- Portada
- volume 16 (2012)
- numéro 1
- Efficiency of semi-automated culture systems on microtubers formation of yam (Dioscorea alata L.)
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Efficiency of semi-automated culture systems on microtubers formation of yam (Dioscorea alata L.)

Notes de la rédaction
Received on April 26, 2011; accepted on December 2, 2011
Résumé
L’efficacité des systèmes de culture semi-automatisés sur la formation de microtubercules d’igname (Dioscorea alata L.). Le clone d’igname ‘Belep’ donne des résultats favorables en ce qui concerne la formation de microtubercules avec le système d’immersion temporaire. Des plants cultivés dans ce type de système de culture ont été les premiers à initier la formation de microtubercules et ont produit le nombre le plus élevé de microtubercules par plant par rapport aux systèmes CIS (Système d'Immersion Constante) et SLS (Système avec Milieu Liquide Statique). Ces microtubercules se caractérisaient par les poids frais et secs les plus élevés et les diamètres les plus grands. Le système d’immersion temporaire donne le plus grand nombre total de microtubercules et des microtubercules utilisables comme matériel de plantation. Les microtubercules obtenus par ce type de système de production se distinguent par leur qualité exprimée par le taux de matière sèche très élevé et par leur concentration en amidon. Pour renouveler les milieux de culture et maintenir une croissance de culture continue pour un matériel de plantation directe, des informations sont fournies quant aux préférences et à la diminution d’ions dans le milieu de culture durant la formation de microtubercules de l’igname dans le système d’immersion temporaire.
Abstract
Yam clone ‘Belep’ in temporary immersion system showed favorable results on microtubers formation. Plants cultivated in this type of culture system were the first ones to initiate microtubers formation and produced the highest microtubers number per plant than with CIS (Constant Immersion System) and SLS (Static Liquid Medium System). These microtubers showed the greatest fresh and dry weights, as well as their diameters. In temporary immersion system, we obtained the highest total microtubers number and competent microtubers as planting material. Microtubers achieved in this type of culture system were distinguished by their quality expressed in the higher dry matter content and starch concentration. In order to carry renewals of culture media and to maintain continuous culture growing for direct planting material, knowledges on preferences and ion depletion in culture medium during microtubers formation in yam cultivated in temporary immersion system were acquired.
Tabla de contenidos
1. Introduction
1Yam (Dioscorea spp.) crop plays a very important role in the global food system. Technologies for microtubers production in yam crop show a great potential as alternatives for propagation (Ovono et al., 2009). Microtubers may be used for planting material production, plant breeding and germplasm conservation programs (Mbanaso et al., 2007; Balogun, 2009).
2The aim of this work was to know the efficiency of semi-automated culture systems on microtubers formation in yam clone 'Belep', as well as to determine in TIS (Temporary Immersion System) the depletion and nutrient preference in culture media for increasing microtubers number and fresh weight.
2. Materials and methods
3For microtubers formation, two culture stages were established. In the growth stage, each system contained nodal segments with axillary buds and the basal medium Murashige and Skoog (1962) (MS) with 30.0 g·l-1 sucrose, a 16 h photoperiod at a density of 42.0-48.0 µmol·m-2·s-1 photosynthetic photon flow (FFF) and at a 25 ± 2.0°C temperature. This culture stage lasted six weeks. During the microtubers formation stage, the basal medium MS with 100 g·l-1 sucrose in constant dark at 23 ± 2.0°C was used. This culture stage was developed during 18 weeks.
4Semi-automated culture systems
51. Temporary Immersion System (TIS), a 15-minute immersion time and an immersion frequency of 6 h (4 immersions per day) were applied;
62. Constant Immersion System (CIS) with aeration through continuous bubbling in culture media;
73. A system with static liquid culture medium (SLS) with passive renewal of the internal atmosphere.
8One hundred nodal segments and 30 ml culture medium volume per nodal segment were placed in each culture system for a total 3,000 ml culture medium volume.
9After 18 weeks of culture in the microtubers formation, 15 plants in each repetition per treatment were selected randomly, and at harvesting time the following parameters were evaluated: microtubers number per plant determined with an analytic balance (SARTORIUS), microtubers fresh weight (gFW) and dry weight (gDW), and microtubers diameters (mm).
10For each repetition per treatment, the total microtubers number per culture flask, suitable microtubers number as planting material (a fresh weight higher than 0.5 gFW, a diameter above 5.0 mm and no watery appearance) and hyperhydrated microtubers number were counted.
11For determining reducing sugars in the culture medium, a protocol described in the manual of analytical techniques, ICIDCA (1974), was applied.
12In relation to ion contents in the culture medium, nitrogen (N) was determined by Nessler's calorimetric method and phosphorus (P) by the calorimetric method of ammonium metavonato, according to the regulations from NRAG 564 (1982). Sample reads were obtained in a spectrophotometer Spekoll 11.
13Potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), copper (Cu), cobalt (Co) and manganese (Mn) were analyzed by dilution, based on the methodology of atomic-absorption spectrometry (Smith et al., 1972). Reads were taken in an atomic-absorption spectrometre SP9 Pye Unicam.
14Data related with microtubers number/plant, microtubers fresh weight, microtubers dry weight and microtubers diameter (mm) were analyzed statistically through a non-parametric test of Kruskall Wallis. Means of total microtubers number by system, competent and hyperhydrated microtubers were compared by simple variance analysis and Turkey’s test was also applied.
3. Results and discussion
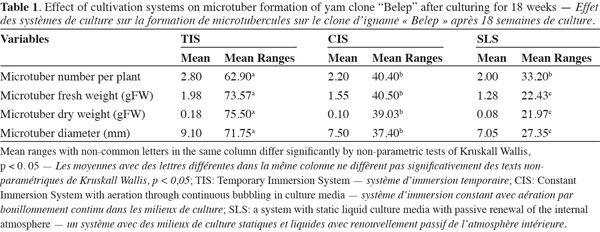
15The type of cultivation system influenced significantly microtubers formation (Table 1). The best results for evaluated variables were obtained in TIS, microtubers number/plant (2.80), microtubers fresh weight (1.98 g), microtubers dry weight (0.18 g) and microtubers diameter (9.10 mm) with significant differences in relation to CIS, microtubers number/plant (2.20), microtubers fresh weight (1.55 g), microtubers dry weight (0.10 g) and SLS, microtubers number/plant (2.00), microtubers fresh weight (1.28 g), microtubers dry weight (0.08 g).
16The use of TIS after culturing for 18 weeks resulted in the highest total microtubers number (269.20) and the highest competent microtubers number as planting material (241.80). Besides, in this culture system, the lowest microtubers number with hyperhydricity symptoms was obtained (3.80). Results obtained in TIS presented significant differences in regard to other evaluated culture systems. The use of TIS increased by 28% the total number of microtubers formed (1.4 times) and by 33.8% the microtubers with a fresh weight superior to 0.5 g (1.5 times) compared with CLS and SLS. With TIS microtubers were obtained at a lower cost and greater efficiency was achieved regarding the planting of microtubers as plant material directly in the field.
17Microtubers achieved in TIS were distinguished by their qualities expressed in higher dry matter content (8.87%) and starch concentration (84.98 g·W-1) in comparison to microtubers obtained in CIS and SLS. Dry matter values as indicators of microtubers quality must be stood out. Dry matter content from microtubers is composed of lignin and polysaccharides in the cell wall, besides protoplast components as proteins, lipids, aminoacids and some inorganic elements such as potassium and calcium, which together with the greater accumulation of reserve substances as starch, makes better conservation possibilities according to Gopal et al. (2008) during storage and further sprouting.
18In the analysis of the reducing sugar concentration in TIS, an initial 9.35% concentration of reducing sugar in culture medium after 18 weeks of culture, was reduced to 0.31%.
19Relative to ion depletion in culture medium during yam microtubers formation in TIS, some elements such as phosphorous were completely depleted after 18 weeks of culture. From the initial ion concentration, nitrogen was below 9.0%, potassium at 28.9% and calcium at 48.7%. Magnesium, manganese, copper and iron were less used as their contents in culture media remained above 50% of the initial concentration. The lowest contents of P, N, K and Ca ions after 18 weeks of culture were due to their involvement in the microtubers formation.
20Results obtained in this study constitute the first report on microtubers formation of yam clone 'Belep' in semi-automated culture system with temporary immersion which is made up of 10 l couple flasks.
4. Conclusion
21Semi-automated culture systems are valuable options to increase yam microtubers formation. Based on results obtained in relation to the number and the quality of microtubers, and the knowledge of the depletion of ions and reducing sugar contents in culture medium, temporary immersion systems were recommended for microtubers formation in yam clone 'Belep'. With TIS microtubers were obtained at a lower cost and greater efficiency.
Bibliographie
Balogun M.O., 2009. Microtubers in yam germplasm conservation and propagation: the status, the prospects and the constraints. Biotechnol. Mol. Biol. Rev., 4(1), 1-10.
Gopal J., Iwama K. & Jitsuyama Y., 2008. Effect of water stress mediated through agar on in vitro growth of potato. In Vitro Cell. Dev. Biol. Plant, 44(3), 221-228.
ICIDCA, 1974. Manual de Técnica Análitica. La Habana: ICIDCA.
Mbanaso E.N.A., Chukwu L.I. & Opara M.U.A., 2007. In vitro basal and nodal microtuberization in yam shoot cultures (Discorea rotundata Poir, cv. 'Obiaoturugo') under nutritional stress conditions. Afr. J. Biotechnol., 6(21), 2444-2446.
Murashige T. & Skoog F., 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant., 15, 473-479, doi:10.1111/j.l399-3054.1962.tb08052.x.
NRAG, 1982. Análisis foliar. La Habana: AGRINFOR, Ministerio de la Agricultura.
Ovono P.O., Kevers C. & Dommes J., 2009. Effects of reducing sugar concentration on in vitro tuber formation and sprouting in yam (Dioscorea cayenensis-D. rotundata complex). Plant Cell Tissue Organ Cult., 99, 55-59.
Smith D.L. & Scherk W.G., 1972. Atomic Absorption Spectrophometry (AAS). Assoc. Off. Anal. Chem. J., 55, 669.
Para citar este artículo
Acerca de: Manuel Cabrera Jova
Instituto Nacional de Investigaciones en Viandas Tropicales (INIVIT). Apartado 6. Santo Domingo CP 53000. Villa Clara (Cuba). E-mail: mcabrera@inivit.cu
Acerca de: Rafael Gómez Kosky
Instituto de Biotecnología de las Plantas. Universidad Central “Marta Abreus” de Las Villas. Carretera a Camajuaní km 5. Santa Clara. Villa Clara (Cuba).
Acerca de: Ernesto Espinosa Cuellar
Instituto Nacional de Investigaciones en Viandas Tropicales (INIVIT). Apartado 6. Santo Domingo CP 53000. Villa Clara (Cuba).
Acerca de: Alberto Espinosa Cuellar
Instituto Nacional de Investigaciones en Viandas Tropicales (INIVIT). Apartado 6. Santo Domingo CP 53000. Villa Clara (Cuba).






