- Accueil
- Volume 11 (2007)
- numéro 3
- Composition of Xylopia aethiopica (Dunal) A. Rich essential oils from Cameroon and identification of a minor diterpene: ent-13-epi manoyl oxide
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Composition of Xylopia aethiopica (Dunal) A. Rich essential oils from Cameroon and identification of a minor diterpene: ent-13-epi manoyl oxide

Notes de la rédaction
Received on 22 August 2006, accepted on 3 July 2007.
Résumé
Composition des huiles essentielles de Xylopia aethiopica (Dunal) A. Rich du Cameroun et identification d’un diterpène : ent-13-epi manoyl oxyde. L’huile essentielle des infrutescences de Xylopia aethiopica (Annonacée), de quatre localités du Cameroun, a été analysée par GC/MS et GC/FID. Plus de soixante composés ont été identifiés avec 47,5–84,0 % de monoterpènes hydrocarbonés, principalement le b-pinène et un mélange de b-phellandrène et de 1,8-cinéole, 6,5–12,9 % de monoterpènes oxygénés, 13,8–30,4 % de sesquiterpènes, et 0,4–0,6 % d’un diterpène non identifié. Les essais de purification sur colonne chromatographique et des analyses par GC/MS et RMN ont permis d’identifier ce diterpène comme étant le ent-13-epi manoyl oxyde qui est rapporté pour la première fois dans l’huile essentielle des fruits de X. aethiopica.
Abstract
Xylopia aethiopica (Annonaceae) essential oil was extracted from fruits collected in four localities in Cameroon, and analysed by GC/MS and GC/FID. More than sixty compounds were identified with 47.5–84.0% of monoterpenes hydrocarbon, mainly b-pinene and b-phellandrene+1,8-cineole, 6.5–12.9% of oxygenated monoterpenes, 13.8–30.4% of sesquiterpenes, and 0.4–0.6 % of a minor unidentified diterpene. Trials of purification by column chromatography, followed by GC/MS and NMR analysis led to the identification of ent-13-epi manoyl oxide which is reported for the first time as a minor component in X. aethiopica essential oil.
Table des matières
1. Introduction
1Xylopia aethiopica is a tree of more than 20 m of height and 60–75 cm of diameter which grows in the forest zone and especially along the rivers in arid areas. The fruit is a slightly hooked cylindrical pod reaching 2–3 mm in width. The mature fruits of green colour take a brown-black coloration after drying and are used as spices (often instead of pepper), in traditional medicine (against the flu, the bronchitis and the dysentery).
2Several studies are reported on biological activity of X. aethiopica. Fruit powder, its essential oil (Okonkwo, Okoye, 1996; Ngamo et al., 2001; Kouninki et al., 2005) or leave essential oil (Asawalam et al., 2006) can be used against cowpea bruchid Callosobruchus maculatus (Fab.) (Coleoptera: bruchidae) or maize weevil Sithophylus zeamais Motsch. (Coleoptera: curculionidae). X. aethiopica is also active against the termites and other bugs who tackle wood (Ladjide et al., 1995). The microbiological activity of X. aethiopica essential oil against Escherichia coli, Staphylococcus aureus or Aspergillus flavus, among other microorganisms, has been well established (Tatsadjieu et al., 2003; Asekun, Adeniyi 2004; Konnings et al., 2004).
3Among the compounds that confer to X. aethiopica its biologic properties one can mention, the diterpenes belonging to the kauranes, the trachylobanes and the kolovanes families (Hasan et al., 1982; Harrigan et al., 1994). It is also noticed that the features of the ether extract of X. aethiopica are favorable to its incorporation in the resins used for the manufacture of the paintings (Ajiwe et al., 1998).
4The composition of fruit essential oil of X. aethiopica given in the literature, shows that it is constituted of monoterpenes hydrocarbon. These compounds are represented mainly by b-pinene 37.0–40.5% (Tomi et al., 1996), 12.0–42.0% (Ayedoun et al., 1996), 18.3% (Jirovetz et al., 1997) 9.9–19.1% (Keita et al., 2003) or by sabinene 36.0% according to Poitou et al. (1996). Germacrene D is the most important sesquiterpene and the oxygenated compounds are mainly the 1,8-cineole and the terpinen-4-ol. A survey undertaken on X. aethiopica essential oil from Egypt showed very particular composition with more than two third of oxygenated compounds 23.4% of terpinen-4-ol, 16.3% of 1,8-cineole and 11.1% of a-terpineol (Karawya et al., 1979). A similar composition with oxygenated monoterpenes (15.1% of 1,8-cineole, 6.6% of terpinen-4-ol) has been reported in essential oil from Nigeria (Asekun, Adeniyi, 2004).
5The aim of the present work is to study the composition of X. aethiopica essential oil from four localities in Cameroon, and to emphasize the occurrence of a diterpene never mentioned in X. aethiopica essential oil which appears to be ent-13-epi manoyl oxide.
2. Experimental
2.1. Essential oil extraction
6Dried fruits of X. aethiopica were obtained from four localities: Bafoussam, Douala, Ngaoundere and Yaounde.
7They were ground and subjected to hydrodistillation during four hours using a Clevenger-type (Figure 1) apparatus. Yellowish essential oils were then obtained.
2.2. Extraction and purification of a natural diterpene
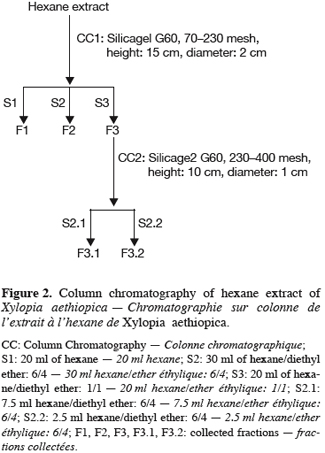
8The isolation of enriched fractions containing a natural diterpene were carried out on n-hexane extracts from ground fruits since its content in the essential oil is too low (<1%). Ground fruits (30 g) were mixed with 200 ml of n-hexane and the raw extract (300 mg) was fractionated by successive column chromatography purifications with silica gel G60 (5% water content). Several preliminary trials have been undertaken and the optimized protocol is summarized in figure 2.
2.3. Gas chromatography and gc-ms analyses
9GC analyses were performed on an Agilent 6890 series apparatus fitted with a split/splitless injector (splitless mode). The operating conditions were as follows: 30 m*0.25 mm HP 5MS (crosslinked 5% phenyl dimethylsiloxane), film thickness: 0.25 µm, temperature programme: from 40°C–230°C at 5°C/min with a final hold of 5 min. at 280°C. Helium at 49.9 KPa was used as carrier gas and the FID detector was maintained at 250°C.
10The oil constituents were identified on the basis of their retention and fragmentation data by using GC/MS analytical conditions similar to that of GC-FID. The mass spectra were recorded on a Agilent 5973 mass spectrometer coupled to an Agilent gas chromatograph (EI mode 70eV, source temperature 230°C, scanned mass ranged 35 to 350 amu). The characteristic fragmentation patterns have been analysed and compared to those of Wiley 275.L database. The retention data (retention indices) were compared to those of Adams (2001) and Joulain and König (1998).
2.4. NMR analysis
11Fraction F3.2, collected during column chromatography, was analysed by NMR (CDCl3, 500MHz) in the Unit of Structural Chemistry and Reaction Mechanisms (CSTR) of the Catholic University of Louvain-la-Neuve (Belgium).
3. Results and discussion
12The four analyzed samples contained mainly monoterpenes hydrocarbons (41.76–77.04%), in particular a-pinene (3.38–13.65%), b-pinene (8.22–44.11%). The b-pinene appears like an important compound in the essential oil of X. aethiopica since it is also the major compound in the oil of Guinea with 37.00 to 40.50% (Tomi et al., 1996), of Mali with 9.90% (Keita et al., 2003) and of Cameroon with 18.30% (Jirovetz et al., 1997). However the sample from Bafoussam is particular with 31.42% of b-phellandrene+1,8-cineole against 8.77 to 17.20% for the other samples. With the forementioned analytical conditions, b-phellandrene and 1,8-cineole coeluted, they were therefore summed in table 1. It is noteworthy that the essential oil originating from Bafoussam contained 14.56% of unidentified compounds each representing less than 1%. The origin of the fruits could explain the differences observed, but also the treatments that the fruits undergo after the harvest. Indeed, according to Ayedoum et al. (1996), the a-pinene and the sabinene can vary respectively from 4 to 16% and 3 to 35% according to whether the fruits are boiled or smoked before the drying.
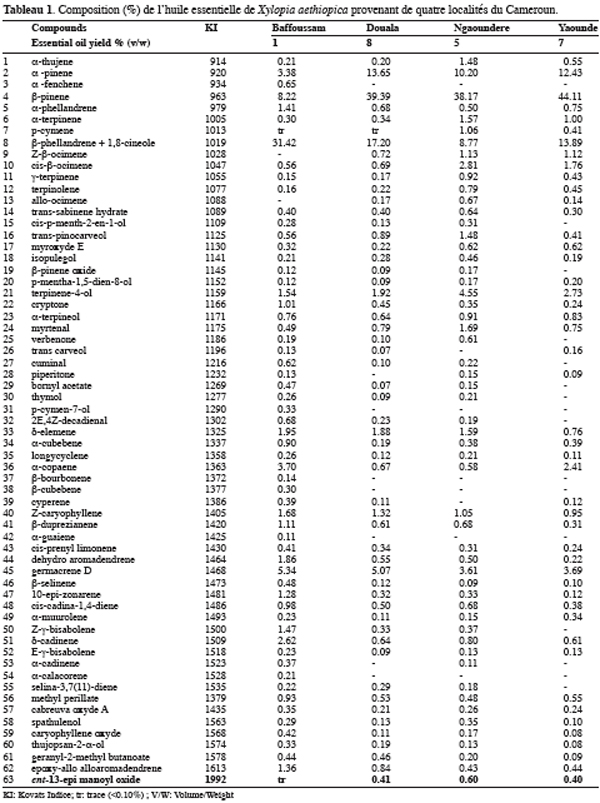
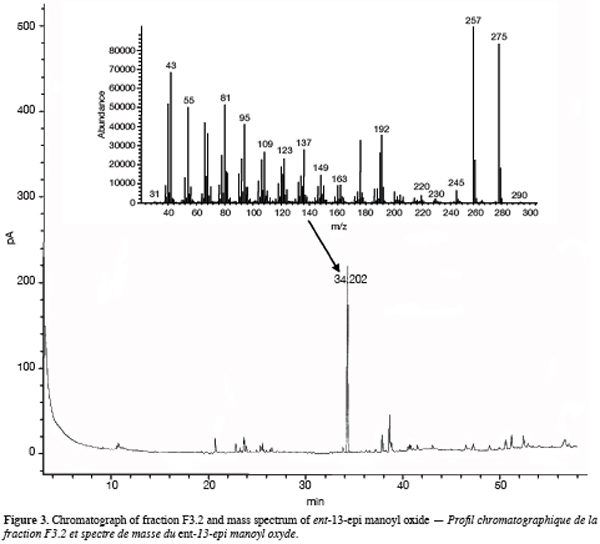
13One particular compound appeared in small amount (less than 1%) at a retention time of 34.2 min. Its molecular ion suggests a diterpene. The molecule, of moderate volatility, was systematically observed after long distillation times and only Jirovetz et al. (1997) mentioned the occurrence of such a compound in the essential oil of X. aethiopica without having proposed any identification. Successive column chromatography purifications (Figure 2) led to the recovery of 58.50 mg of an enriched fraction called F3.2 (Figure 3) which contained exclusively diterpenes, notably a derivative of manoyl oxide type. In agreement with Angelopoulou et al. (2001), the recorded mass spectra and the m/z (mass to charge ratio)=275 and 257 intensity ratio (ion m/z=257 higher than ion m/z=275), oriented identification toward the ent-13-epi manoyl oxide (Figure 4) rather than manoyl oxide where the two ions are almost equal. Comparing to the 1H NMR spectrum of pure ent-13-epi manoyl oxide (Demetzos et al., 2002), the recorded 1H NMR spectrum (Figure 5) showed the occurrence of the doublets H-15 cis and H-15 trans. A selective irradiation at 6 ppm confirmed the presence of H-14 since the doublets H-15 were reduced to two singlets by suppression of H-14/H-15 coupling. This confirmed well that the molecule of interest is the ent-13-epi manoyl oxide. Successive injections of the essential oil and the purified product revealed the same kovats index (KI=1992).
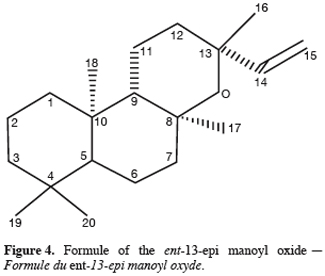
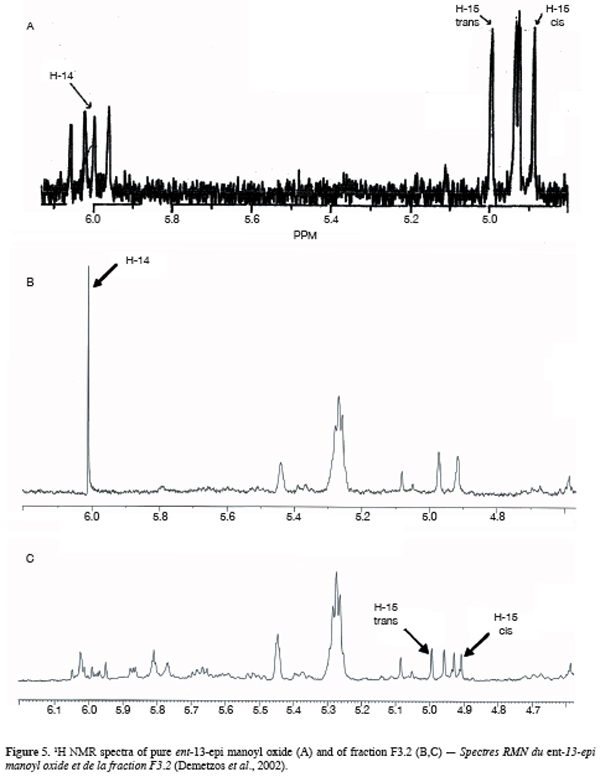
4. Conclusion
14More than 60 compounds were identified in the four samples of X. aethiopica essential oils which show the complexity of this natural extract with insecticide activity (Ngamo et al., 2001; Kouninki et al., 2005). The main chemical compounds are: b-pinene, b-phellandrene+1,8-cineole, a-pinene, terpinen-4-ol and germacrene D with different content in each sample. With GC-MS and NMR investigations, it was possible to unambiguously identify the ent-13-epi manoyl oxide, a diterpene which is reported for the first time in X. aethiopica essential oils. Nevertheless due to its very low proportion in all analysed hydrodistillates it is not established that this molecule could play a significant role in the essential oil activity.
15Acknowledgments
16The authors gratefully acknowledge Prof A. Schanck (Unité de Chimie structurale et des Mécanismes réactionnels) of the Catholic University of Louvain (UCL, Belgium) for NMR analyses; and Belgian University Cooperation to the Development (CUD) for financial support of the project “STOREPROTECT”.
Bibliographie
Adams RP. (2001). Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Carol Stream, Illinois, USA: Allured Publishing Corporation, 456 p.
Ajiwe EIV., Okeke AC., Ogbuagu OJ., Ojukwu U., Onwukeme IV. (1998). Characterisation and applications of oils extracted from Canerium schweinfurttii, Vitex doniana and Xylopia aethiopica fruits/seeds. Bioresour. Technol. 64, p. 249–252.
Angelopoulou D., Demetzos C., Dimas C., Perdetzoglou D., Loukis A. (2001). Essential oils and hexane extracts from leaves and fruits of cistus monspeliensis. Cytotoxic activity of ent-13-epi-manoyl oxide and its isomers. Planta Med. 67, p. 168–171.
Asawalam EF., Emosairue SO., Hassanali A. (2006). Bioactivity of Xylopia aethiopica (Dunal) A. Rich. essential oil constituents on maize weevil Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Electron. J. Environ. Agric. Food Chem. 5 (1), p. 1195–1204.
Asekun OT., Adeniyi BA. (2004). Antimicrobial and cytotoxic activities of the fruit essential oil of Xylopia aethiopica from Nigeria. Fitoterapia 75 (3-4), p. 368–370.
Ayedoun AM., Adeoti BS., Sossou PV., Leclerq PA. (1996). Influence of fruit conservation methods on the essential oil composition of Xylopia aethiopica (Dunal) A. Richard from Benin. Flavour Fragrance J. 11 (4), p. 245–250.
Demetzos C., Kolocouris A., Anastasaki T. (2002). A simple and rapid method for the differentiation of C-13 manoyl oxide epimers in biologically important samples using GC-MS analysis supported with NMR spectroscopy and computational chemistry results. Bioorg. Med. Chem. Lett. 12, p. 3605–3609.
Harrigan GG., Bolzani V. da S., Gunatilaka LAA., Kingston IGD. (1994). Kaurane and trachylobane diterpenes from Xylopia aethiopica. Phytochemistry 36 (1), p. 109–113.
Hasan MC., Healey MT., Waterman GP. (1982). Kolavane and kaurane diterpenes from the stem bark of Xylopia aethiopica. Phytochemistry 21 (6), p. 1365–1368.
Jirovetz L., Buchbauer G., Ngassoum MB. (1997). Investigation of the essential oils from the dried fruits of Xylopia aethiopica (West African “peppertree”) and Xylopia parviflora from Cameroon. Ernahrung 21 (7-8), p. 324–325.
Joulain D., König WA. (1998). The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. Hamburg, Germany: EB-Verlag, 658 p.
Karawya MS., Abdel SM., Hifnawy MS. (1979). Essential oil of Xylopia aethiopica fruit. Planta Med. 37, p. 57–59.
Keita B., Sidibe L., Figueredo G., Chalchat JC. (2003). Chemical composition of the essential oil of Xylopia aethiopica (Dunal) A. Rich. from Mali. J. Essent. Oil Res. 15 (4), p. 267–269.
Konnings GH., Agyare C., Ennison B. (2004). Antimicrobial activity of some medicinal plants from Ghana. Fitoterapia 75 (1), p. 65–67.
Kouninki H., Haubruge E., Noudjou FE., Lognay G., Malaisse F., Ngassoum MB., Goudoum A., Mapongmetsem PM., Ngamo LS., Hance T. (2005). Potential use of essential oils from Cameroon applied as fumigant or contact insecticides against Sitophilus zeamais Motsch. (Coleoptera: Curculionidae). Commun. Agric. Appl. Biol. Sci. 70 (4), p. 787–792.
Lajide L., Escoubas P., Mitzutani J. (1995). Termite antifeedant activity in Xylopia aethiopica. Phytochemistry 40 (4), p. 1105–1112.
Ngamo LS., Ngassoum MB., Jirovetz L., Ousman., Nukenine EC., Mukala OE. (2001). Protection of stored maize against Sitophilus zeamais (Motsch.) by use of essential oil of spices from Cameroon. Proceedings of the 53rd International Symposium on Crop protection, Gent Belgium, 8 May 2001. Meded. Fac. Landbouwkd. Toegepaste Biol. Wet. 66 (2a), p. 473–478.
Okonkwo EU., Okoye WI. (1996). The efficacity of four seed powders and the essential oils as protectants of cowpea and maize grains against infestation by Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchidae) and Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae) in Nigeria. Int. J. Pest Manage. 42 (3), p. 143–146.
Poitou F., Masotti V., Guigues de Souza S., Viano J., Gaydou M. (1996). Composotion of the essential oil of Xylopia aethiopica dried fruits from Benin. J. Essent. Oil Res. 8 (3), p. 329–330.
Tatsadjieu LN., Ngang JJE., Ngassoum MB., Etoa FX. (2003). Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloides and Zanthoxylum leprieurii from Cameroon. Fitoterapia 74 (5), p. 469–472.
Tomi F., Casanova J., Nianga M. (1996). Identification of the seed oil of Xylopia aethiopica from Guinea using 13C-NMR spectroscopy. J. Essent. Oil Res. 8, p. 429–431.
Pour citer cet article
A propos de : Félicité Noudjou
Faculté universitaire des Sciences agronomiques de Gembloux. Passage des Déportés, 2. B-5030 Gembloux (Belgique). E-mail : noudjou.f@fsagx.ac.be
A propos de : Habiba Kouninki
Université de Ngaoundéré. BP 455, Ngaoundéré (Cameroun).
A propos de : Thierry Hance
Unité d’écologie et de Biogéographie. Université Catholique de Louvain. 4-5, place Croix du Sud. B-1348 Louvain-la-Neuve (Belgique).
A propos de : Éric Haubruge
Université de Ngaoundéré. BP 455, Ngaoundéré (Cameroun).
A propos de : Léonard ST. Ngamo
Unité d’écologie et de Biogéographie. Université Catholique de Louvain. 4-5, place Croix du Sud. B-1348 Louvain-la-Neuve (Belgique).
A propos de : Pierre M. Maponmestsem
Faculté universitaire des Sciences agronomiques de Gembloux. Passage des Déportés, 2. B-5030 Gembloux (Belgique).
A propos de : Martin Ngassoum
Université de Ngaoundéré. BP 455, Ngaoundéré (Cameroun).
A propos de : François Malaisse
Université de Ngaoundéré. BP 455, Ngaoundéré (Cameroun).
A propos de : Michel Marlier
Université de Ngaoundéré. BP 455, Ngaoundéré (Cameroun).
A propos de : Georges Lognay
Faculté universitaire des Sciences agronomiques de Gembloux. Passage des Déportés, 2. B-5030 Gembloux (Belgique).






