching equilibrium.
20We can conclude that the saturation of montmorillonite with copper makes the adsorption of imazethapyr by this clay quicker and more efficient. As for the complex Cu-M, the adsorption kinetics of imazethapyr on Cu-HA-M is also higher and was over after 30 min (Figure 2). No decrease of imazethapyr concentration was observed after reaching equilibrium.
21For each of the three adsorbents, we noted rapid adsorption of imazethapyr during the first minutes of contact, followed by a slower adsorption phase. According to Pignatello (1989), the weak kinetics of adsorption were due to a slow diffusion of the herbicide into the micropores of the adsorbents, especially of the clay fraction and organic matter (Mechrafi, 2002). This fact can also result from limitations of kinetic adsorption (Mechrafi, 2002). The equilibrium between the liquid and solid phases apparently took more time to be established. The adsorbed quantities were then more important when the concentration of the solution was higher (Figure 5, § 3.3) and no saturation of adsorption sites happened at the studied concentration range.
3.2. pH effect on the adsorption of imazethapyr
22The adsorption of imazethapyr on montmorillonite although small, appears to be pH dependent (Figure 3). This result is in good agreement with those obtained by Loux et al. (1989) for H/Al-montmorillonite.
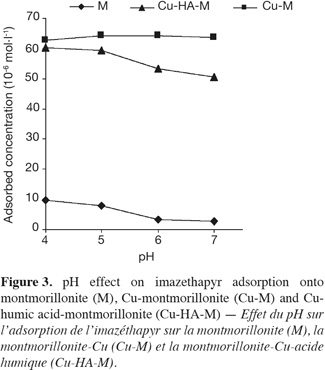
23The pH effect on the interaction of imazethapyr and montmorillonite can be explained in terms of ionization of the carboxylic group of this herbicide (Gennari et al., 1998; Stougaard et al., 1990). At high pH values above 5, imazethapyr should exist predominantly in an anionic form (–COO-) as shown in figure 1c which will be repulsed by the negative charges of the clay. At low pH conditions, the presence of uncharged non-ionic molecules (–COOH) increases allowing an increasing interaction with the negative charges of the montmorillonite colloids (Figure 1a).
24Imazethapyr would be largely ionized at pH 6, and no significant anion retention would be expected at higher pH values if the retention mechanism was exclusively ionic.
25Contrary to the results obtained for montmorillonite, no pH effect was observed for the adsorption of imazethapyr on the complex Cu-M. We concluded that the amount of herbicide adsorbed was the same for the four pH values. Therefore, it can be concluded that at low pH values, imazethapyr exists predominantly as the form (–COOH) (Figure 1a) which is largely adsorbed on the negatively charged colloids of clay and in the interlayer space which is greater with copper treatment. At higher pH values, the form (–COO-) (Figure 1c) of imazethapyr increased in solution and was adsorbed by the positively charged copper.
26Several researchers have been studying the complexation of copper and imidazolinone herbicides (Duda et al., 1996; Fursova et al., 2003). It was shown that possible binding mechanism can be created between the ions Cu++ and the two nitrogen atoms 1 and 2 (Figure 4) of the imidazolinone molecules. Duda et al. (1996) reported that imazapyr, an herbicide from imidazolinone family, can form the complex CuA2H2 with copper up to pH 6 (Figure 4). A is the deprotonated form of the pesticide.
27Finally, as with montmorillonite, it appears from figure 3 that pH had an effect on imazethapyr adsorption by the complex Cu-HA-M. This result is in agreement with the data regarding adsorption of imazethapyr by humic acids (Gennari et al., 1998).
28We observed that the adsorption of imazethapyr decreased by 15% with pH increasing from 4 to 7. Also, the amount adsorbed at pH 6 and 7 decreased slightly. This could be due to a steric effect that the macromolecules of humic acid are supposed to apply on the copper molecules linked to the clay and/or repulsive action of ionized carboxylic group of humic acid at higher pH.
3.3. Adsorption studies
29Adsorption isotherms of imazethapyr, respectively on montmorillonite, Cu-M and Cu-HA-M are shown in figures 5, 6 and 7. These isotherms were well described by the Freundlich equation:
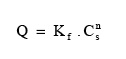
30where, n and Kf values are reported in table 2.
31Values for n were > 1 in all the cases indicating type S isotherms according to the classification of Giles et al. (1960) (Table 2). This type of isotherm is obtained when the solid presents more affinity for the solvent rather than for the solute at low concentrations. Meanwhile, when the concentration increases in the aqueous phase, layers of molecules can be formed on the surface of the clay support that causes cooperative adsorption. Similar results were obtained for the adsorption of carbofuran, and fenamifos (El M'Rabet, 2002).
32Kf values were affected by the chemical characteristics of the support (Table 2) and it increased in the order M < Cu-HA-M << Cu-M. According to Jamet et al. (1975), we can classify these adsorbents into two categories:
33– 1 < Kf < 4: for the montmorillonite indicating that this clay presents a medium affinity to imazethapyr;
34– Kf > 4: for the complexes Cu-M and Cu-HA-M indicating an important degree of affinity of imazethapyr to these two supports.
35The results of this study confirm that the adsorption capacity increases with the saturation of montmorillonite with copper. This is probably due to the increase of the interlayer space of the clay resulting of the copper treatment, which permits to the pesticide to be adsorbed not only on the surface but also in the interlayer space. The X-ray analysis showed that d001 of the clay increased from 9.6 Å for the montmorillonite to 12.44 Å for the complex Cu-M. These results are in agreement with those obtained from studies on the adsorption of pesticides on homoionic montmorillonite (El M'Rabet, 2002).
36For the complex Cu-HA-M, adsorption is greater than with montmorillonite alone and less than with Cu-M. This finding indicates that adsorption in this case happens also on the macro-molecules of humic acid which are probably responsible for a steric effect that prevents insertion of imazethapyr molecules into the interlayer space.
4. Conclusion
37The effects of Cu and Cu-humic acids on the adsorption of imazethapyr herbicide by montmorillonite were studied and the results obtained are as follows:
38– The pH seems to affect adsorption of imazethapyr on montmorillonite and Cu-HA-M. However, no pH effect was observed for the complex Cu-M due probably to a complexation of imazethapyr with copper at higher pH values;
39– The kinetics of imazethapyr was faster when adsorbing on the two complexes Cu-M and Cu-HA-M than for the montmorillonite;
40– The Freundlich equation gives the best fit to the data over the concentration range studied, with increasing affinity for the supports in the order of M < Cu-HA-M << Cu-M.
41– The higher adsorption of imazethapyr on the complex Cu-M may be attributed to the intercalation of imazethapyr in the interlayer space of the clay and to the adsorption on the copper linked to the montmorillonite.
Bibliographie
Cox L. et al., 1998. Sorption of imidacloprid on soil mineral and organic components. Soil Sci. Soc. Am., 62, 911-915.
Décision du Ministère de l'Environnement du Canada, 1994. Document n°E 94-03, Canada, March 30, 1994.
Duda A.M. et al., 1996. Copper (II) complexes of imidazolinone herbicide Imazapyr. J. Agric. Food Chem., 44, 3698-3702.
El M'Rabet M., 2002. Contribution à l'étude de l'adsorption du carbofuran et du phénamiphos par les complexes argilo-humiques et par les sols et de la biodégradation du carbofuran. Thèse d'État : Université Ibn Tofail, Faculté des Sciences, Kénitra (Maroc).
El Madani M. et al., 2003. pH effect and kinetic studies of the binding behaviour of imazethapyr herbicide on some Moroccan soils. Fresenius Environ. Bull., 12(9), 1114-1119.
Fursova E., Romanenko G., Ikroskii V. & Ovchanrenko V., 2003. Copper (II) complexes with imidazol-4-yl derivatives of 2-imidazoline nitroxides. Polyherdron, 22, 1957-1964.
Gennari M., Nègre M. & Vindrola D., 1998. Adsorption of the herbicides Imazapyr, Imazethapyr and Imazaquin on soils and humic acids. J. Environ. Sci. Health, B33(5), 547-567.
Giles C.H. & Mac Ewan T.H., 1960. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solid. J. Chem. Soc., 3, 3973-3993.
Houng K.H. & Lee D.Y., 1998. Comparisons of linear and nonlinear Langmuir and Freundlich curve-fit in the study of Cu, Cd and Pb adsorption on Taiwan soils. J. Soil Sci., 163(2), 115-121.
Jamet P. & André M., 1975. Étude de l'adsorption et de la désorption de la pyrazone par différents types de sols. Weed Res., 15, 113-121.
Koskinen W.C. & Harper S.S., 1990. The retention process: mechanisms. In: Cheng H.H., ed. Pesticides in the soil environment: processes, impacts, and modeling. Madison, WI, USA: Soil Science Society of America, 51-77.
Liu W., Gan J. & Yates S.R., 2002. Influence of herbicide structure, clay acidity and humic acid coating on acetanilide herbicide adsorption on homoionic clays. J. Agric. Food Chem., 50, 4003-4008.
Loux M.M., Liebl R.A. & Slife F.W., 1989. Adsorption of imazaquin and imazethapyr on soils, sediments and selected adsorbants. Weed Sci., 37, 712-718.
Maqueda C., Undabeytia T. & Morillo E., 1998. Retention and release of copper on montmorillonite as affected by the presence of a pesticide. J. Agric. Food Chem., 46, 1200-1204.
Mechrafi E., 2002. Adsorption, désorption et mobilité des herbicides au contact des adsorbants organiques et minéraux. Thèse de doctorat : Université Mohammed V, Faculté des Sciences, Rabat (Maroc).
Nègre M., Schulten H.R., Gennari M. & Vindrola D., 2001. Interaction of imidazolinones herbicides with soil humic acids. Experimental results and molecular modelling. J. Environ. Sci. Health, B36(2), 107-125.
Pal O.R. & Vanjara A.K., 2001. Removal of malathion and butachlor from aqueous solution by clays and organoclays. Sep. Purif. Technol., 24, 167-172.
Pignatello J.J., 1989. Sorption dynamics of organic compounds in soils and sediments. In: Sawhney B.L. & Brown K., eds. Reactions and movement of organic chemicals in soils. Madison, WI, USA: Soil Science Society of America; American Society of Agronomy, 45-80.
Satrallah A. et al., 2002. Adsorption-desorption and mobility of 14C- Ethofumesate in Moroccan soils. J. Environ. Protect. Ecol., 3(2), 390-399.
Schnitzer M., 2000. A lifetime perspective on the chemistry of soil organic matter. Adv. Agron., 68, 1-58.
Stougaard R.N., Shea P.J. & Martin A.R., 1990. Effect of soil type and pH on adsorption, mobility and efficacy of imazaquin and imazethapyr. Weed Sci., 38, 67-73.
Om dit artikel te citeren:
Over : El Habib El Azzouzi
Université Mohammed V-Agdal. Faculté des Sciences de Rabat. BP 1014. Avenue Ibn Battouta. MA-Rabat (Maroc). E-mail: elazzouzim@hotmail.com
Over : Meryem El Madani
Université Mohammed V-Agdal. Faculté des Sciences de Rabat. BP 1014. Avenue Ibn Battouta. MA-Rabat (Maroc).
Over : Mohamed Mekkaoui
Université Mohammed V-Agdal. Faculté des Sciences de Rabat. BP 1014. Avenue Ibn Battouta. MA-Rabat (Maroc).
Over : Mohamed Alaoui El Belghiti
Université Mohammed V-Agdal. Faculté des Sciences de Rabat. BP 1014. Avenue Ibn Battouta. MA-Rabat (Maroc).
Over : Rabet
Institut Agronomique et Vétérinaire Hassan II- APESA. BP 6022-Instituts. MA-Rabat (Maroc).
Over : Hafida Mountacer
Université Hassan I. Faculté des Sciences et Techniques de Settat. BP 72. MA-Settat (Maroc).
Over : Abderrahim El Hourch
Université Mohammed V-Agdal. Faculté des Sciences de Rabat. BP 1014. Avenue Ibn Battouta. MA-Rabat (Maroc).
Over : Abdellah Zrineh
Université Mohammed V-Agdal. Faculté des Sciences de Rabat. BP 1014. Avenue Ibn Battouta. MA-Rabat (Maroc).
Over : Mohammed El Azzouzi
Université Mohammed V-Agdal. Faculté des Sciences de Rabat. BP 1014. Avenue Ibn Battouta. MA-Rabat (Maroc).






