- Startpagina tijdschrift
- Volume 11 (2007)
- numéro 2
- Catalase inhibition accelerates dormancy release and sprouting in potato (Solanum tuberosum L.) tubers
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Catalase inhibition accelerates dormancy release and sprouting in potato (Solanum tuberosum L.) tubers

Nota's van de redactie
Received on 25 October 2006, accepted on 10 January 2007.
Résumé
L’inhibition de la catalase accélère la levée de dormance et la germination des tubercules de pomme de terre. L’implication du métabolisme du peroxyde d’hydrogène (H2O2) dans la levée de dormance et la germination des tubercules de pomme de terre (Solanum tuberosum L.) a été étudiée en utilisant trois approches complémentaires. Dans la première approche, l’évolution de la cinétique de germination, du contenu en H2O2 et de l’activité d’enzymes antioxydants a été suivie pendant le stockage des tubercules. Les changements les plus importants ont eu lieu au niveau du « bourgeon/germe ». En particulier, la levée de dormance a été accompagnée d’une augmentation transitoire mais remarquable du contenu en H2O2. Dans la deuxième approche, l’effet d’un inhibiteur (thiourée) de catalase (CAT, EC 1.11.1.6) ou d’une application exogène de H2O2 sur le comportement germinatif du tubercule a été évalué. Les deux traitements ont permis une réduction de la durée de dormance et une germination rapide et synchronisée des tubercules par rapport au contrôle, ainsi qu’une augmentation du nombre de germes par tubercule. Dans la troisième approche, l’effet de l’inhibition de la CAT sur la dormance et la germination des tubercules de pomme de terre a été évalué en utilisant la transgénèse. Des plantes partiellement réprimées dans leur activité CAT ont été produites et, encore une fois, l’inhibition de la CAT a permis une accélération de la cinétique de germination et une augmentation du nombre de germes dans les tubercules transgéniques comparés au type sauvage. Il parait donc que la dormance et la germination du tubercule peuvent être contrôlées par la manipulation du métabolisme du H2O2 via l’inhibition de l’activité CAT. Les éventuels mécanismes par lesquels les inhibiteurs de CAT ou H2O2 lèvent la dormance et favorisent la germination des tubercules de pomme de terre sont discutés en relation avec ce qui est connu chez d’autres modèles végétaux (graines et bourgeons d’arbres fruitiers).
Abstract
The involvement of hydrogen peroxide (H2O2) metabolism in dormancy release and sprouting of potato (Solanum tuberosum L.) tubers has been investigated using three complementary approaches. In the first approach, the evolution of the sprouting kinetics, H2O2 content and antioxidant enzyme activities were examined during tuber storage. The most important changes occurred at the « bud/sprout » level. In particular, dormancy release was accompanied by a transient but remarkable increase in H2O2 content. In the second approach, the effect of a catalase (CAT, EC 1.11.1.6) inhibitor (thiourea) or of exogenous H2O2 application on tuber sprouting behaviour was assessed. Both treatments resulted in a reduction of the dormancy period and in rapid and synchronised sprouting of the treated tubers when compared to the control as well as in increased sprout number per tuber. In the third approach, the effect of CAT inhibition on potato tuber dormancy and sprouting was evaluated using the transgenic technology. Plants partially repressed in their CAT activity were produced and, once again, CAT inhibition resulted in acceleration of the sprouting kinetics and in increased sprout number of the transgenic tubers compared to those from the wild type. It thus appears that tuber dormancy and sprouting can be controlled in potato by the manipulation of H2O2 metabolism via the inhibition of CAT activity. The possible mechanisms whereby CAT inhibitors or H2O2 overcome dormancy and promote sprouting in the potato tuber are discussed in relation to what is known in other plant models (seeds and fruit tree buds).
Inhoudstafel
1. Introduction
1At harvest and for a finite period thereafter, potato tubers will not sprout and are considered as dormant (Burton, 1989). Dormancy is defined as « the physiological state of the tuber in which autonomous sprout growth will not occur, even when placed under ideal conditions for sprouting » (Reust, 1986). The length of this dormant period is dependent on the genotype as well as on both pre- and post-harvest conditions (Burton, 1989). Tuber dormancy is desirable when potatoes must be stored (industrial processing); however, excessively long dormancy poses a problem in sprouting of seed tubers (early crop installation). Accelerated or delayed sprouting of the harvested tubers may be favoured depending on the intended purpose. Controlling the length of dormancy period could therefore be of considerable economic importance. Unfortunately, the underlying mechanisms regulating the maintenance and breakage of tuber dormancy are still poorly understood.
2There is evidence that endogenous plant hormones play a pivotal role in the initiation, maintenance and release of potato tuber dormancy (Coleman et al., 1992; Wiltshire, Cobb, 1996; Suttle, 2004b). Endogenous ethylene has been shown to play an important role in the induction of tuber dormancy (Suttle, 1998). The sustained presence of abscisic acid was found to be essential for both induction and maintenance of tuber dormancy (Suttle, Hultstrand, 1994). Cytokinins have been suggested to be involved in the release of tuber dormancy (Hemberg, 1985; Coleman, 1987) and gibberellins in the regulation of subsequent sprout growth (Suttle, 2004a).
3In contrast to the above-mentioned hormonal regulation, little attention has been given to the possible involvement of reactive oxygen species (ROS) and antioxidants in the control of potato tuber dormancy. ROS [including superoxide anions (O2-), hydrogen peroxide (H2O2), and hydroxyl radicals (OH)] generation is ubiquitous in biological systems, and occurs either as unavoidable by-products of metabolic reactions or through signal-regulated processes under both normal and stress conditions (Bolwell, 1996). Major plant ROS-scavenging mechanisms include superoxide dismutase (SOD, EC 1.15.1.1), ascorbate peroxidase (APX, EC 1.11.1.11) and catalase (CAT, 1.11.1.6) (for review, see Mittler, 2002). SOD is considered as a key player within the antioxidant defence system as it regulates the cellular concentration of O2- and H2O2. H2O2 is eliminated by CAT and APX; their different affinities for H2O2 (CAT, mM range and APX, µM range) suggest that they belong to two distinct classes of H2O2- scavenging enzymes: CAT might be responsible for the removal of excess ROS during stress, whereas APX might be responsible for the fine modulation of ROS for signalling (Mittler, 2002). A relationship between ROS metabolism and dormancy breakage in both plant seeds (Hendricks, Taylorson, 1975; Fontaine et al., 1994) and vegetative buds (Wang et al., 1991; Or et al., 2002; Pérez, Lira, 2005) has been repeatedly reported. In particular, application of H2O2 or of CAT inhibitors releases dormancy in these plant tissues. In potato, however, little is known about the involvement of ROS metabolism in tuber dormancy release (Rojas-Beltran et al., 2000).
4The present work has thus been undertaken with the general aim to evaluate the relevance of ROS and antioxidants in the control of potato (cv. Désirée) tuber dormancy. To this end, three complementary approaches have been adopted:
5– time-course analysis of sprouting, H2O2 content and antioxidant enzyme activities in harvested tubers during storage;
6– direct application of H2O2 and of a chemical inhibitor of CAT (thiourea) to harvested tubers and assessment of their impact on sprouting;
7– generation of transgenic potato plants deficient in their CAT activity and characterisation of their tuber sprouting behaviour.
2. Materials and methods
2.1. Experiment 1: Time-course analysis of potato tuber sprouting, H2O2 content and antioxidant enzyme activities during storage
8Potato tubers (Solanum tuberosum L. cv. Désirée) were field-grown under the standard cultural conditions of west temperate Europe (Belgium). After harvest, healthy and uniform tubers (35–45 mm) were selected and allowed to undergo skin set at 20°C in the dark for 10 days. After that, they were placed to sprout in the dark under constant temperature (20°C) and relative humidity (RH, 90%). In total, 13 lots of ca. 200 tubers each were used. For sampling, two cylindrical (8x8 mm) pieces of the parenchyma tissue including either the apical « bud/sprout » or the most proximal one were cut from each tuber. At each sampling (each week), the number of sprouted buds was recorded for each category (apical or proximal) using one tuber lot (200 tubers). Immediately after that, « buds/sprouts » and parenchyma tissues were separated for both apical and proximal cylindrical parts of the tuber, ground to a fine powder in liquid nitrogen and stored at -80°C until use.
9For this first part of our work, sprouting kinetics, H2O2 content and activity staining of SOD, CAT, and APX isoforms were quantified in both the apical and proximal « buds/sprouts » and parenchyma tissues of tubers (see hereafter).
2.2. Experiment 2: Direct application of H2O2 and of thiourea to harvested tubers and assessment of their impact on sprouting
10Potato tubers, cv. Désirée, were greenhouse-grown under natural light supplemented with Sylvania mercuric lamps (HSB-BW/500) to reach a minimum photon flux density of 250 µmol.m-2.s-1 under a photoperiod of 16 h. Day/night temperature and RH averaged respectively 30–20°C and 45–65%. Immature tubers (100 d after sowing) were carefully harvested, graded (± 30 mm) and stored in the dark at 20°C and 90% RH for 1 d before treatments. Tubers were then treated with 250 mM thiourea, 20 mM H2O2, or with water by dipping them into the corresponding solutions for 2 h, after exposing the parenchyma around the apical bud by a limited cutting (8 mm diameter x 8 mm depth). Another set of tubers treated with water was kept intact and referred to as control. For each treatment (thiourea, H2O2, water, and control), 45 tubers were used. Once treated, tubers were stored under sprouting conditions (dark, 20°C, and 90% RH) and examined daily.
11For this experiment, the quantified parameters were the sprouting kinetics and the sprout number per sprouted tuber (n=30).
2.3. Experiment 3: Generation of transgenic potato plants deficient in their CAT activity and characterisation of their tuber sprouting behaviour
12Cat2 from Nicotiana plumbaginifolia and SU2 from Gossypium hirsutum, both coding for the CAT2 isoform (which is predominant in the stem vascular tissues) were used in this part of the present work. Two constructs (see Chamnongpol et al., 1996 for more details) pCat2AS (Cat2, antisense orientation) and pCatGH (SU2, sense orientation) were kindly provided by Prof. D. Inzé (Gent University, Belgium). The two constructs were found to efficiently repress CAT activity in tobacco plants (Chamnongpol et al., 1996). They were mobilised into Agrobacterium tumefaciens (strain LB4404) and used to transform internodal explants of potato, cv. Désirée, as described by Beaujean et al. (1998) with slight alterations (M’Hamdi et al., 2003).
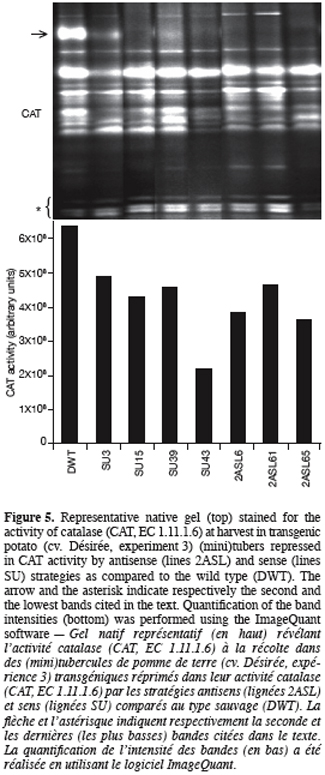
13Once generated, transgenic potato plants were characterised for their tuber activity staining of CAT isoforms, sprouting kinetics and their sprout number per sprouted tuber. Since the transgenic plants produced only few (mini)tubers, CAT activity was determined considering whole tuber tissues instead of « bud/sprout » tissues only as in experiment 1.
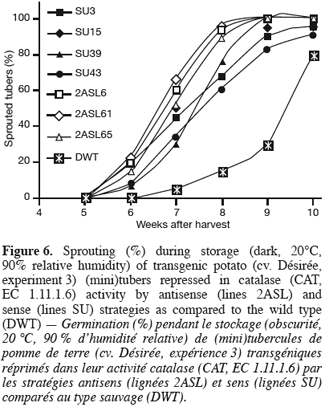
2.4. Quantified parameters
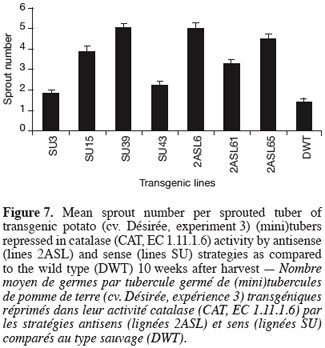
14For sprouting kinetics determination (experiments 1, 2 and 3), the percentage of sprouted tubers was recorded at weekly intervals. In accordance with established guidelines (Reust, 1986), a tuber was considered as sprouted when it had at least one sprout 2 mm long. The moment of 80% sprouting was used to characterise the end of dormancy (Van Ittersum et al., 1992). Sprout number (experiments 2 and 3) corresponds to the mean number of sprouts produced considering the sprouted tubers only.
15For H2O2 content estimation (experiment 1), fresh tuber tissues were frozen in liquid nitrogen and ground to a fine powder. Then, ca.1 g of the frozen powder and 250 mg of activated charcoal were homogenised for 2 min in 4 ml cold 5% (w/v) trichloroacetic acid. The mixtures were then centrifuged at 12,100 g for 30 min at 4°C. The amount of H2O2 in the resulting extracts was quantified by the chemiluminescence reaction with luminol as suggested by Warm and Laties (1982).
16For antioxidant enzyme activities (experiments 1 and 3), frozen tuber powder was homogenised in one volume of extraction buffer [50 mM potassium phosphate (pH 7.6), 10 mM sodium metabisulfite, 1 mM ascorbic acid, 1 mM ethylenediamine-tetraacetic acid (EDTA), 20% (w/v) sorbitol, and 2% (w/v) polyvinylpolypyrrolidone (PVP)] and centrifuged at 12,000 g for 20 min at 4°C. The supernatant was collected and the protein content was determined according to Bradford (1976) using the Bio-Rad Protein Assay.
17Native polyacrylamide gels were prepared essentially according to the procedure of Laemmli (1970) but without sodium dodecyl sulphate. Each gel was composed of a stacking gel and a separation gel of respectively 5% and 10% acrylamide. Equal amounts of protein (80 µg) from tuber extracts were subjected to electrophoresis at a constant current of 8 mA per gel, overnight, and the temperature set at 4°C. SOD activity was detected essentially according to the riboflavin-nitroblue tetrazolium staining method (Beauchamp, Fridovich, 1971). After electrophoresis, the gels were quickly rinsed with deionised water and then incubated in a mix of two staining solutions in the dark. The mix consisted of 65 ml of 1 mM EDTA, 50 mM potassium phosphate buffer (pH 7.8), containing 0.38 mM nitroblue tetrazolium and 330 µl of N,N,N',N'-Tetramethylethylenediamine (TEMED), and 30 ml of 0.13 mM riboflavin-5'-phosphoric acid. After 20 min under gentle agitation, the gels were rinsed two times with water and then exposed to high light until appearance of white bands (exhibiting SOD activity) on a purple background. When maximum contrast was achieved, the reaction was stopped by rinsing the gels with water. For CAT activity staining (John G. Scandalios, personal communication), gels were first incubated in the dark for 30 min in a freshly prepared solution containing 50 mM potassium phosphate (pH 7.0), 0.1 mM EDTA, and 0.5 mM nitroblue tetrazolium. The gels were after that transferred to a second fresh solution (50 mM potassium phosphate (pH 7.0), 0.1 mM EDTA, 5 mM H2O2, and 10 mM ascorbic acid) in light until appearance of white bands on purple background. The gels were finally rinsed in deionised water several times once the desired staining intensity was reached. The intensity of the resolved bands was quantified by densitometric analysis using the ImageQuant TL software (Amersham Biosciences). APX activity was determined as described by Mittler and Zilinskas (1993). In this case, and unlike SOD and CAT, 2 mM ascorbate and 10% (w/v) glycerol were added to the separation gel and the entire gel was pre-run for 30 min to allow ascorbate entry into the gel. After electrophoresis, the gels were immersed in 50 mM sodium phosphate, pH 7.0 and 2 mM ascorbate for 30 min. They were then soaked in 50 mM sodium phosphate, pH 7.0, 4 mM ascorbate and 2 mM H2O2 for an additional 30 min before a 4 min-washing with 50 mM sodium phosphate, pH 7.0. Finally, the gels were incubated in 50 mM sodium phosphate, pH 7.8, 28 mM TEMED and 2.45 mM nitroblue tetrazolium under gentle agitation until appearance of APX activity as achromatic bands on a purple blue background. Once maximum contrast was attained, the reaction was stopped by rinsing the gels with water.
3. Results
18Three complementary experiments have been performed in the present study in order to evaluate the possible link between the metabolism of ROS, in particular H2O2, and the breakage of tuber dormancy in potato (Solanum tuberosum L. cv. Désirée).
3.1. Time-course analysis of potato tuber sprouting, H2O2 content and antioxidant enzyme activities during storage (experiment 1)
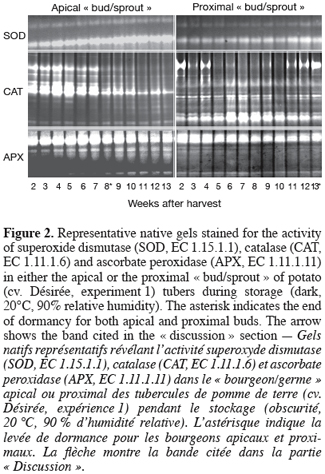
19For this first experiment, sprouting kinetics (Figure 1), H2O2 content (Figure 1) and activity staining of SOD, CAT and APX (Figure 2) were analysed during storage in the apical and proximal « buds/sprouts » and their underlying parenchyma tissues of field-grown potato tubers harvested at maturity.
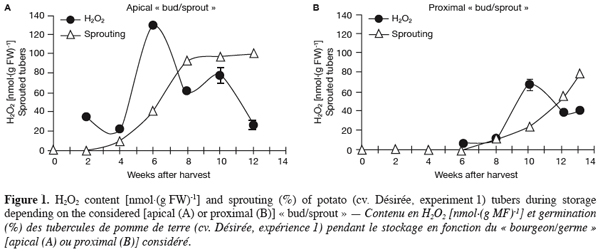
20Three weeks after harvest, all tubers analysed were still dormant (Figure 1). Indeed, potato tubers exhibit all types of dormancy (endo-, para-, and ecodormancy) as defined by Lang et al. (1987). At harvest and during a certain period thereafter, all buds (eyes) of the tuber are endodormant. After that, endodormancy is lost (sprouting of typically the most apical bud) and paradormancy (apical dominance) of the lateral buds (including the proximal one) keeps them at rest. When stored under low temperatures, bud growth is then prevented by ecodormancy. In the present study, as expected, sprouting of the apical bud (Figure 1a) was earlier, faster and higher than that of the proximal bud (Figure 1b).
21In parallel to sprouting, H2O2 content was estimated in both apical and proximal parts of the tuber during storage. The amounts recorded in parenchyma tissues beneath both bud types were around 30 nmol.(g FW)-1 and essentially did not change whatever the sampling time (data not shown). In the « buds/sprouts », however, a transient but large increase in H2O2 content was observed in both apical and proximal buds and coincided with their respective sprouting phases (Figure 1). Maximum values of 129.6 nmol.(g FW)-1 in apical « buds/sprouts » (Figure 1a) and of 72.8 nmol.(g FW)-1 in proximal « buds/sprouts » (Figure 1b) were obtained respectively 6 and 10 weeks after harvest.
22SOD activity gradually increased throughout storage time in both apical and proximal « buds/sprouts », the activity being higher in the former than in the latter whatever the sampling time (Figure 2).
23CAT activity progressively increased from the second to the fourth week of storage in the apical « buds/sprouts » and then decreased most particularly beyond the seventh week (Figure 2) when sprouting was more than 90% (Figure 1a). Between the fourth and the seventh week (sprouting phase, Figure 1a), the densitometric analysis revealed that the decrease in CAT activity was more than 50% (data not shown). In the case of the proximal « buds/sprouts », the pattern of CAT activity was quite different from that observed at the apical « bud/sprout » level. Quantification of the band intensities revealed that CAT activity decreased by ~60% between the fourth and the tenth week after storage and then increased for the remaining experimental time (data not shown).
24APX activity in both kinds of « buds/sprouts » was in general high and showed only slight changes during the sampling time (Figure 2).
25Unlike « buds/sprouts », the corresponding parenchyma tissues did not really show any alteration in the activity of SOD, CAT and APX throughout the post-harvest storage of tubers (detailed data not presented).
3.2. Direct application of H2O2 and of thiourea to harvested tubers and assessment of their impact on sprouting (experiment 2)
26Results from experiment 1 indicating that H2O2 accumulation accompanies the termination of dormancy (Figure 1) led us to test whether or not provoking H2O2 accumulation will influence tuber sprouting.
27In the present experiment, harvested greenhouse-grown potato tubers, cv. Désirée, were treated with 20 mM H2O2 (H2O2), 250 mM thiourea (a CAT inhibitor, Thiourea) or with water (H2O) (Figure 3 and Figure 4) by dipping them into the corresponding solutions for 2 h. As the periderm of potato tubers is almost impermeable to chemicals, gases and liquids (Beukema, van der Zaag, 1990), parenchyma tissues just around the apical bud of each tuber were exposed to the different solutions by a limited cutting (8 mm diameter x 8 mm depth). In a preliminary study, this kind of wounding has been shown to have only a small effect on the sprouting behaviour of tubers (data not shown). A set of intact tubers was also treated with water and referred to as control (Control, Figure 3 and Figure 4). Once treated, all tubers were stored under sprouting conditions and daily examined.
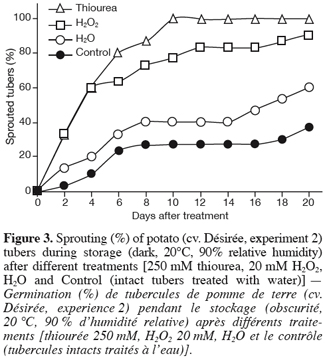
28As expected, the way the tubers were treated (limited cutting) did not significantly affect the sprouting of the tubers (H2O vs. Control) (Figure 3). The expected accumulation of H2O2 in tuber tissues either by its direct application or indirectly by treating tubers with a CAT inhibitor (thiourea) resulted in accelerated sprouting of the treated tubers when compared to water. According to Van Ittersum et al. (1992), a tuber batch is stated as sprouted when 80% of the tubers display at least one 2 mm sprout (« 80% and 2 mm » criterion). Tubers treated with thiourea and H2O2 showed 80% of sprouting after respectively 6 and 10 d while this percentage was never reached (at least within the 20 d following treatment) by the water-treated (H2O and Control) tubers, which displayed a delayed, slow and partial sprouting within the time-frame of the experiment (Figure 3). In other words, dormancy of thiourea- and H2O2- treated tubers was broken respectively 6 and 10 d after treatment while that of water-treated tubers (H2O and Control) was still unreleased even at the end of the experimental period (20 d after treatment).
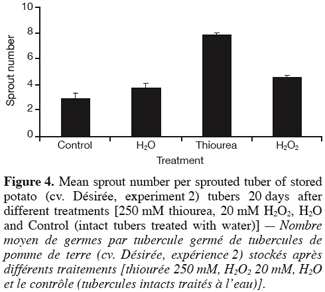
29Furthermore, figure 4 displays that the mean sprout number per sprouted tuber was remarkably increased by thiourea, and to a lesser extent by H2O2. As with sprouting, the effect of « wounding » (H2O vs. Control) on the number of sprouts was limited.
3.3. Generation of transgenic potato plants deficient in their CAT activity and characterisation of their tuber sprouting behaviour (experiment 3)
30Transgenic potato plants partially repressed in their CAT activity were generated through antisense (2AS lines, using the Nicotiana plumbaginifolia Cat2 gene) and sense (SU lines, using the Gossypium hirsutum SU2 gene) technologies. In fact, CAT was used as a target of transformation as it showed the most important changes during dormancy and sprouting compared to SOD and APX (Rojas-Beltran et al., 2000 and this work, figure 2). All lines obtained in the present work grew normally under our greenhouse conditions. Tubers produced by the different transformants were collected and analysed for their CAT activity staining, sprouting kinetics and their sprout number per sprouted tuber.
31Figure 5 displays that CAT activity of the transformed lines shows a significant reduction, the extent of which depending on the isoform. The second band from the top corresponding to a major band in the wild type pattern (indicated by an arrow, figure 5, top) is missing in the lanes of almost all transformed lines regardless of the strategy used (sense or antisense). Data derived from densitometric analysis indicate that despite a significant increase in the intensity of the lowest bands (indicated by an asterisk, figure 5, top), the overall CAT activity was reduced in the transgenic tubers compared to the wild type (Figure 5, bottom). The most important reduction (66%) was obtained with SU43 tubers.
32The sprouting kinetics during storage of greenhouse-grown tubers shows that sprouting was earlier in the transgenic lines than in the wild type (Figure 6). Within transformants, sprouting of the antisense (2AS) lines seems more accelerated than that of the sense (SU) ones.
33Figure 7 displays the mean sprout number per sprouted tuber 10 weeks after harvest. The genetic repression of CAT resulted in a significant but variable increase in the number of sprouts produced reaching 5 sprouts per sprouted tuber in 2ASL6 and SU39 compared to 1.4 sprouts per sprouted tuber in the wild type.
4. Discussion
34In a previous study performed on two potato cvs (Désirée, Bintje), the activity of antioxidant enzymes during tuber dormancy has been analysed on both the apical (1/3 of the tuber) and proximal (2/3 of the tuber) parts of the tuber (Rojas-Beltran et al., 2000). It was found that, during tuber dormancy, SOD activity was relatively constant while CAT activity was decreased whatever the cultivar and the tuber part. Based on their results, Rojas-Beltran et al. (2000) have discussed the possible relationship between ROS metabolism and dormancy regulation of the potato tuber. The present work has been particularly focused on the implication of H2O2 metabolism in the release of potato tuber dormancy using different approaches.
4.1. Evidence for the possible implication of CAT inhibition/H2O2 accumulation in the breakage of potato tuber dormancy
35With reference to the « 80% and 2 mm » criterion, i.e. end of dormancy when 80% of tubers had at least 1 sprout 2 mm long (Van Ittersum et al., 1992), (endo)dormancy release of the tuber lot used in the first experiment occurred around the eighth week after harvest (Figure 1a). At that time, only 13% of the proximal buds have sprouted (Figure 1b); sprouting of the proximal bud was first slow between the sixth and the tenth week and rapid after that, probably due to the removal of the apical bud action (paradormancy) (Lang et al., 1987). The sprouting of both bud types, although occurring at different times, was accompanied by a transient but significant increase in H2O2 content (Figure 1) suggesting that this compound may play an important role in the sprouting of potato tubers.
36SOD activity gradually increased throughout the experiment whatever the tuber « bud/sprout » (Figure 2) and may account, at least in part, for the observed accumulation of H2O2 (Figure 1). However, it does not seem to be correlated with dormancy release of the potato tuber and can be part of overall increased activity in the « bud/sprout » tissues during the initiation of sprouting and sprout growth. The observation of the pattern of CAT activity (Figure 2) supplemented by the densitometric analysis (detailed data not shown) revealed that the reduction in CAT activity started during (apical « bud/sprout ») and even before (proximal « bud/sprout ») the onset of sprouting and thus preceded the moment of maximum H2O2 accumulation (Figure 1). This indicates that the transient accumulation of H2O2 may result, at least partially, from a reduction in CAT activity. The other H2O2-removing enzymes (APX, figure 2 and diaminobenzidine-peroxidases, our unpublished data) do not seem to contribute to the rise in H2O2 content.
37The treatment of harvested greenhouse-grown potato tubers with a CAT inhibitor (thiourea) or with H2O2 resulted in rapid and more synchronised sprouting as well as in higher sprout number production (especially with thiourea) compared to H2O-treated tubers (Figure 3 and Figure 4). Dormancy of tubers treated with thiourea or H2O2 was released 7 d before that of tubers treated with water (H2O and Control). Such effect of CAT inhibitor or H2O2 treatments on dormancy and sprouting does not seem to be a peculiar characteristic of potato tuber buds since several studies have shown similar responses in both seeds and fruit tree buds, providing additional evidence that common regulatory mechanisms of dormancy could occur in these different plant tissues (Bewley, 1997; Anderson et al., 2001; Suttle, 2000). For instance, it has been shown that thiourea, among other dormancy breaking agents, promoted the germination of lettuce (Lactuca sativa L.) and pigweed (Amaranthus albus L.) seeds (Hendricks, Taylorson, 1975) and increased the bud breaking of grapevine (Vitis vinifera L.) (Nir et al., 1986; Nir, Lavee, 1993), the effect being accompanied by a reduction in CAT activity. As a result of CAT inhibitor treatment, it is expected that H2O2 accumulates, and this was actually shown in grapevine buds (Nir, Lavee, 1993; Pérez, Lira, 2005). Like with potato tubers, the treatment of barley (Hordeum vulgare L.) seeds with H2O2 leads to their dormancy breakage (Fontaine et al., 1994). The fact that different CAT inhibitors on one hand and exogenous H2O2 on the other hand exert similar effects on dormancy release of both seeds and buds (Hendricks,Taylorson, 1975; Nir, Lavee, 1993; Or et al., 2002; Pérez, Lira, 2005) indicates that CAT inhibition and consequently the rise in H2O2 content may be one of the early and relevant events leading to the termination of plant dormancy. This may probably be true for potato tubers in which dormancy was released and sprouting improved by the use of different CAT inhibitors (thiourea, aminotriazole and hydrogen cyanamide) and H2O2 (Figure 3 and Figure 4 and our unpublished data). However, these effects represent only indirect proof of the relationship between the chemical inhibition of CAT and the induction of dormancy breakage since
38– CAT activity was not determined in this part of our work and
39– due to the other possible effects of thiourea treatment.
40In the present work and unlike the above-mentioned ones, CAT inhibition has been realised not only chemically by applying a CAT inhibitor but also genetically by producing transgenic plants. Potato plants partially repressed in their CAT activity were produced using both antisense and sense strategies (Figure 5). In plants, molecular analysis of CAT gene expression has mainly been performed in model species such as Arabidopsis thaliana, N. plumbaginifolia, Oryza sativa and Zea mays. Unlike animals, plants contain multiple CAT isozymes (mainly three, CAT1, CAT2, and CAT3) that are encoded by small gene families (Scandalios, 1990; Willekens et al., 1995; McClung, 1997). These isozymes can be resolved by native gel electrophoresis (McClung, 1997; Lingqiang, Scandalios, 2002; this work), their total number being dependent on multiple factors such as the species, the organ, the developmental stage, and the sensitivity of the staining method. Our genetic inhibition of CAT activity resulted in acceleration of the sprouting kinetics (Figure 6) and in an increased number of sprouts per tuber (Figure 7) of the transgenic tubers compared to those from the wild type. A further characterisation (specific activity of CAT and H2O2 content) of these transgenic lines (except SU39) once again showed that CAT activity was decreased in these lines compared to the wild type, the decrease ranging from 13 to 52% (M’Hamdi, 2004). In addition to CAT inhibition, H2O2 content was increased by 1.8 to 2.5-fold over the wild type, depending on the lines. It appears thus that tuber dormancy and sprouting can be controlled in potato by the manipulation of H2O2 metabolism. Interestingly, a CAT2/CAT3 double mutant from maize (Zea mays L.) also exhibited a higher germination rate than normal lines (Scandalios, 1994). It is noteworthy that positive effects on the tuber sprouting quality, similar to those resulting from the chemical inhibition (experiment 2), are obtained with transgenic potato plants when the repression mainly concerned a precise band in the pattern of CAT activity staining (marked by an arrow, figure 5, top), suggesting a sort of specialisation of potato CAT isoforms. This point represents a specific contribution of the transgenic material in comparison with the chemical inhibition, that is all CAT isoforms may not have a role in the regulation of potato tuber dormancy. According to the isozyme pattern reported for other species (e.g. maize, Lingqiang, Scandalios, 2002), and the fact that the missing band on the isozyme pattern of antisense plants (suppression of a specific CAT subunit activity, CAT2) is quantitatively less intense or even not detectable on the pattern of certain sense plants (suppression of at least the activity of CAT2 due to the co-suppression process) (Chamnongpol et al., 1996), suggests that the band in question could correspond to the homotetramer of the CAT2 subunit. In the wild type material, the lacking band in the transgenic tubers is not exclusively present in « bud/sprout » tissues (see the arrow, figure 2) but was also detected in the (mini)tuber tissues (see the arrow, figure 5), the band being larger in the latter than in the former (our unpublished data). We would like to emphasise that the differences in CAT activity among transgenic lines and wild type potato tubers might be more relevant and more tightly correlated to the length of dormancy if bud tissues were separately analysed. This requires a high number of greenhouse-grown (mini)tubers and represents a future development of our research.
4.2. Possible modes of action of CAT inhibition/H2O2 accumulation on tuber dormancy release and sprouting initiation in potato
41Our results on potato tubers and previous ones on other plant seeds and tree buds (Hendricks, Taylorson, 1975; Nir, Lavee, 1993; Fontaine et al., 1994; Or et al., 2002; Pérez, Lira, 2005) indicate that H2O2 accumulation may play a crucial role in the mechanism of dormancy breakage. However, the relationship between these two processes is still not well understood.
42It has been suggested that CAT inhibitor or exogenous H2O2 treatments induce dormancy breakage by favouring the oxidative pentose phosphate pathway (PPP) (Hendricks, Taylorson, 1975; Nir, Lavee, 1993; Fontaine et al., 1994). In the present work, it is likely that, as in seeds and fruit tree buds, CAT inhibition or exogenous H2O2 application on potato tubers could result in an increase in the level of endogenous H2O2 in bud tissues which might activate the PPP and thus leads to dormancy breakage and initiation of sprouting. However, results of the first part of this work (APX, figure 2) and those not shown here (glutathione reductase activity, glutathione and ascorbate contents) do not plead in favour of the oxidative PPP activation. Additional experiments with PPP inhibitors are thus required in order to clearly elucidate the role played by this important pathway.
43It is possible that the activation of the PPP is only part of the metabolic pathways induced by H2O2 and leading to the termination of dormancy. In fact, two other mechanisms have been suggested for seeds, and may occur in the tuber, to explain the promotion of their germination by H2O2. In the first mechanism, a build up of H2O2 would yield oxygen, used by respiration and other oxidation processes (Roberts, 1969) and by monooxygenases implicated in gibberellin biosynthesis (Fontaine-Roux et al., 1997). The second suggested mechanism may involve a peroxidase interacting with H2O2 to oxidise germination inhibitors (Ching, 1959). Other effects of H2O2 in cellular mechanisms involved in germination cannot, however, be excluded. H2O2 is indeed an important molecule in plants since, on the one hand, its production is dependent on the rate of several major physiological processes and environmental factors, and, on the other hand, the endogenous level of H2O2 modulates the expression of many genes and is involved in the control of growth and differentiation (for review, see Penel, 1997). Although there has been rapid progress in recent years on how ROS control various plant processes, there are still many uncertainties and gaps in our understanding of how H2O2 interacts with hormones during dormancy/sprouting. In a recent review, Bailly (2004) has reported that the control of dormancy by hormones such as ABA and ethylene could be connected to H2O2 signalling and such kind of interplay constitutes a challenge for future research in this area.
44When a stored tuber is transferred to conditions favouring its sprouting, the pattern of the sprout growth will depend on the physiological age of the tuber (Beukema, van der Zaag, 1990). There are five stages of tuber physiological ageing: dormancy (no sprout growth), apical dominance (one sprout growth), multiple sprouting, branching (branched sprout growth), and senility (« little potato » growth). The treatment of dormant potato tubers with CAT inhibitors, including thiourea, have allowed us not only to break dormancy, accelerate sprouting and increase the number of sprouts (Figure 3 and Figure 4), but also to produce branched sprouts and even a direct secondary tuberization when high concentrations are used (unpublished data). This indicates that CAT inhibition may break dormancy (dormancy, apical dominance) and increase the number of sprouts (apical dominance, multiple sprouting) by accelerating tuber ageing. It is known that ageing of tubers is accompanied by a progressive increase in oxidative stress (Kumar, Knowles, 1996) and the use of CAT inhibitors or exogenous H2O2 may accelerate the induction of oxidative stress and consequently advances the physiological age of the tuber. Enhancing physiological ageing of seed potatoes has the potential to substantially improve both total and marketable yields, especially for short-season growing areas (Asiedu et al., 2003). In seeds, accelerated ageing was found to be closely related to a decrease in the activities of detoxifying enzymes, including SOD and CAT (Bailly et al., 1996). It thus seems that dormancy and ageing could be controlled by ROS, including H2O2 and superoxide anions (O2-). This conclusion is substantiated by the transgenic material produced in the course of this work, which showed altered sprouting behaviour and paves the way for novel approaches of post-harvest potato management.
45Acknowledgements
46We acknowledge Prof. D. Inzé (VIB, Gent, Belgium) for providing the molecular constructs used in the genetic transformation. We thank Rouvière C., Martiat JC., Everaert O. and Locicero A. for their excellent technical assistance.
Bibliographie
Anderson JV., Chao WS., Horvath DP. (2001). A current review on the regulation of dormancy in vegetative buds. Weed Sci. 49, p. 581–589.
Asiedu SK., Astatkie T., Yiridoe EK. (2003). The effect of seed-tuber physiological age and cultivar on early potato production. J. Agron. Crop Sci. 189, p. 176–184.
Bailly C. (2004). Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 14, p. 93–107.
Bailly C., Benamar A., Corbineau F., Côme D. (1996). Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plant. 97, p. 104–110.
Beauchamp CO., Fridovich I. (1971). Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal. Biochem. 44, p. 276–287.
Beaujean A., Sangwan RS., Lecardonnel A., Sangwan-Norrel BS. (1998). Agrobacterium-mediated transformation of three economically important potato cultivars using sliced internodal explants: an efficient protocol of transformation. J. Exp. Bot. 49, p. 1589–1595.
Beukema HP., van der Zaag DE. (1990). Introduction to potato production. Wageningen, The Netherlands: Pudoc, 208 p.
Bewley JD. (1997). Seed germination and dormancy. Plant Cell 9, p. 1055–1066.
Bolwell GP. (1996). The origin of the oxidative burst in plants. Biochem. Soc. Trans. 24, p. 438–442.
Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, p. 248–254.
Burton WG. (1989). The Potato (3th ed.). Essex, UK: Longman Scientific & Technical, 742 p.
Chamnongpol S., Willekens H., Langebartels C., Van Montagu M., Inze D., Van Camp W. (1996). Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis-related expression under high light. Plant J. 10, p. 491–503.
Ching TM. (1959). Activation of germination in Douglas fir seeds by hydrogen peroxide. Plant Physiol. 34, p. 557–563.
Coleman WK. (1987). Dormancy release in potato tubers: a review. Amer. Potato J. 64, p. 57–68.
Coleman WK., Hawkins G., Melnerney J., Goddard M. (1992). Development of a dormancy release technology: a review. Amer. Potato J. 69, p. 437–445.
Fontaine O., Huault C., Pavis N., Billard JP. (1994). Dormancy breakage of Hordeum vulgare seeds: effects of hydrogen peroxide and scarification on glutathione level and glutathione reductase activity. Plant Physiol. Biochem. 32, p. 677–683.
Fontaine-Roux O., Billard JP., Gaspar T., Huault C. (1997). Inhibition of germination of barley seeds by inhibitors of gibberellic acid synthesis: reversal by oxygen or hydrogen peroxide. Saussura 28, p. 59–64.
Hemberg T. (1985). Potato rest. In Li PH. (ed.). Potato Physiology. Orlando, USA: Academic Press, p. 353–388.
Hendricks SB., Taylorson RB. (1975). Breaking of seed dormancy by catalase inhibition. Proc. Natl Acad. Sci. USA 72, p. 306–309.
Kumar GNM., Knowles NR. (1996). Oxidative stress results in increased sinks for metabolic energy during aging and sprouting of potato seed-tubers. Plant Physiol. 112, p. 1301–1313.
Laemmli UK. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, p. 680–685.
Lang GA., Early JD., Martin GC., Darnell RL. (1987). Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. Hort. Sci. 22, p. 371–377.
Lingqiang MG., Scandalios JG. (2002). Catalase gene expression in response to auxin-mediated developmental signals. Physiol. Plant. 114, p. 288–295.
M’Hamdi M. (2004). Control of dormancy and sprouting of potato tubers (Solanum tuberosum L. cv. Désirée) by genetic modification of the catalase activity. PhD thesis, Gembloux, Belgium: Agricultural University.
M’Hamdi M., Rouvière C., Rojas-Beltran J., du Jardin P. (2003). Optimization of potato genetic transformation by Agrobacterium tumefaciens using hygromycin resistance as a selective marker. Biotechnol. Agron. Soc. Environ. 7, p. 183–188.
McClung CR. (1997). Regulation of catalases in Arabidopsis. Free Rad. Biol. Med. 23, p. 489–496.
Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, p. 405–410.
Mittler R., Zilinskas BA. (1993). Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal. Biochem. 212, p. 540–546.
Nir G., Lavee S. (1993). Metabolic changes during cyanamide induced dormancy release in grapevines. Acta Hort. 329, p. 271–274.
Nir G., Shulman Y., Fanberstein L., Lavee S. (1986). Changes in the activity of catalase (EC 1.11.1.6) in relation to the dormancy of grapevine (Vitis vinifera L.) buds. Plant Physiol. 81, p. 1140–1142.
Or E., Vilozny I., Fennell A., Eyal Y., Ogrodovitch A. (2002). Dormancy in grape buds: isolation and characterization of catalase cDNA and analysis of its expression following chemical induction of bud dormancy release. Plant Sci. 162, p. 121–130.
Penel C. (1997). Production and roles of hydrogen peroxide. In Greppin H., Penel C., Simon P. (eds.). Travelling shot on plant development. Geneva: University of Geneva, p. 1–20.
Pérez FJ., Lira W. (2005). Possible role of catalase in post-dormancy bud break in grapevines. J. Plant Physiol. 162, p. 301–308.
Reust W. (1986). EAPR working group « Physiological age of the potato ». Potato Res. 29, p. 268–271.
Roberts EH. (1969). Seed dormancy and oxidation processes. In Woolhouse HW. (ed.). Dormancy and survival. New York: Academic Press, p. 161–192.
Rojas-Beltran JA., Dejaeghere F., Abd Alla Kotb M., du Jardin P. (2000). Expression and activity of antioxidant enzymes during potato tuber dormancy. Potato Res. 43, p. 383–393.
Scandalios JG. (1990). Response of plant antioxidant defense genes to environmental stress. Adv. Genet. 28, p. 1–41.
Scandalios JG. (1994). Regulation and properties of plant catalases. In Foyer CH., Mullineaux PM. (eds.). Causes of photooxidative stress and amelioration of defence systems in plants. Boca Raton, USA: CRC Press, p. 275–315.
Suttle JC. (1998). Involvement of ethylene in potato microtuber dormancy. Plant Physiol. 118, p. 843–848.
Suttle JC., Hultstrand JF. (1994). Role of endogenous abscisic acid in potato microtuber dormancy. Plant Physiol. 105, p. 891–896.
Suttle JC. (2000). The role of endogenous hormones in potato tuber dormancy. In Viémont JD., Crabbé J. (eds.). Dormancy in plants: from whole plant behaviour to cellular control. New York: CABI Publishing, p. 211–226.
Suttle JC. (2004a). Involvement of endogenous gibberellins in potato tuber dormancy and early sprout growth: a critical assessment. J. Plant Physiol. 161, p. 157–164.
Suttle JC. (2004b). Physiological regulation of potato tuber dormancy. Amer. J. Potato Res. 81, p. 253–262.
Van Ittersum MK., Aben FCB., Keijzer CJ. (1992). Morphological changes in tuber buds during dormancy and initial sprout growth of seed potatoes. Potato Res. 35, p. 249–260.
Wang SY., Jiao HJ., Faust M. (1991). Changes in ascorbate, glutathione, and related enzyme activities during thidiazuron-induced bud break of apple. Physiol. Plant. 82, p. 231–236.
Warm E., Laties GG. (1982). Quantification of hydrogen peroxide in plant extracts by the chemiluminescence reaction with luminal. Phytochemistry 21, p. 827–831.
Willekens H., Inze D., Van Montagu M., van Camp W. (1995). Catalases in plants. Mol. Breed. 1, p. 207–228.
Wiltshire JJJ., Cobb AH. (1996). A review of the physiology of potato tuber dormancy. Ann. Appl. Biol. 129, p. 553–569.
Om dit artikel te citeren:
Over : Mohammed Bajji
Faculté universitaire des Sciences agronomiques de Gembloux. Unité de Biologie végétale. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Mahmoud M’Hamdi
Faculté universitaire des Sciences agronomiques de Gembloux. Unité de Biologie végétale. Passage des Déportés, 2. B-5030 Gembloux (Belgium). Ecole supérieure d’Agriculture du Kef. 7119 Kef (Tunisia).
Over : Frédéric Gastiny
Faculté universitaire des Sciences agronomiques de Gembloux. Unité de Biologie végétale. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Jorge A. Rojas-Beltran
Faculté universitaire des Sciences agronomiques de Gembloux. Unité de Biologie végétale. Passage des Déportés, 2. B-5030 Gembloux (Belgium). Universidad Mayor de San Simon, Final Jordan (Edificio multiacademico). Cochabamba (Bolivia).
Over : Patrick du Jardin
Faculté universitaire des Sciences agronomiques de Gembloux. Unité de Biologie végétale. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: dujardin.p@fsagx.ac.be






