- Startpagina tijdschrift
- Volume 15 (2011)
- numéro spécial 1
- New developments in classical microscopy; what can be expected for the official control?
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
New developments in classical microscopy; what can be expected for the official control?

Abstract
The official control of animal proteins in feed is focused on the prevention of Bovine Spongiform Encephalopathy (mad cow disease). The current legislation of the European Union is planned to avoid the feeding of animal by-products to the same species as its origin (ban of cannibalism, or species-to-species ban). With respect to the official control, the circumscription of the term species in legislation should be defined, and species-specific markers should be available. Markers will include primer sets, antibodies, near-infrared profiles or visual characteristics. The method of classical light microscopy is currently the only accepted method in the framework of the official detection of animal proteins. Besides the necessary development of complementary methods, either as stand alone methods or in combination, the visual characteristics used for a microscopic examination of meat and bone meal particles should be fully explored. Multivariate analysis of a range of characteristics of lacunae in bone fragments revealed that discrimination is possible between mammalian and avian bone fragments. Translation to features for every day practical use should be carried out very carefully, and only comprehensively collected information on a range of features will give a first indication of the source. Characteristics of hairs and feather filaments can be used to identify the origin of animal particles. An in situ identification method has been developed for antibody conjugation with troponin I in muscle fibers on a microscopic slide. A proof of principle is presented. Interlaboratory transferability and validation have still to be achieved. The development and testing of light microscopy markers in the framework of the SAFEED-PAP project revealed that a fine tuning of existing microscopic characteristics appears to be possible.
Inhoudstafel
1. Introduction
1Bovine Spongiform Encephalopathy (mad cow disease) is generally believed to be caused by contamination of animal feeds containing animal by-products contaminated with prions (Prince et al., 2003). Therefore, an impressive set of regulations on processing, storage, incineration, and use in the feed production chain is in force. The microscopic analysis of feed samples for the detection of animal materials such as bone fragments, muscle fibers a.o. is applied from the beginning of the regulations (Directive 98/88/EC and successors). In the last years a range of other methods for detection, identification and confirmation are developed (latest overview in Fumière et al., 2009; see other contributions in this volume). Nevertheless, light microscopy remains until now as the only one method officially accepted for detection of animal proteins by the European Commission. Regulation 152/2009/EC provides the most recent overview of officially recognized methods for the official control of feed. The strengths of the microscopic detection method are, among others, sufficient detection at contamination levels as low as 0.02% (Engling et al., 2000; van Raamsdonk et al., 2009), the indication of the type of the detected materials, and the insensibility for the sterilization temperature. Animal materials, both fully or partly prohibited, such as meat meal, meat and bone meal, feather meal and fish meal can easily be distinguished from legally applied ingredients, e.g. milk powder, blood meal and gelatin. Weaknesses are the poor abilities to identify the species origin of the materials found, and the need to have skilled laboratory analysts, both for applying the method correctly, and for a reliable distinction between the allowed and prohibited ingredients.
2The European project Stratfeed was started to develop methods and markers for identification of animal proteins. A further development of the microscopic method and its markers was included in the following SAFEED-PAP project. In this paper a brief overview of the further developments in the area of microscopy of these markers is reported.
2. Background
3There is no legal limit for meat and bone meal (MBM) in feed. In all cases where animal protein from a certain source is prohibited, a null tolerance is applied. A range of Directives and Regulations apply to the use or prohibition of animal proteins.
4All animal proteins are prohibited for feeding to ruminants by TSE Regulation 999/2001/EC. This is the permanent ban. Feeding of by-products to the same species as the source is prohibited by the Animal By-Product Regulation 1774/2002/EC (article 22: Ban of cannibalism, or species-to-species ban). Feeding of meal processed from caught fish is allowed for feeding farmed fish, even if material of the own species might be included. This species-to-species ban is meant to be permanent, but currently “hidden” behind the Extended Feed ban. According to this Extended feed ban (Regulation 1234/2003/EC) all animal proteins from farmed animals are prohibited for feeding to farmed animals again. This quite severe measure is a compromise as long as animal specific identification methods are not fully developed and validated. The complete ban on feeding animal proteins to ruminants is relaxed by Regulation 956/2008/EC which allows the feeding of fishmeal to unweaned farmed ruminants.
5A more detailed view on the legislation points out that the feeding of animal proteins, including those of ruminant source, is allowed for pets and fur animals. However, the exemptions are mentioned in different Regulations:
6– pets (dogs, cats, etc.): Regulation 999/2001/EC, article 7;
7– fur animals: Regulation 1234/2003/EC, Amendment on Annex IV of Regulation 999/2001/EC; Regulation 1774/2002/EC, article 22.
8Furthermore derogations exist for a range of materials such as blood meal, blood plasma, hydrolyzed proteins, gelatin, milk products, egg products, tricalciumfosfate. These by-products are listed in Regulation 999/2001/EC Annex IV, last amended by Regulation 1292/2005/EC and Regulation 956/2008/EC.
9Insignificant amounts of bone fragments are tolerated in ingredients of a vegetative source intended for feeding according to Regulation 163/2009/EC, provided that a risk assessment indicates that a low risk level applies. This derogation was intended for accepting the presence of bone fragments in root and tuber crops, possibly originating from rodents or related animals.
10A principal problem is the lack of a definition of the term “species”. Besides eternal discussions among taxonomists concerning species concepts, the legislation is not clear on the definition of the term “species”. A biological species is indicated by a Latin binomen, such as Sus scrofa for wild boar, and by the name Sus scrofa domesticus for domesticated pig, which is an indication of subspecific rank. If this species circumscription is applied to the term “species” in legislation, then Sus scrofa would be allowed to consume by-products of e.g. Sus barbatus (bearded pig), one of the other pig species. In all cases the term “species” in legislation might point to a group of species, but it is not specified what taxonomic grouping this should be. In practice it is assumed to mean that pigs might consume non-ruminant/non-pig and avian material, and poultry might consume mammalian material, to name the two most prominent non-ruminant farmed animals. The term “ruminant” might either apply to the official order Ruminantia (Table 1) or to the group of ruminating animals, which includes camels, llamas and alpacas as well. In the latter case, identification markers for discriminating “ruminants” in wide sense from pigs is very difficult. The explanation of the ban is also less clear for horses, turkeys, ostrich, reptiles, etc. Some of these species are getting increasing interest in farming activities.
11Some examples can be given to illustrate the difficulty of distinguishing species groups for avoiding unintentional contamination in the framework of the species-to-species ban. Regulation 163/2009/EC anticipates on the situation that remains of mammals living on production fields can unintentionally show up in plant material in the form of “bone spicules”. No indication of species concerned is given in the Regulation. This could include mice species, rat and vole (belonging to the rodents) as well as rabbit, hare and mole, which belong to other species groups (Table 1). A comparably undefined situation applies to contaminations of fish meal by remains of sea mammals. Whales, dolphins, seals and related animals belong to different groups. The development of markers for identification should be based on an established view on animal group definitions.
12In the following paragraphs microscopic markers will be discussed according to their ability to distinguish between groups of animals at different classification levels.
3. Recognition of bone fragments
13The microscopic method comprises of several steps. After grinding the sample with mesh size of 2 mm, an amount of preferably 10 g is mixed in tetrachloroethylene (TCE). Most ingredients such as plant materials, hair filaments, feather filaments and muscle fibers remain floating. Only minerals, bone particles, teeth fragments and fish scales will together form the sediment. Usually the sediment comprises of 100-300 mg, depending on the amount of minerals in the original feed. The investigation of the relatively small amount of material of the sediment still represents the original 10 g of sample material. The sedimentation procedure is to be considered as a concentration step of a factor 20 or higher. In addition, the floating material and a part of the original sample can be investigated. Further details on the microscopic method can be found in Regulation 152/2009/EC, and in Gizzi et al. (2003), van Raamsdonk et al. (2007) and ARIES (2010).
14Based in the above brief description of the microscopic method, bone fragments are the primary target of microscopic examination. It is common and legal practice to distinguish easily between materials from the superclass of bone fish and the superclass of tetrapods (in the sense of terrestrial animals).
15Several different markers are being used for the characterization of bone fragments (e.g. shape, size and density of lacunae, visibility of connecting canals). One of the first publications on this topic is from Pinotti et al. (2004). A range of 32 characters pertaining to the lacunae is examined further by using image analysis techniques. The analyses were based on measurements of 30 individual lacunae (13 originating from 4 mammal samples, 17 from 4 poultry samples). The major characters of the variation between mammal and poultry material are the area polygon (area covered by a single lacuna) and the perimeter (length of the lacuna outline). For 28 lacunae (93.3%) a correct identification was made, in two occasions (6.7%) the lacunae from poultry bone fragments were incorrectly classified as being mammalian (Pinotti et al., 2004).
16In the framework of the SAFEED-PAP research 863 lacunae were measured using both manual and automatic methods in reference samples containing poultry and mammalian meat meal and bone meal (Pinotti et al., 2008; Campagnoli et al., 2009). In this case 26 characters were determined on the lacunae, of these 23 differed significantly (P < 0.001, ANOVA) between mammalian and poultry bone. Their results indicated that gradual differences exist between mammalian and poultry bone characteristics. Box-plots of means and medians of a range of variables indicated that absolute discrimination between different species groups is not possible when based on single parameters, mainly due to the overlap in the datasets. Combinations of variables might give further possibilities for discrimination.
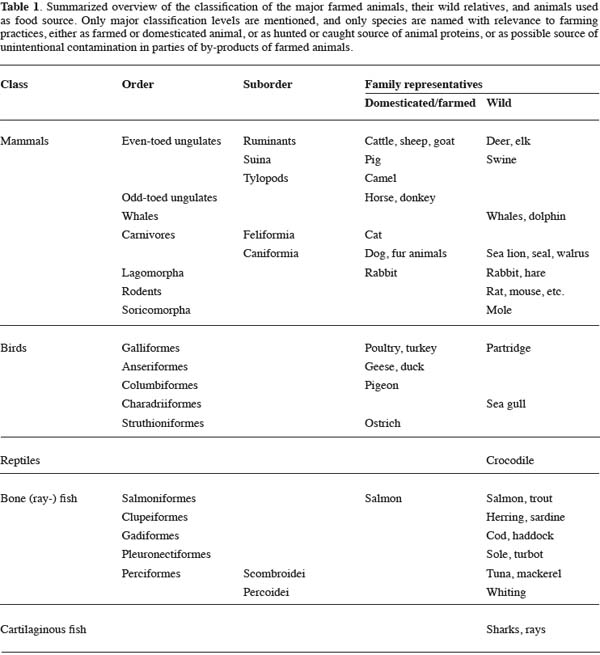
17In a further study an even more extended set of lacunae measurements was established with data on 1,143 lacunae of 25 different samples. The 56 characters were ranged in groups with correlation coefficients of ± 0.85 or higher. Only one parameter of each group was chosen for the final analyses, in order to avoid too much redundancy in the dataset. Multivariate analysis in the form of a Principal Component Analysis (PCA) was applied to the resulting dataset with eight characters for every lacuna. This way of examining a bone fragment with a number of lacunae does not reflect the way in practice to describe the overall view of a bone fragment. Usually a microscopist will examine and evaluate the bone fragment as a whole, reflecting on a range of different features. Therefore, a new dataset was constructed consisting of eight averages for the variables of each of the 25 samples. The result of the PCA on this 25 x 8 dataset is shown in figure 1. It appears that a combination of characters can differentiate in general between mammalian and poultry bone fragments. The main division along the x-axis is predominantly supported by three characters: the total area covered by a lacuna, the width of a lacuna, and the smoothness of the border of a lacuna. In all cases “lacuna” means the average representation of the lacunae in a bone fragment, since the PCA was based on a dataset with averages. The next very important step is to translate these results to the everyday laboratory practice for providing useful markers for giving at least a first indication about the nature of the fragments found. This translation should be carried out carefully.
18The spatial distribution of lacunae in a bone fragment was analyzed in SAFEED-PAP using a second dataset. First indications of this dataset reveal that also in these cases only gradual differences between mammalian and avian material exist.
19As a summarizing first indication, mammalian bone fragments might show larger and relatively wider lacunae, with a more erratic border compared to avian bone fragments. The lower smoothness of the border of lacunae in mammalian bone fragments might be related to the situation that on average the small canaliculae, connecting the lacunae, are more visible. The connections of the canaliculae with the lacunae are visible as irregularities. A more detailed presentation and discussion of the results will be published separately.
4. Hair and feather filaments
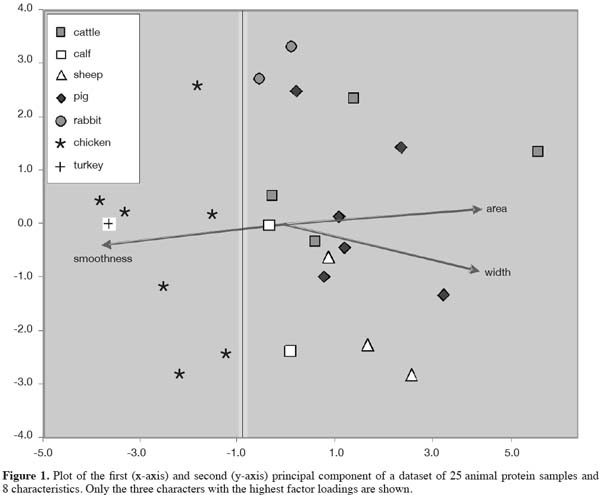
20The simple presence of hairs or of feather filaments points to an identification at the level of classes (mammals vs birds). More detailed analysis of hairs was obtained by the CRA-W in the framework of the SAFEED-PAP project and under the request of the European Commission. A visual study of “rodent” (see background in the beginning of this article), ruminant and pig hairs was carried out showing differences at level of orders. Furthermore species identification of Rodentia species is possible.
21In normal practice hairs are usually not detected in samples in regular monitoring programs (personal communications). If some small fragments of hairs or of feathers are anyway present in meat and bone meals (MBM), the detection is difficult; a dedicated reagent for detection of hairs and feathers is not routinely applied. This low occurrence might be due to a low abundance in EU produced MBM, as an effect of the European rendering process (133°C, 3 atm during 20 min). The Annex of Commission Regulation 242/2010/EC states that “The product must be substantially free of hooves, horn, bristle, hair and feathers, as well as digestive tract content.” Presence of low amounts of animal proteins in feeds can, however, be due to the situation that occasionally rodents enter the production facilities and the product flow. In these cases hairs can be expected as well and they can be used to discriminate between these unintentional side effects and the presence of processed animal proteins in the sense of the European legislation.
22It is known that the major groups of mammals (i.e. ungulates including ruminants, carnivores including fur animals and pets, and rodents in wide sense, table 1) can be distinguished using hair characteristics (Brunner et al., 1974; Teerink, 1991). In the occasions that a feed sample in practice contains one or a few particles of animal origin, it would be an advantage to discriminate at least between ruminant and rodent material if hairs can be identified (Figure 2). In the case of a negative result for the identification of ruminant material, a confirmed presence of rodents might be a complementary result, explaining the source of the animal proteins present in the feed. In the case of a presence of ruminant material, the finding of other, including rodent, material indicates the presence of a mixture of animal materials. The general principle of identifying the origin of animal constituents, also in the case that these particles might not be prohibited, is included in the global analytical scheme of Fumière et al. (2009).
23Staining of a part of the sieve fractions of the whole feed sample with cystin reagent, in order to enhance the visibility of keratin as major component of hairs, is only a facultative step in the procedure as described in Regulation 152/2009/EC. The different types of hairs as indicated in literature although rare, can even be found after heat treatment.
24Hair characteristics and documentation have been collected in the framework of SAFEED-PAP. The results obtained by SAFEED-PAP partner CRA-W will be used as part of the expert system Animal Remains and Identification System (ARIES).
5. Combination of methods
25Combination of methods can be achieved in order to join the strengths of several methods, whereas the disadvantages can be minimalised. The different detection and identification methods such as PCR (DNA detection), immunoassays (protein detection), near-infrared microscopy and light microscopy can be combined in various ways. One possibility for combining the strengths of different methods sequentially is the application of a method for identification after a positive detection is achieved by an initial (screening) method. One example is to run PCR on a sediment, indicated as positive after light microscopy (Toyoda et al., 2004; Fumière et al., 2006). It is also possible to combine two approaches in one method. In situ detection or hybridization is known for years as a powerful method for detection and identification of small quantities and small particles (Jin et al., 1997; Leitch et al., 2004; Harrison, 2007). The combination of light microscopy and either PCR or immunochemical analysis adds the possibility of identifying individual particles to the achievements of the microscopic method: its sensitivity to detect animal proteins at low contamination levels and its specificity. An on the spot identification of microscopically detected particles with respect to the source (species group) would enhance the support of the legislation, especially the species-to-species ban (1774/2002/EC).
26A method combining light microscopy with an identification technique (in situ identification) has been developed in SAFEED-PAP. Muscles were chosen as primary target, because this is a well recognizable type of particles in animal proteins, and a primary target for the examination of sieve fractions in the microscopy method. Muscle material shows the combination of high abundance and the presence of an identification mark with high specificity (DNA, protein). Both rt-PCR and immunochemical detection are suitable for application to muscle material. Immunochemical techniques were chosen for the identification, since antibodies are available for troponin I as well as other muscle proteins, whereas a second antibody labeled with a staining enzyme is available as well. In the framework of the SAFEED-PAP project several antibodies are raised. An antibody with a specific response and sensitivity for ruminants (cattle and sheep) has been used. This antibody shows a minor reaction to pig proteins, and no signal for poultry and fish materials.
27The design of a combination method comprises several steps. The chosen target, present at low frequencies if any is found, has to be concentrated and selected from the feed sample. A solvent is required with a relatively low density, which allows to get a flotation with the muscle fibers, and a sediment with the majority of the other particles. A second step is to immobilize the particles from the concentrate on a microscopic slide. The dried flotation is sprinkled on a slide which is coated with Norland Resin 81 (r), and hardened with UV light in order to immobilize the particles. Optimal circumstances have to be established for hybridization of the first and second antibody, and for the staining procedure. Therefore, slides are at first blocked with a buffer containing indifferent proteins, and washed at several points in the procedure with a TRIS-buffer. It appears that several enzyme-substrate combinations connected to the secondary goat-anti-mouse antibody can be used effectively, either with alkaline phosphatase (blue staining; figure 3) or with horse-radish peroxidise (red staining). These systems can be used simultaneously, allowing a theoretical discrimination system for muscle fibers from different target animal species.
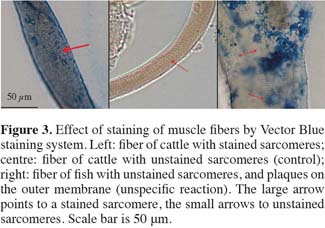
28It can be assumed that muscle fibers are not evenly sterilized during the rendering process, and that as a result the susceptibility of the troponin I complex is also unevenly distributed. In images of muscle fibers fully applied to the first and second antibody conjugations and the staining procedure, predominantly the sarcomeres are stained (Figure 3: left). In the control (without the first antibody incubation step) no color reaction is found (Figure 3: centre). Since the troponin I complex is found in the sarcomeres, this result indicates a specific reaction between the proteins and the antibody. The result after applying the same antibody against fish muscle fibers resulted in an irregular staining pattern (Figure 3: right), which is under a compound microscope not in focus with the sarcomeres. This could be indicated as an a-specific color reaction. The first results are encouraging, but transferability and validation of the method still need to be realized. More detailed reports on the development of the in situ combination method will be published separately.
6. Strategy of control
29Several approaches for the control to support the species-to-species ban can be designed (Baeten et al., 2005; van Raamsdonk et al., 2007; Fumière et al., 2009). These approaches should include detection, identification and, if required, confirmation steps. The available methods should be applied in one of those three steps according to their strengths. A second prerequisite is that the grouping of species (Table 1) will direct the choice for certain identification methods and for the use of group-specific markers (primer sets, antibodies, visual characteristics). The scheme of Fumière et al. (2009) looks complete and precise. As indicated in their paper, a tolerance level is currently not part of European legislation, but it is included in the scheme. Furthermore, a sample in which animal constituents are found can be routed through four different investigations including the detection method leading to the positive result, two identification steps and a final confirmation method. In this paper a simplified investigation scheme will be presented which includes the in situ identification method.
30It is likely to accept light microscopy as primary detection method for its advantages listed in the start of this paper. Also for the primary discrimination between fish and terrestrial animals light microscopy is preferred. Reliable identification at lower taxonomic levels needs other methods, although a first indication can already be achieved with microscopic characteristics. A range of primer sets for rt-PCR is readily available (Hormisch, 2004; Broll et al., 2007; Shinoda et al., 2008; Rojas et al., 2009). The development of reliable antibodies asks for a relatively high investment, but at lower levels of classification (e.g. mammalian orders) and for very quick methods these antibodies are a good choice.
31A strategy as presented in figure 4 could be imagined. The application of PCR on sediments containing bone fragments avoids largely the problem of positive signals from milk and blood products. The readily available antibodies for ruminant material can be applied in situ, giving the opportunity to have the advantage of a very low level of detection (one muscle fiber).
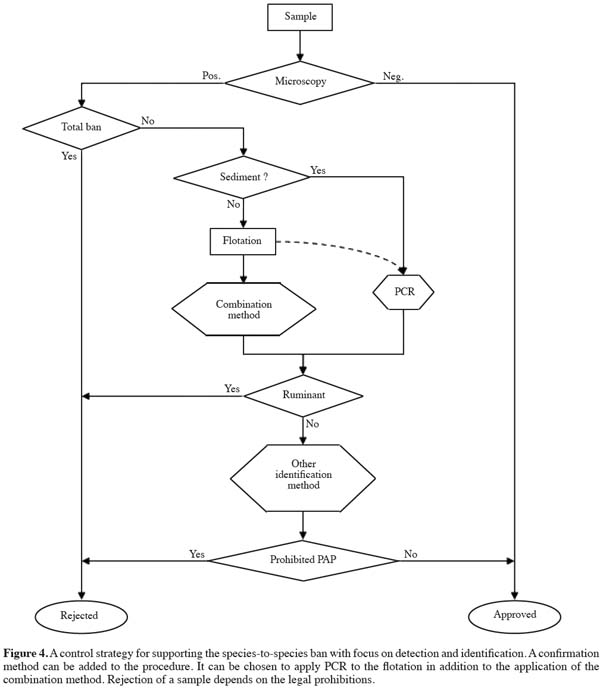
32Confirmation could be necessary in selected cases. Mass Spectroscopy methods are in development for this purpose.
7. Conclusion
33The development and testing of light microscopy markers in the framework of the SAFEED-PAP project revealed that a fine tuning of existing microscopic characteristics could be achieved. Visual markers could be applied at several classification levels (Table 1): the discrimination between fish and terrestrial animals, a first indication of the discrimination between mammalian and avian material (bone fragments, hairs vs feathers), the identification of different (groups of) mammalian orders (hair types), and the discrimination between ruminant vs non-ruminant material (muscle fibers) supported by a specific antibody conjugation and staining procedure.
34Currently hairs are found in samples from monitoring programs at a very low frequency. It has to be investigated whether these rare occurrences are due to the situation that a special color reaction is normally not applied, or that the frequency of occurrence is really low. A further analysis of the applicability of hair identification is recommended.
35The current application of the in situ hybridization method is based on a ruminant antibody. Antibodies raised against other animal species (groups) could be applied as well, resulting in a broader range of application. Investments in the development of other antibodies could be very valuable if validated.
36All these strategic possibilities resulting from the SAFEED-PAP research are based on the further development of markers in the framework of the official method, which allows them to be used under Regulation 152/2009/EC. The visual microscopic method and the in situ combination method can be used in a broader framework with other identification and confirmation methods (Figure 4).
Bibliographie
ARIES, 2010. Animal Remains Identification and Evaluation System, version 2.0. Decision support system for the identification of animal proteins. Wageningen, The Netherlands: RIKILT Institute of Food Safety, http://aries.eti.uva.nl., (December 2010).
Baeten V. et al., 2005. Comparison and complementarity of the methods. In: Stratfeed, strategies and methods to detect and quantify mammalian tissues in feedingstuffs. Luxembourg: Office for Official Publication of the European Communities.
Broll et al., 2007. Rapid identification of plant and animal species in foods. In: van Amerongen et al. Rapid methods for food and feed quality determination. Wageningen, The Netherlands: Wageningen Academic Publishers.
Brunner H. & Coman B., 1974. The identification of mammalian hair. Melbourne, Australia: Inkata Press.
Campagnoli A. et al., 2009. Combining microscopic methods and computer image analysis for lacunae morpho-metric measurements in poultry and mammal by-products characterization. Biotechnol. Agron. Soc. Environ., 13(S), 25-28.
Engling F.P., Jörgenson J.S., Paradies-Severin I. & Hahn H., 2000. Evidence of animal meal in feeds. Kraftfutter Feed Mag., 1, 14-17.
European Commision, 1998. Commission Directive EC/88/1998 establishing guidelines for microscopic identification and estimation of constituents of animal origin for the official control of feedingstuffs. Off. J. Eur. Union, L318, 27.11.1998, 45-50.
European Commission, 2003. Commission Regulation (EC) No 1234/2003 of 10 July 2003 amending Annexes I, IV and XI to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Regulation (EC) No 1326/2001 as regards transmissible spongiform encephalopathies and animal feeding. Off. J. Eur. Union, L173, 11.07.2003, 6-13.
European Commission, 2005. Commission Regulation (EC) No 1292/2005 of 5 August 2005 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as regards animal nutrition. Off. J. Eur. Union, L205, 06.08.2005, 3-11.
European Commission, 2008. Commission Regulation (EC) No 956/2008 of 29 September 2008 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Off. J. Eur. Union, L260, 30.09.2008, 8-11.
European Commission, 2009. Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union, L54, 26.02.2009, 1-130.
European Commission, 2009. Commission Regulation (EC) No 163/2009 of 26 February 2009 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Off. J. Eur. Union, L 55, 27.02.2009, 17-18.
European Union, 2001. Regulation (EC) No 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Off. J. Eur. Union, L147, 31.05.2001, 1-40.
European Union, 2002. Regulation (EC) No 1774/2002 laying down health rules concerning animal by-products not intended for human consumption. Off. J. Eur. Union, L 273, 10.10.2002, 1-95.
Fumière O. et al., 2006. Effective PCR detection of animal species in highly processed animal by-products and compound feeds. Anal. Bioanal. Chem., 385, 1045-1054.
Fumière O. et al., 2009. Methods of detection, species identification and quantification of processed animal proteins in feedingstuffs. Biotechnol. Agron. Soc. Environ., 13(S), 59-70.
Gizzi G. et al., 2003. An overview of tests for animal tissues in animal feeds used in the public health response against BSE. Rev. Sci. Tech. Off. Int. Épizooties, 22(1), 311-331.
Harrison P., 2007. In situ hybridization (basics). Abingdon, UK: Taylor & Francis.
Hormisch, 2004. Traceability of processed animal proteins with varying texture in feed: determination with microscopic and polymerase chain reaction methods. Biotechnol. Agron. Soc. Environ., 8, 257-266.
Jin L. & Lloyd R.V., 1997. In situ hybridization: methods and applications. J. Clin. Lab. Anal., 11(1), 2-9.
Leitch A.R., Schwarzacher T. & Jackson D., 2004. In situ hybridization. Abingdon, UK: Routledge.
Pinotti L. et al., 2004. Microscopic method in processed animal proteins identification in feed: applications of image analysis. Biotechnol. Agron. Soc. Environ., 8(4), 249-251.
Pinotti L. et al., 2008. Image analysis applied to classic microscopic method in animal meal characterization. Veterinary Res. Commun., 32 (Suppl. 1), 355-357.
Prince M.J. et al., 2003. Bovine spongiform encephalopathy. Rev. Sci. Tech. Off. Int. Épizooties., 22(1), 37-60.
Rojas et al., 2009. Authentication of meats from quail (Coturnix coturnix), pheasant (Phasianus colchicus), partridge (Alectoris spp.), and guinea fowl (Numida meleagris) using polymerase chain reaction targeting specific sequences from the mitochondrial 12S rRNA gene. Food Control, 20, 896-902.
Shinoda et al., 2008. Developing PCR primers using a new computer program for detection of multiple animal-derived materials in feed. J. Food Prot., 71, 2257-2262.
Teerink B.J., 1991. Hair of west-European mammals. Cambridge, UK: University Press.
Toyoda A. et al., 2004. PCR detection of bovine mitochondrial DNA derived from meat and bone meal in feed. J. Food Prot., 67(12), 2829-2832.
van Raamsdonk L.W.D. et al., 2007. New developments in the detection of animal proteins in feeds. Feed Sci. Technol., 133, 63-83.
van Raamsdonk L.W.D. et al., 2009. Animal proteins in feed. IAG ring test 2009. Report 2009.017. Wageningen, The Netherlands: RIKILT.
Om dit artikel te citeren:
Over : Leo van Raamsdonk
RIKILT ‐ Institute of Food Safety. Wageningen UR. Bornsesteeg, 45. PO Box 230. NL-6700 AE Wageningen (The Netherlands). E-mail: Leo.vanraamsdonk@wur.nl
Over : Luciano Pinotti
University of Milan. Department of Veterinary Sciences and Technology for Food Safety - VSA. Via Trentacoste, 2. I-20134 Milano (Italy).
Over : Pascal Veys
Walloon Agricultural Research Centre (CRA-W). European Union Reference Laboratory for Animal Proteins in Feedingstuffs. Chaussée de Namur, 24. B-5030 Gembloux (Belgium).
Over : Monique Bremer
RIKILT ‐ Institute of Food Safety. Wageningen UR. Bornsesteeg, 45. PO Box 230. NL-6700 AE Wageningen (The Netherlands).
Over : Wilma Hekman
RIKILT ‐ Institute of Food Safety. Wageningen UR. Bornsesteeg, 45. PO Box 230. NL-6700 AE Wageningen (The Netherlands).
Over : Anniek Kemmers
RIKILT ‐ Institute of Food Safety. Wageningen UR. Bornsesteeg, 45. PO Box 230. NL-6700 AE Wageningen (The Netherlands).
Over : Anna Campagnoli
University of Milan. Department of Veterinary Sciences and Technology for Food Safety - VSA. Via Trentacoste, 2. I-20134 Milano (Italy).
Over : Claudia Paltanin
University of Milan. Department of Veterinary Sciences and Technology for Food Safety - VSA. Via Trentacoste, 2. I-20134 Milano (Italy).
Over : Camino Belinchón Crespo
Walloon Agricultural Research Centre (CRA-W). European Union Reference Laboratory for Animal Proteins in Feedingstuffs. Chaussée de Namur, 24. B-5030 Gembloux (Belgium).
Over : Jef Vliege
RIKILT ‐ Institute of Food Safety. Wageningen UR. Bornsesteeg, 45. PO Box 230. NL-6700 AE Wageningen (The Netherlands).
Over : Victor Pinckaers
RIKILT ‐ Institute of Food Safety. Wageningen UR. Bornsesteeg, 45. PO Box 230. NL-6700 AE Wageningen (The Netherlands).
Over : Jan Sten Jørgensen
Danish Plant Directorate. Skovbrynet, 20. DK-2800 Lyngby (Denmark).






