- Startpagina tijdschrift
- Volume 15 (2011)
- numéro 4
- Effect of liquid media culture systems on yam plant growth (Dioscorea alata L. ‘Pacala Duclos’)
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Effect of liquid media culture systems on yam plant growth (Dioscorea alata L. ‘Pacala Duclos’)

Nota's van de redactie
Received on June 28, 2010; accepted on January 11, 2011
Résumé
Effet des systèmes de culture en milieu liquide sur la croissance des plants d'igname (Dioscorea alata L. ‘Pacala Duclos’). Le type de système de culture en milieu liquide influence la croissance des plants d’igname (clone ‘Pacala Duclos’). Dans des systèmes de culture avec renouvellement forcé de l’atmosphère interne des flacons de culture, le Système d’Immersion Temporaire (SIT) et le Système d’Immersion Constante (SIC), dont l’aération est assurée par la formation continue de bulles dans le milieu liquide, de meilleurs résultats furent observés au niveau des indicateurs morphologiques et physiologiques des plantes en comparaison avec des plantes provenant de systèmes de culture avec renouvellement passif de l’atmosphère interne en flacons de culture ou Système Liquide Statique (SLS). Dans le Système d’Immersion Temporaire, les meilleurs résultats furent obtenus après six semaines de culture et concernaient la longueur totale (20,8 cm), le nombre de bourgeons axillaires (8,6), le poids frais (2,1 g) et le poids sec (0,18 g) par plante, ainsi que le contenu en pigments photosynthétiques (chlorophylle a, b et totale), la photosynthèse nette (15,3 μmol C02.m-2.s-1), la transpiration totale (5,97 mmol H2O.m-2.s-1), la conductance stomatique (457 μmol H2O.m-2.s-1) et le contenu en amidon des feuilles (45,77 mg.gMF-1). Le sucre réducteur dans le milieu de culture avec le Système d’Immersion Temporaire a été complètement épuisé, et les nutriments minéraux en moindre quantité (phosphore, azote, magnésium, calcium, fer et manganèse) dans le milieu de culture de ce système ont pu être mis en relation avec la croissance de la plante. Les résultats de ce travail pourraient contribuer au développement d’un protocole pour la propagation in vitro de ce clone d’igname.
Abstract
The culture system type in liquid media influences yam plant growth (‘Pacala Duclos’ clone). In culture systems with forced renewal of internal atmosphere in culture flasks, Temporary Immersion System (TIS) and Constant Immersion System (CIS) with aeration through continuous bubbling in culture medium, higher results were obtained in morphological and physiological plant indicators in comparison with plants obtained in culture systems with passive renewal of internal atmosphere in culture flasks or Static Liquid System (SLS). In Temporary Immersion System, the best results were obtained after six weeks of culture in relation to total length (20.8 cm), axillary bud number (8.6), fresh weight (2.1 g) and dry weight (0.18 g) per plant, as well as photosynthetic pigment content (chlorophyll a, b, and total), net photosynthesis (15.3 μmol C02.m-2.s-1), total transpiration (5.97 mmol H2O.m-2.s-1), stomatal conductance (457 μmol H2O.m-2.s-1) and leaf starch content (45.77 mg.gMF-1). Reducing sugar in culture medium with Temporary Immersion System was completely depleted, and mineral nutrients of lower contents (phosphorus, nitrogen, magnesium, calcium, iron, and manganese) in culture medium from this culture system could be related with plant growing. The results of this work could contribute to develop protocol for in vitro plant propagation of this yam clone.
Inhoudstafel
1. Introduction
1Yam (Dioscorea spp.) crop has a wide range of uses in human life: staple food (as fresh consumption and processed products), animal food, and as raw material for industrial purposes (Tamiru et al., 2008). However extensive crop development in the principal yam producing countries of Central America and the Caribbean has been limited mainly because of lacking physiological, genetic, and healthy planting material.
2In vitro plant propagation may help to overcome constraints related with the availability of high quality planting material (Wheatley et al., 2003; Vaillant et al., 2005; Rodriguez, 2006). However almost all in vitro propagation protocols developed in yam up to now have shown erratic and low plant growth from nodal segments due to the use of semisolid culture media, conventional small-size culture flasks, and missing knowledge on the morpho-physiological development of in vitro plants (Malaurie et al., 1995; Medero et al., 1999; Chu et al., 2002; Borges et al., 2004; Balogun, 2009). So it has been necessary to look for alternatives with nodal segment explants in liquid media culture systems for plant growth, but little is known about the effect of these culture systems on in vitro plant physiology and morphogenesis (Cabrera et al., 2008).
3This work was aimed at knowing the effect of liquid media culture system on yam plant growing (clone ‘Pacala Duclos’) and to determine in temporary immersion systems the nutrient depletion and preference in culture media, and to relate them with plant growth.
2. Materials and methods
2.1. Plant material
4Plants of ‘Pacala Duclos’ (Dioscorea alata L.) clone were cultivated in a culture medium containing inorganic salts and vitamins as proposed by Murashige and Skoog (1962) (MS). Cistein (20 mg.l-1), sucrose (30 g.l-1) and agar-E at a final concentration of 5 g.l-1 were added to culture medium. Nodal segments showing axillary buds were obtained from in vitro plants in the third subculture.
5The conditions of cultivation were a temperature of 25 ± 2°C and artificial illumination by means of white fluorescent (Sylvania, Daylight F40T12/D 40 W) lamps that provided an intensity of 60 µmol.m-2.s-1. The photoperiod corresponded to 16 h of light and 8 h of darkness.
2.2. Semi-automated culture systems
6Temporary Immersion System (TIS). A setup of two 10 l flasks (Clearboys, Nalgene Company, USA), one serving as culture container and the other one as medium reservoir, was used. Flasks were connected with 6.0 mm silicone hoses passing through the cap down to the containers’ bottoms. A flux of culture medium between the containers was controlled by two three-way electro valves. A programmable timer controlled immersion time and frequency. Hydrophobic filters (0.22 µm, MIDISART 2000, SARTORIUS Company) were fitted to each flask to ensure air sterility. Air pressure (1 kg.cm-2) from a compressor was regulated by a manometer. A 10-minute immersion time and an immersion frequency of 8 immersions per day were used.
7Constant Immersion System with aeration through continuous bubbling in culture medium (CIS). This culture system consisted of a 10 l culture flask (Clearboys, Nalgene Company, USA) in which nodal segments and culture medium were placed. Air coming from a compressor flew through 6.0 mm silicone tubes down to the bottom of the container. Hydrophobic filters (0.22 µm, MIDISART 2000, SARTORIUS Company) were placed at the entrance and outlet of the culture flask to ensure air sterility. A compressor ensured a 25 ml.min-1 constant air flow.
8Static Liquid culture System medium (SLS). A 10 l culture flask (Clearboys, Nalgene Company, USA) was used to grow nodal segments in a static liquid culture medium. Culture flasks were closed with caps, and hydrophobic filters (0.22 µm, MIDISART 2000, SARTORIUS Company) were fitted at the entrance and outlet of connectors to guarantee air sterility.
9One hundred nodal segments were placed, each with an axillary bud, and a volume of 30 ml culture medium per nodal segment in each system of studied cultivation, for a total volume of 3 l culture medium. Experiments were repeated five times for each culture system.
2.3. Evaluation of morphological and physiological markers
10After culturing for six weeks, 20 plants in each repetition per treatment were selected at random, and the following parameters were evaluated: total plant length (cm), axillary bud number per plant, and fresh (gFW) and dry weight (gDW) using an analytic balance (SARTORIUS). Before determining dry weight, plants were placed in a drying oven (SUTJESKA) at 70°C during 48 h. Besides the total plant number with hyperhydricity symptoms per replication was obtained in each culture system.
11In order to determine the photosynthetic pigment content, 10 g of fresh leaves were taken. Three samples with three repetitions per treatment, thus a total of nine values, were considered. Chlorophylls a and b were extracted according to the protocol described by Porra (2002). Chlorophylls a, b, and total were counted following a procedure described by Lichtenthaler (1987).
12In order to measure the photosynthetic, transpiration, and stomatal conductance activities, the youngest and completely expanded leaves from in vitro yam plants were used. Measurements were obtained with a CIRAS-2 (Portable Photosynthesis System, Europe, PP Systems, and UK) coupled to an universal tray (PLC6) 4 h after starting the photoperiod treatment. The tray area was completely covered with the youngest and totally expanded leaves (2.5 cm2). As yam plant is a C3 plant, a value of photo-synthetically active radiation (PAR) of 600 W.m-2 was chosen. Regarding carbon dioxide concentration and relative humidity, environmental values of 375 µmol.mol-1 and 90% were considered respectively. Measurements were carried out in five plants with 10 repetitions for a total of 50 values. In the case of photosynthesis, values were given in µmol CO2.m-2.s-1, transpiration in mmol H2O.m-2.s-1, and the stomatal conductance in µmol H2O.m-2.s-1.
13To determine leaf starch concentration, 1 g of fresh weight was ground in liquid nitrogen using a mortar and a pestle. Three samples with three repetitions per treatment for a total of nine values were taken. Sugars were extracted in 5 ml ethanol at 80%. Extracts were centrifuged during 20 min at 10,000 g, 4°C to the vacuum in centrifuge Beckman (JB-21). Resultant pellets were resuspended in KOH 0.2 mol.l-1 promoting the alkaline hydrolysis during 12 h at 90°C. Later dissolving was carried out in sodium citrate with the β-amilo glycosidase (EC 3.2.1.3) (SIGMA) because of its enzymatic degradation according to a protocol described by Thomas et al. (1983). Starch was quantified using a calibration curve of potato starch (SIGMA). Values were expressed in mg.gMF-1.
2.4. Reducing sugar determination and mineral nutrients in culture medium in Temporary Immersion System
14The responses from in vitro plant growth in temporary immersion systems were related with final reducing sugar contents and mineral nutrients in culture medium, and so, the following evaluations of each culture media were carried out: final reducing sugar concentration and mineral nutrient contents (N, P, K, Ca, Mg, Fe, Cu, Mn and Zn) at the beginning and at the end of plant growth.
15To determine reducing sugars in culture medium, a protocol described in the manual of analytical techniques, ICIDCA (1974), was applied.
16In relation to ion contents in culture medium, nitrogen (N) was determined with the Nessler´s calorimetric method, and phosphorus following the calorimetric method of ammonium metavanadate, according to the regulations from NRAG 564 (1982). Samples were read in a spectrophotometer Spekoll 11.
17Potassium, calcium, magnesium, iron, copper, cobalt, and manganese were analyzed by dilution, based on the methodology of atomic-absorption spectrometry (Smith et al., 1972). Readings were taken in an atomic-absorption SP-9 Pye Unicam spectrometer.
18The data relating to total plant length (cm), axillary bud number per plant, fresh weight (gFW) and dry weight (gDW) were statistically analyzed with a non-parametric test of Kruskall Wallis. Chlorophyll a, b, and total content means, photosynthetic activity, stomatal conductance, transpiration, and leaf starch concentration were compared with a simple variance analysis, and Turkey’s test was applied.
3. Results and discussion
3.1. Evaluation of morphological and physiological markers
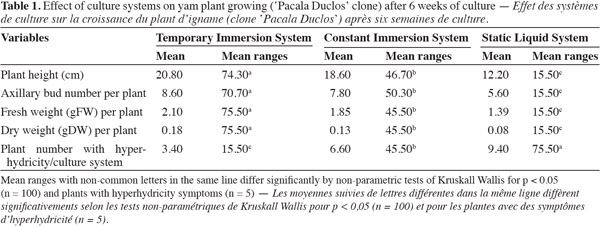
19The type of cultivation system influenced significantly the growth of yam nodal segments. In the cultivation systems with forced renovation of the internal atmosphere of the cultivation (TIS and CIS) flask, superior results were obtained in the morphological indicators of the plants in comparison with those that were cultivated in the cultivation system with passive renovation of the internal atmosphere of the cultivation (SLS) flask. After six weeks of cultivation in TIS the highest values were obtained for total length, axillary bud number, fresh weight and dry weight per plant, and significant differences appeared as compared with plants growing in CIS and SLS (Table 1). The plants cultivated in SIT were characterized by an intense green coloration and featured expanded and well developed leaves (Figure 1).

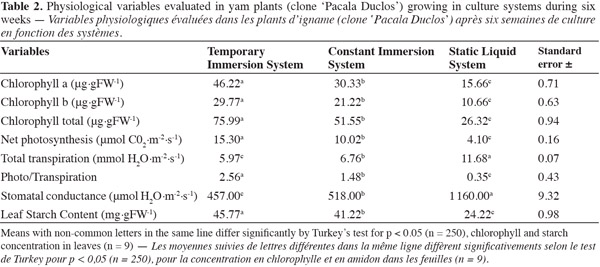
20Evaluating the different physiological variables made it possible to corroborate the development response of yam plants from TIS in comparison with plants cultivated in the other culture systems. In culture systems with forced renewal of internal culture flask atmosphere (TIS and CIS), results were better in physiological markers in comparison with plants grown in culture systems with passive renewal of internal culture flask atmosphere (SLS). The best results in relation to photosynthetic pigment content (chlorophyll a, b, and total), net photosynthesis, total transpiration, net photosynthesis relation and total transpiration, stomatal conductance, and leaf starch content were obtained after six weeks of culturing in TIS (Table 2).
21The highest photosynthetic activity of plants from TIS and consequently the possibility of inducing greater photo-mixotrophic growth can be explained by the functioning of this culture system type. Escalona et al. (2007) pointed out that the periodic renovation of the internal atmosphere of the cultivation flask and the intermittent contact of the plants with culture medium in SIT resulted in the plants improving their relationship between photosynthesis and transpiration. These cultivation conditions allowed the cultivated yam plants in TIS to present better stomatal functionality and relationship regarding the movement of gases with the entrance of CO2 and the exit of water, which was reflected in a better relationship between photosynthesis and transpiration in comparison with the plants cultivated in the other cultivation systems.
22According to Kozai et al. (2005) the culture conditions for photo-mixotrophic growing may induce chlorophyll synthesis in leaves from in vitro plants. Therefore the leaves of yam plants cultivated in TIS had a higher photosynthetic pigment content at the end of the growing stage compared with plants cultivated in any of the above-mentioned culture systems.
23Aragon (2005) analyzed the chlorophyll content (a, b, and total) and its relation with photosynthesis during the rooting stage of plants from ‘CEMSA ¾’ clone in TIS under photo-mixotrophic conditions, high illumination (250 µmol.m-2.s-1), and high CO2 concentration (1,200 µmol.mol-1). Plants with more chlorophyll a (63.54 µg.gMF-1), b (35.67 µg.gMF-1), and total (99.21 µg.gMF-1) exhibited higher net photosynthesis values (2.41 µmol CO2.m-2.s-1) and less total transpiration (1.53 mmol H2O.m-2.s-1).
24On the other hand, apple sprouts (Malus domestica Borkh.) were compared in continuous immersion bioreactors and in temporary immersion bioreactors by Chakrabarty et al. (2006). Sprouts cultivated in temporary immersion bioreactors showed higher chlorophyll a and b, fluorescence of chlorophyll, and net photosynthesis. Sprouts also presented higher fresh weight, dry weight, and length, and less hyperhydricidity.
25Periodical renewal of the internal culture flask atmosphere in TIS and the intermittent contact of plants with culture medium made it possible to improve relations between photosynthesis and transpiration. These culture conditions caused yam plants cultivated in TIS to feature better stomatal conductance and a better relation according to gas movement with CO2 entrance and water output, and this performance is reflected in a better relation between photosynthesis and transpiration in comparison with plants cultivated in other culture systems.
26According to Afreen (2005) the lowest conductance values registered in the leaves of plants cultivated in TIS in comparison with leaf values of plants cultivated in CIS and SLS were a sign of better integrality in leaf structural development. This suggested that the mesophyll had more defined parenchyma palisade tissues, higher dimension in the intercellular spaces, higher chloroplast content, better epidermal cell development, and that epicuticular wax was present. Besides their stomas were more functional, as they would have permitted enough CO2 to enter while water was retained as much as possible. The higher morphological development in leaves of plants cultivated in TIS made them more functional from the physiological point of view which was reflected in a better relation between net photosynthesis and total transpiration. The highest starch content in yam plant leaves was also observed in TIS. As is well known, the starch accumulated in leaves of in vitro plants may generate precursors due to the hydrolysis of sucrose in culture medium (Kozai et al., 2005) and to the carbonated skeletons formed through photosynthesis that may cause hexosas to become starch (Mendrano et al., 2001). In this case, the higher photosynthetic activity and the better functional mechanism to avoid unnecessary water losses in yam plants cultivated in TIS favored the highest accumulation of this compound with regard to other treatments. Researchers such as Aragon et al. (2009) determined that in plantain “CEMSA ¾” in TIS, the photomixotrophic growing conditions stimulated the carbohydrate accumulation in the starch content.
27The culture conditions created in TIS resulted in the best plant growing in yam. According to Berthouly et al. (2005) and Escalona (2006), factors are combined to allow an intermittent contact of culture medium with plant material, so as to make possible an efficient nutrient contribution and a periodical renewal of the internal atmosphere in culture flasks. They have been able to increase plant growing and sprout proliferation in crops such as Coffea arabica L. and Coffea canephora L., pineapple (Ananas comosus L.), sugar cane (Saccharum officinarum L.), Eucalyptus grandis L., Anthurium andraeanum Schott, and in other Araceae species in comparison with protocols developed for these crops in culture media in semi-solid or static liquid state. The previous results permitted to explain why yam plants cultivated in TIS with higher chloropyll content (a, b, and total), higher photosynthetic activity, lower transpiration and stomatal conductance, as well as a better relation photosynthesis / transpiration, showed a better response on morphological indicators, such as total plant length, axillary bud number, fresh and dry weight per plant in comparison with plants growing in CIS and SLS.
3.2. Reducing sugar determination and mineral nutrients in culture medium in Temporary Immersion System
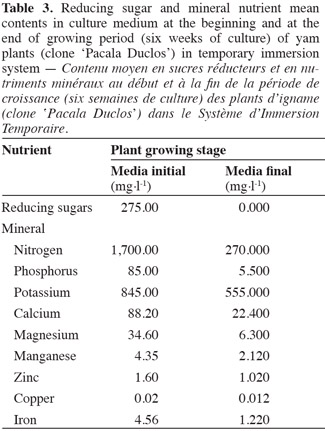
28At the end of the 6-week plant growth period, the reducing sugars were completely depleted in the culture medium (Table 3). These results raise the question whether or not this compound turned into a limiting factor for plant development or influenced to a certain extent the photosynthetic activity recorded. Kozai et al. (2005) stated that plants taking exogenous carbohydrates from culture medium or endogenous carbohydrates produced by photosynthesis for growing showed a photomixotrophic metabolism, a process which could have occurred in yam plants cultivated in TIS.
29Similar results in relation to photomixotrophic metabolism have been described in this culture system in crops such as pineapple by Escalona et al. (2003). Although sprouts were developed at high luminous intensity (225 µmol.m-2.s-1) and they carried out photosynthesis, in vitro plant growing depended more on reducing sugars and mineral nutrients from culture medium than on photosynthesis. However Aragon (2005) stated that in vitro plantain plants cultivated in temporary immersion bioreactors presented photomixotrophic growing, although not all the sugar in culture medium was depleted, and a high net photosynthesis (13.60 µmol CO2.m-2.s-1) under culture conditions of high light intensity and high CO2 concentration were shown.
30At the end of the growing period, the mineral nutrients with lower contents in culture medium in relation to initial values were: phosphorus, nitrogen, magnesium, calcium, iron, and manganese, while mineral nutrients such as potassium, copper, and zinc were maintained above 50% of the initial values (Table 3).
31Different metabolic processes and functions in plant cells, where mineral nutrients of lower contents in culture medium are involved, may be related with the growth experimented by plants cultivated in TIS.
32George et al. (2008) explained that phosphorus participates in the energetic metabolism and cell biosynthesis taking part in the glucose–6–phosphate and adenosinetriphosphate (ATP), in membrane stability, and is considered as an activator of adenosine diphosphate carboxylase enzyme, the most important enzyme involved in starch synthesis. This element also takes part in vital metabolic processes, such as photosynthesis and respiration. Nitrogen participates in the complex organic molecule synthesis, such as amino acids, proteins, and porphirine, which is a molecule from so important compounds since the metabolic point of view, as chlorophyll and in essential enzymes of the cytochrome group for photosynthesis and respiration. Besides the authors have also stated that calcium is involved in the structure and physiological properties of cellular membranes, and in the middle lamella of cell wall. Potassium takes part in the synthesis and metabolism of complex carbohydrates, because of its regulatory effect on several enzymes involved in carbon metabolism. Magnesium is also involved in the chlorophyll molecules of pigment systems I and II involved in photosynthesis.
33Although Mn, Zn, Cu, and Fe mineral nutrients showed less variation in respect to initial concentrations in culture medium, Chajer et al. (2008) have also pointed out their roles as activators and regulatory cofactors of several enzymes that could have been involved in the growing of yam plants.
4. Conclusion
34Liquid media culture systems influenced the growing of yam plants (clone ‘Pacala Duclos’). Plants grown in TIS during six weeks showed the best results in the morphological indicators such as total length, axillary bud number, fresh weight and dry weight per plant, and in the physiological indicators evaluated, such as photosynthetic pigment content (a, b, and total chlorophyll), net photosynthesis, total transpiration, relation to net photosynthesis and total transpiration, stomatal conductance, and leaf starch content. Reducing sugars in culture medium of TIS were depleted, and the mineral nutrients with lower contents in culture medium in this culture system could be compared with plant growing.
35Acknowledgements
36We would like to thank Dr. Carlos E. Aragon and Dr. Justo Gonzalez Olmedo from Bio-plant Centre, Ciego de Avila University for their advisory services and evaluation of physiological variables and to Lic. Ramón Perez for translating the article into English language.
Bibliographie
Afreen F., 2005. Physiological and anatomical characteristics of in vitro photoautotrophic plants. In: Hvoslef-Eide A.K. & Preil W., eds. Liquid culture systems for in vitro plant propagation. Dordrecht, The Netherlands: Springer, 62-90.
Aragón C.E., 2005. Estudio de los principales indicadores del metabolismo del carbono en plantas de plátano (CEMSA ¾) propagadas en Biorreactores de Inmersión Temporal y durante su posterior aclimatización. Tesis para optar por el grado académico de Master en Biotecnología Vegetal: Universidad de Ciego de Ávila, Centro Bioplantas (Cuba).
Aragón C. et al., 2009. Effect of sucrose, light and carbon dioxide on plantain micropropagation in temporary immersion bioreactors. In Vitro Cell. Dev. Biol. Plant, 41, 550-554.
Balogun M.O., 2009. Microtubers in yam germplasm conservation and propagation: the status, the prospects and the constraints. Biotechnol. Mol. Biol. Rev., 4(1), 1-10.
Berthouly M. & Etienne H., 2005. Temporary immersion systems: a new concept for use liquid medium in mass propagation. In: Hvoslef-Eide A.K. & Preil W., eds. Liquid culture systems for in vitro plant propagation. Dordrecht, The Netherlands: Springer, 165-195.
Borges M., Meneses S., Aguilera N. & Vázquez J., 2004. Regeneration and multiplication of Dioscorea alata germplasm maintained in vitro. Plant Cell Tissue Organ Cult., 76(1), 87-89.
Cabrera M. et al., 2008. Multiplicación de segmentos nodales del clon ñame ‘Blanco de Guinea’ (Dioscorea cayenensis - D. rotundata) en sistemas de cultivo semi-automatizados. Rev. Colomb. Biotecnol., 10(2), 97-103.
Chajer A.K., Sharma M., Kachhwaha S. & Kothari L., 2008. Micronutrient optimization results into highly improved in vitro plant regeneration in kodo (Paspalum scrobiculatum L.) and finger (Eleusine coracana (L.) Gaertn.) millets. Plant Cell Tissue Organ Cult., 94, 105-112.
Chakrabarty D. et al., 2006. The dynamics of nutrient utilization and growth of apple root stock ‘M9 EMLA’ in temporary versus continuous immersion bioreactors. Plant Growth Regul., 43, 184-189.
Chu P.E. & Ribeiro R.C.L.F., 2002. Growth and carbohydrate changes in shoot cultures of Dioscorea species as influenced by photoperiod, exogenous sucrose and cytokinin concentrations. Plant Cell Tissue Organ Cult., 70, 241-249.
Escalona M., 2006. Temporary immersion beats traditional techniques on all fronts. In: Prophyta. The annual 2006. Roelofarendsveen, The Netherlands: Prophyta Foundation; Velsen-Zuid, The Netherlands: Blue Bird Publishers, 48-49.
Escalona M., Samaon G., Borroto C. & Desjardins Y., 2003. Physiology of effects of temporary immersion bioreactors on micropropagated pineapple plantlets. In Vitro Cell Dev. Biol. Plant, 39, 651-656.
Escalona M. et al., 2007. Physiology of effects of temporary immersion bioreactor (TIB) on micropropagated plantlets. Acta Hortic., 748, 95-101.
George E.F. & Klerk G.J., 2008. The components of plant tissue culture media I: macro- and micro-nutrients. In: George E.F. et al., eds. Plant propagation by tissue culture. 3rd ed. Dordrecht, The Netherlands: Springer, 65-113.
ICIDCA, 1974. Manual de Técnica Análitica. La Habana: ICIDCA.
Kosai T. & Kubota C., 2005. Unit and terminology use for the studies of photoautotrophic micropropagation. In: Kozai T., Afreen F. & Zobayed S.M.A., eds. Photoautotrophic (sugar-free medium) micropropagation as a new propagation and transplant production system. Dordrecht, The Netherlands: Springer, 1-5.
Lichtenthaler H.K., 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol., 148, 350-382.
Malaurie B., Pungu O. & Trouslot M., 1995. Effect of growth regulators concentrations on morphological development of meristem tips in Dioscorea cayenensis-D. rotundata complex and D. praehensilis. Plant Cell Tissue Organ Cult., 41, 229-235.
Medero V., Del Sol L. & García M., 1999. Metodología para la propagación del clon de ñame Blanco o Pelú. In: BioCat 99. Taller Caribeno de Biotecnologia Vegetal: resúmenes, 5-7 de Octubre, Granma, Cuba. Bayamo, Cuba: Centro de Estudios de Biotecnologia Vegetal, 12.
Mendrano H. & Flexas J., 2001. Fijación de dióxido de carbono y biosíntesis de fotoasimilatos. In: Azcon-Bieto J. & Talon M., eds. Fundamentos de fisiología vegetal. 1ra ed. Barcelona, Spain: McGraw-Hill, 173-187.
Murashige T. & Skoog F., 1962. A revised medium for rapid growth and biossays with tobacco tissue culture. Physiol. Plant., 15, 473-497.
NRAG, 1982. Análisis foliar. Ciudad de La Habana, Cuba: AGRINFOR, Ministerio de la Agricultura, 10-32.
Porra R.J., 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res., 73, 149-156.
Rodríguez S., 2006. Evaluación y recomendación de clones de boniato, yuca, ñame, plátanos y bananos resistentes o tolerantes a los factores adversos de la producción (FAP) y su manejo integrado. Cuba: Programa Nacional Científico Técnico, 3-67.
Smith D.L. & Schrenk W.G., 1972. Atomic Absorption Spectrophometry (AAS). Assoc. Off. Analytical Chemists J., 55, 669.
Tamiru M., Becker H.C. & Maass B.L., 2008. Diversity, distribution and management of yam landraces (Dioscorea spp.) in Southern Ethiopia. Genet. Resour. Crop Evol., 55, 115-131.
Thomas W., Rufty J. & Steven C., 1983. Changes in starch formation and activities of sucrose phosphate synthase and cytoplasmic fructose-1-6-bisphosphatase in response to source-sink alterations. Plant Physiol., 72, 474-480.
Vaillant V., Bade P. & Constant C., 2005. Photoperiod affects the growth and development of yam plantlets obtained by in vitro propagation. Biol. Plant., 49(3), 355-359.
Wheatley A.O., Ahmad M.H. & Asemota H.N., 2003. Development of salt adaptation in vitro greater yam (Dioscorea alata) plantlets. In Vitro Cell Dev. Biol. Plant, 39, 346-353.
Om dit artikel te citeren:
Over : Manuel Cabrera Jova
Instituto Nacional de Investigaciones en Viandas Tropicales (INIVIT). Apartado 6. Santo Domingo CP 53000. C-Villa Clara (Cuba). E-mail: ernestoe@inivit.cu
Over : Rafael Gómez Kosky
Universidad Central “Marta Abreus” de Las Villas. Instituto de Biotecnología de las Plantas. Carretera a Camajuaní km 5. Santa Clara. C-Villa Clara (Cuba).
Over : Ernesto Espinosa Cuellar
Instituto Nacional de Investigaciones en Viandas Tropicales (INIVIT). Apartado 6. Santo Domingo CP 53000. C-Villa Clara (Cuba). E-mail: ernestoe@inivit.cu






