- Startpagina tijdschrift
- volume 16 (2012)
- numéro 1
- Structure, properties and obtention routes of flaxseed lignan secoisolariciresinol, a review
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Structure, properties and obtention routes of flaxseed lignan secoisolariciresinol, a review

Nota's van de redactie
Received on May 11, 2011; accepted on November 8, 2011
Résumé
Structure, propriétés et voies d'obtention du sécoisolaricirésinol, une lignane de la graine de lin (synthèse bibliographique). Après avoir rappelé la structure et la nomenclature de la famille des lignanes, cette revue a pour objectif de décrire les principales propriétés, les méthodes d'analyse et les techniques d'obtention d'une lignane du lin, le sécoisolaricirésinol (SECO). Deux voies d'obtention du SECO sont décrites : l'extraction au départ de matières premières naturelles et la synthèse chimique. Pour la première, le travail met l'accent sur les procédés verts développés jusqu'à présent. Pour la deuxième voie, la synthèse chimique s'inspirant de la biosynthèse naturelle est principalement détaillée.
Abstract
Following a brief description of the structure and nomenclature of the lignan family, this review focuses on the flaxseed lignan secoisolariciresinol (SECO). The main properties, the analysis methods and two routes for the preparation of SECO, i.e. extraction from renewable raw material and (hemi)-synthesis, are reviewed. Green methods recently developed for the first route and chemical syntheses inspired from biosyntheses for the second one are the main subjects of this paper.
Inhoudstafel
1. Introduction
1Following the description of the structure and nomenclature of lignans, the aim of this article is to give an overview of the properties, the analysis methods and the two routes developed to obtain the flaxseed lignan secoisolariciresinol (SECO, 6). The first route involves an extraction step from its biological source (flaxseed). This topic has already been surveyed (Hosseinian et al., 2009a). Hence, the present work will give a brief overview of the main conventional techniques and will be more focused on greener processes. The second route to obtain SECO (6) is chemical synthesis. Conventional conjugated addition can be compared to more recent oxidative coupling methods. These latter ones are based on biosynthetic pathways that were elucidated only recently.
2. Lignan structure
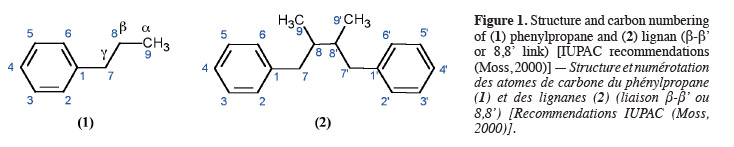
2The lignan family is a large group of naturally abundant molecules that can be found in a plethora of superior plants. Lignans were first defined in 1936 as phenylpropanoids dimers where two phenylpropane units (C6C3) (1) are linked by their carbon 8 (β-β' link) (2) as represented in figure 1 (Haworth, 1936). This definition is largely accepted although some authors prefer to describe lignans as “1,4-Diarylbutane” compounds (Cassidy et al., 2000). During the seventies, the lignan family was extended to a series of compounds where the momomers are linked differently (Gottlieb, 1978). These “new lignans” are named “neolignans”. Lignans fundamental structures are accurately described in the IUPAC Recommendations published in 2000 (Moss, 2000).
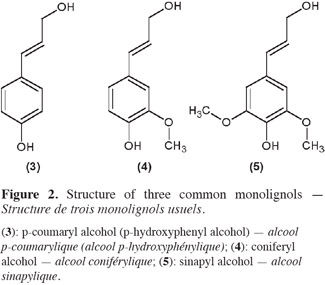
3The most frequent phenylpropane units constitutive of lignans, often called monolignol units, are p-coumaryl (3), coniferyl (4) and sinapyl alcohols (5) (Lainé et al., 2007). These three phenylpropanoids species vary only by methoxylations on their aromatic ring (see structures in figure 2). Assembly of monolignols gives rise to natural lignin which occurs in many woody plants (Umezawa, 2003).
4Although lignans have been the subject of many studies over the years, research continues concerning their natural diversity and abundance in the vegetal kingdom and their biological properties and biosynthesis pathways (Beejmohun et al., 2007; Bonzanini et al., 2009; Hosseinian et al., 2009b; Attoumbré et al., 2010). Lignans have also been the topic of several reviews (Westcott et al., 2003; Albertazzi et al., 2008; Touré et al., 2010).
5Among lignans, secoisolariciresinol (SECO) (6) is of particular interest (Figure 3). It is formed by two coniferyl alcohols (monolignol units) linked by their carbon number 8. Particularly abundant in flaxseed, this molecule can also be found, for example, in soybean, peanut (Mazur et al., 1998), broccoli, cashew nut, mung bean (Schwartz et al., 2006), many fruits such as kiwi (Milder et al., 2005) and pomegranate (Bonzanini et al., 2009), triticale straw (Hosseinian et al.,2009b), greater burdock (Cai et al., 2006) or Forsythia intermedia (Umezawa et al., 1991).
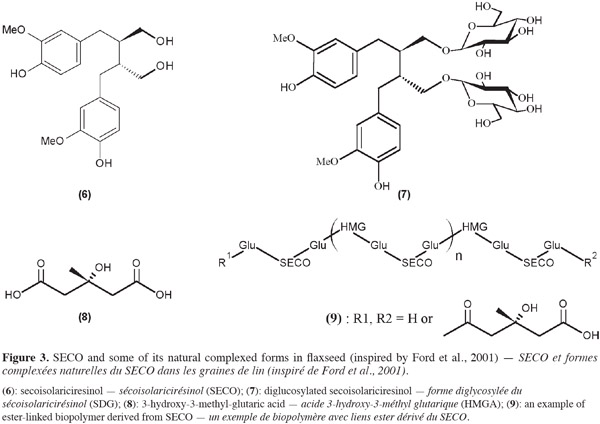
6SECO (6) like other lignans may occur in different natural forms. They can be free (aglycone) or attached to other molecules such as glucose or organic acids to form more complex structures. In flaxseed, SECO is mostly mono or diglycosylated [SMG or SDG (7)]. SMG and SDG are linked by 3-hydroxy-3-methyl-glutaryl units (HMG), derived from 3-hydroxy-3-methyl-glutaric acid (HMGA) (8), to form an ester-linked biopolymer (9) (Figure 3) (Ford et al., 2001).
3. Main properties
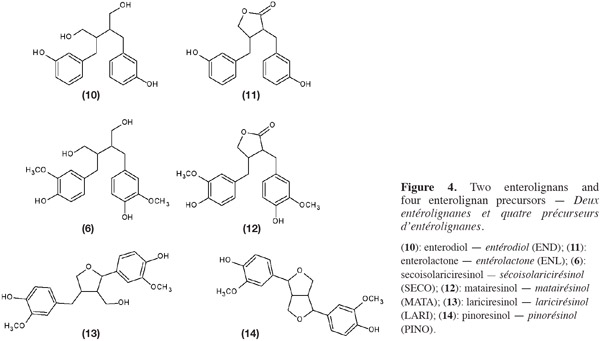
7SECO (6) is known to have many physiological properties and health benefits. Indeed, SECO is converted into enterolignans [enterodiol (END) (10) and enterolactones (ENL) (11)] by the anaerobic intestinal microflora (Wang et al., 2000; Wang et al., 2010). Three other components of flaxseed, matairesinol (MATA) (12), lariciresinol (LARI) (13) and pinoresinol (PINO) (14) (Figure 4), are also converted into enterolignans. These four coumpounds are mammalian oestrogens precursors, also called phyto-oestrogens (Raffaelli et al., 2002; Bartkiene et al., 2011).
8Due to the structural similarity of enterolignans with mammalian oestrogens, these compounds are potentially interesting for combating some hormone-dependent cancers. Over the past ten years, this topic has been extensively studied and reviewed (Adlercreutz, 1995; Thompson et al., 1996; Wang, 2002a; Apers et al., 2003; Duncan et al., 2003; Boccardo et al., 2006; Albertazzi et al., 2008; Mesa-Siverio et al., 2008). Some epidemiologic investigations have shown that the risk of breast, prostate and colon cancers is lower in countries or regions in which the diet is particularly rich in lignans. However, others were considered conclusively “negative”, i.e. failed to demonstrate any protective effect on carcinogenesis (Boccardo et al., 2006). Several cell culture and animal experiments have also shown the positive effect of enterolignans against these cancers. The protective role of lignans can be in part explained by the fact that they can bind to oestrogen receptors. However, their mechanism of action has been shown to be more complex, they can also influence intracellular enzymes and protein synthesis. They can stimulate the production of the sex hormone-binding globulin in the liver which results in reducing the concentration of free hormones in the plasma. They interact with sex steroid binding protein and act as inhibitors of several steroid metabolizing enzymes such as aromatase and cholesterol 7α-hydrolase, these inhibitions being respectively positive against the breast and colon cancers. Their antioxidant activity is also one of the possible anticarcinogenic mechanism of the compounds. For example they can reduce the endogenous generation of oxidized DNA bases. SECO (6), SDG (7), END (10) and ENL (11) were demonstrated to have antioxidant activity (e.g. lowering lipid oxidation or DPPH free radical quench) in various in vitro systems (Kitts et al., 1999; Hu et al., 2007).
9Phyto-oestrogens were also suggested to play a role in protection against diabetes and cardiovascular diseases (Duncan et al., 2003; Albertazzi et al., 2008).
10Despite these numerous studies, many questions still remain unanswered (potency, toxicity, bioavailability, etc.) and further studies are required to fully understand the role of enterolignans in human health and disease and to establish dietary recommendations for example.
4. Structure analysis
11For more than twenty years, NMR techniques, along with the classical melting point determination and IR spectroscopy, have been used to confirm the structure of extracted and synthesized lignans (Pelter et al., 1992; Eklund et al., 2002). More recently, mass spectrometers coupled or not with a chromatographic separation has become the technique of choice to analyze lignans. NMR and MS techniques can also be combined (LC-NMR-MS) to elucidate structures of optically active molecules (Fritsche et al., 2002) such as the two enantiomers of SDG (7) occurring in flaxseed.
12RP-HPLC is the most used analytical technique for detection and quantification of lignans (Willfor et al., 2006). The most common method for analysis of SECO (6) and its glycosylated forms consists of separation on an octadecyl silica gel (ODS) column followed by detection by UV and MS (Eliasson et al., 2003; Beejmohun et al., 2007; Attoumbré et al., 2010). Other detection techniques such as Coulometric Electrode Array Detection can also be used (Schwartz et al., 2006). As with UV and MS detection, simplicity, universal applicability and good sensitivity are the main advantages of this technique. Furthermore, it can give additional information such as the presence of electrochemically active groups in the sample. Chiral chromatography allows the separations of lignan enantiomers, and for example, a semi-micro chiral cellulose carbamate-based column is able to distinguish (+)-SECO and (-)-SECO (6) (Okunishi et al., 2004). Gas chromatography coupled to a mass spectrometer has also been used to detect and quantify lignans in specific food applications (Peñalvo et al., 2004).
13Among identification methods of phytoestrogens, non-chromatographic techniques such as MALDI-TOF-MS, radioimmunoassay or ELISA are powerful and sometimes more adapted for analysis of complex media such as biofluids (Wang et al., 2002b).
5. Extraction
14The choice of method for the extraction of lignans depends on their molecular structure. Less polar lignans can be extracted by hexane but in contrast, SECO (6), with a higher polarity, can be extracted by polar solvents such as aqueous methanol or ethanol.
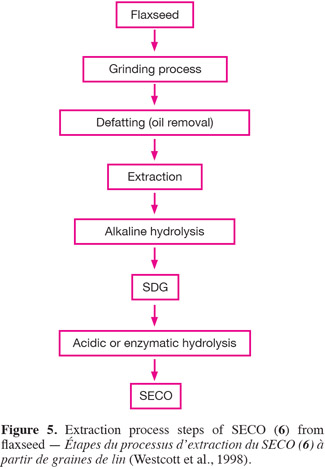
15SDG (7) and SECO (6) were first identified in flaxseed by Bakke et al. (1956). Westcott et al. (1998) patented an optimized extraction and purification method of flaxseed lignans. Their general extraction steps for SECO are schematized in figure 5. Following grinding, the flour obtained is usually defatted with organic non polar solvents (e.g. hexane) to facilitate alcoholic extraction of SDG (7). After defatting, the lignans are extracted by a primary alcohol (aqueous methanol or ethanol, with an alcohol content of 55 to 75% v/v being preferred). The crude extract undergoes an alkaline treatment (sodium or potassium hydroxide 1N, 3-7% w/v) which breaks the SDG-HMG link and releases free SDG (7). An extraction yield of about 30 mg SDG per gram of defatted flaxseed flour (DFF) is obtained with a mixture of ethanol and water (65/35, v/v). To obtain the aglycone form of the SECO (6), SDG (7) must undergo an acidic or an enzymatic treatment in order to break the glycosidic link (Bakke et al., 1956; Schwartz et al., 2006).
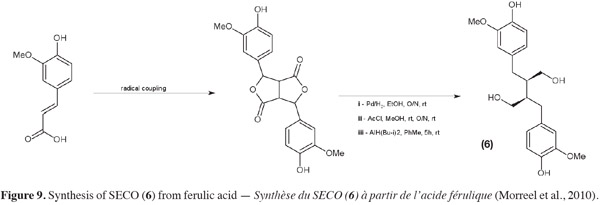
16For safety and environmental issues, replacement of the organic solvents is a growing requirement. Supercritical fluids, such as supercritical carbon dioxide (SC-CO2), which have gas-like diffusivity and liquid-like density have been used successfully as greener alternatives to non-polar solvents such as hexane to defat flaxseed flour (Bozan et al., 2002). Pressurized low polarity water (PLPW) extraction (also named subcritical water extraction) has also been applied to obtain lignans such as SDG (7) from flaxseed (Cacace et al., 2006). The defatted flaxseed flour (DFF) undergoes a high temperature (circa 140°C) treatment under high pressure (circa 5.2 MPa) to ensure liquid state of water. In this subcritical state, water's dielectric constant and polarity decrease and reach similar values to those of a methanol-water mixture. Toxicity of this method is lower than a methanolic extraction but the effect of the temperature on extracts must be taken into account. SDG (7) extraction yield is quite good (10 mg SDG per gram of seeds) but some phenolic compounds may be damaged.
17Real usefulness of organic solvents to extract SDG (7) from flaxseed is not established. For example, a direct alkaline hydrolysis process that does not require the use of organic solvent has provided an extraction yield of 14 to 31 mg·g-1 dry DFF depending on the origin of the flaxseeds (Eliasson et al., 2003). This process can be microwave-assisted (Nemes et al., 2011). Different parameters were studied to optimize the microwave-assisted extraction such as the microwave (MW) power level (between 30 and 360 W), the time of residence in the MW cavity (between 1 and 25 min), the molar concentration of sodium hydroxide (between 0.25 and 1 M) and the time of MW power application (30 or 60 s·min-1). The yields obtained with the optimized MW-assisted extraction method (0.5 M NaOH for 3 min with a MW power level of 135 W and MW power application of 30 s·min-1) were 6% higher than those obtained with the Elianson's reference hydrolysis method which is much longer (1 M NaOH for 1 h at room temperature) and were 10% higher than the control method (0.5 M NaOH for 3 min at room temperature). This organic solvent and corrosive product free process is a promising green method to obtain SDG (7) from flaxseed.
6. Synthesis and modification of SECO
18Two trends can be observed for lignans synthesis. The first one exploits traditional organic chemistry routes including asymmetric synthesis. The second one, more recent, develops chemical pathways inspired from biosynthesis.
19A great challenge in chemical lignan formation is asymmetric synthesis. Several methods can be used to synthetize chiral building blocks that can be assembled to create controlled-configuration lignans (Itoh et al., 1997). For a review of the most frequent lignan synthesis pathways the reader can refer to Del Signore and Mathias Berner's paper (2006).
20In 2005, Davin and Lewis highlighted the flaxseed lignan biosynthesis mechanism. It is based on an enzyme-assisted oxidative coupling of two monolignol units (for example, two coniferyl alcohol units). The identification of the key compounds and main steps of the natural biosynthesis leads to the development of original chemical synthesis routes. As an example, Wang et al. (2006) have investigated the chemical transformation of coniferyl alcohol or its derivatives into SECO (6) via an oxidative coupling. In this example, SECO synthesis can be subdivided into three main stages: obtention of the lignan precursor unit, oxidative coupling and reduction and dealkylation steps (Figure 6). The first stage, the lignan precursor (16) synthesis from commercially available 4-hydroxy-3-methylbenzaldehyde (15), can be summarized into four steps:
21(i) reduction of the aldehyde function;
22(ii) alkylation with tert-butanol of the aromatic carbon number 5;
23(iii) reformation of the aldehyde function by oxidation;
24(iv) transformation into an ester derivative via a Wittig reaction.
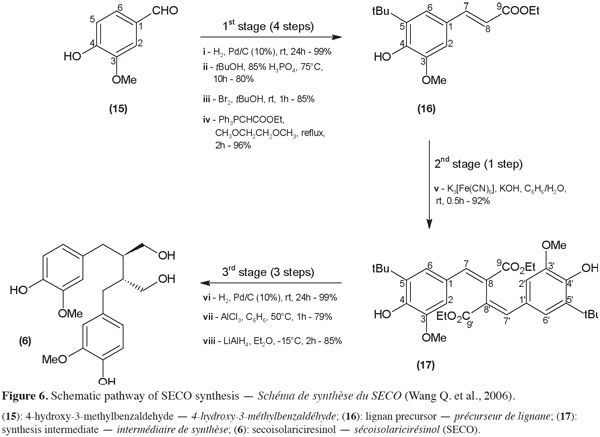
25An overall yield of 64% was obtained for this first stage. During the second stage, two molecules of the lignan precursor are then linked by their carbon number 8 (17) by oxidative coupling (v). The third stage leads to (±)-SECO (6) with a yield of 36% and consists of three steps:
26(vi) reduction of the 7-8 (7'-8') unsaturated link;
27(vii) removal of the tert-butyl groups;
28(viii) final reduction of the ethyl esters.
29The critical step in this biomimetic approach is the oxidative coupling. When the link between the two lignan precursor units is formed, a lack of selectivity leading to compounds other than the one with a β-β' link is observed. Indeed, the radical obtained by deprotonation of a coniferyl derivative is delocalized over the molecule and causes uncertainty about which carbon will be involved in the coupling reaction. The major product was reported to be the β-5 linked compound. To increase the β-β' link, Wang et al. (2006) blocked the position 5 of the phenyl ring by alkylation (step 2 of stage 1). This reaction step risks decreasing the yield of the lignan precursor synthesized but the final product should be easier to isolate.
30Flaxseed lignans such as SECO (6) can also be obtained by chemical modification of another flaxseed lignan such as MATA (12). The latter can be synthesized or extracted from natural sources such as leaves and stems of Forsithia intermedia (Eich et al., 1996) or from Norway spruce as hydroxyMATA (18) (Eklund et al., 2002) which will lead to hydroxySECO.
31Mäkelä et al. (2000) synthesized MATA (12) via a Michael conjugated addition (and not oxidative coupling) which is a classical route for flaxseed lignans synthesis. The main step of the reaction is generally the tandem addition of a carbanion to butenolide followed by an in situ benzylation (1st stage of figure 7). Many examples exist in the literature (Pelter et al., 1992; van Oeveren et al., 1994; Ward et al., 2001) and some are cited in Del Signore and Mathias Berner's review (2006).
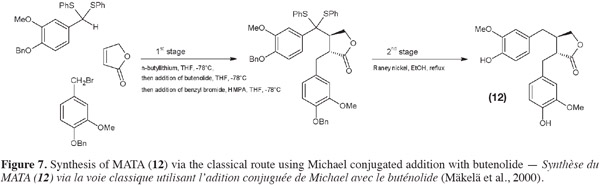
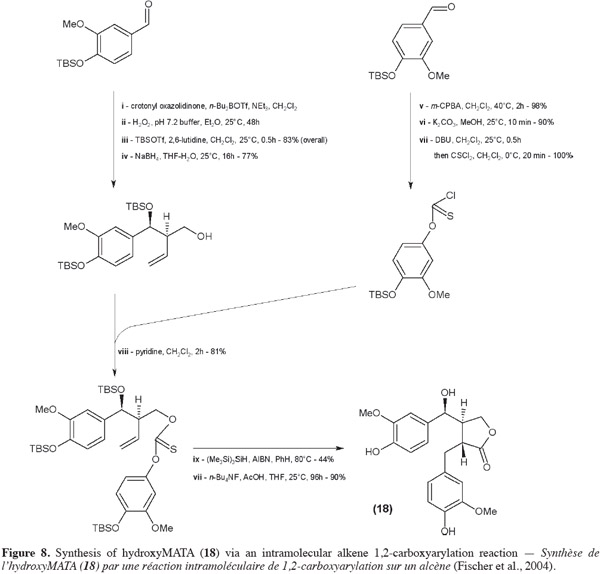
32Sefkow et al. (2003) replaced the classical butenolide reactant by malic acid. It reacts twice with the benzyl bromide and then the product obtained is reduced into hydroxyMATA. Fryatt et al. (2005) whose goal was to obtain 13C-labeled MATA (20) used diethyl malonate (instead of butenolide). HydroxyMATA (18) can be synthesized by following a radical carboxyarylation approach (Fischer et al., 2004). Starting materials are two molecules of silyl ether protected 4-hydroxy-3-methoxybenzaldehyde (Figure 8): one of them undergoes an aldol reaction (ii) followed by a protection (iii) and a reduction (iv) and the second is “chlorothionated” (vii) before their assembly (viii); intramolecular radical reaction (ix) followed by removal of the protecting groups (x) gave the desired hydroxyMATA (18).
33SECO (6) is obtained by reduction of MATA (12) with a Lewis-base catalyst such as LiAlH4 that catalyses the reduction of the lactone into the diol function (Eich et al., 1996; Ward et al., 2001; Eklund et al., 2002; Fryatt et al., 2005). Thus, Ward et al. (2001) synthesized the SECO (6) with a 62% yield, whereas Eklund et al. (2002) obtained hydroxySECO with a 78% yield, and a small yield of 1% was reached by Fryatt et al. (2005) for the synthesis of the 13C labeled SECO.
34More recently, Morreel et al. (2010) have designed a synthesis of SECO (6) starting from ferulic acid (Figure 9) which is particularly interesting as it is the most abundant hydroxycinnamic acid found in plants. The intermediate 8-8'-dilactone, resulting from radical coupling of the ferulic acid, was reduced into 8-8' diacid (i), then esterified with methanol to give the 8-8-dimethylester (ii) and finally reduced (iii) into SECO (6).
35Enzymes can also be used to catalyze the transformation of lignans into SECO (6) (Chu et al., 1993; Dinkova-Kostova et al., 1996). Pinoresinol/Lariciresinol Reductase, an enzyme found in Forsythia intermedia, is able to catalyze the sequential reduction of PINO (14) into LARI (13) then into SECO (6). These steps are performed with conversion yields of 18% and 37.7% respectively. The knowledge of the possibility of modification of this enzyme opens door for research concerning level control of phytoestrogens associated with hormone dependant diseases.
7. Conclusion
36Lignans, including SECO (6), are abundant in superior plants and present properties particularly interesting to the health sector. The food and cosmetic fields could also potentially exploit their antioxidant activity.
37The two approaches to obtain SECO (6) are extraction from natural raw materials and chemical synthesis. Organic solvents are often used to extract this lignan from biomass. Greener processes have been developed in the past few years but further work is needed to improve the extraction yields. The chemical synthesis alternative, allows control of the structure of the desired compounds and some of the purification steps are thus avoided. Another advantage of organic synthesis is that it gives access to a larger range of structures and this may open new applications opportunities. Only a few studies have focused on enzymes applied in SECO (6) synthesis. However, this synthesis pathway has considerable potential. Indeed, the specificity of enzyme activity leads to stereochemically-controlled compounds that should not require tedious purification steps and the process may have a lower environmental footprint.
38Acknowledgments
39The work was funded through the ARC grant “Superzym”, financed by the French Community of Belgium which is gratefully acknowledged for its financial support. Magali Deleu thanks the Fonds National de la Recherche Scientifique (FNRS) from Belgium for her Research Associate position.
Bibliographie
Adlercreutz H., 1995. Phytoestrogens – Epidemiology and a possible role in cancer protection. Environ. Health Perspect., 103, 103-112.
Albertazzi P. & Purdie D.W., 2008. The nature and utility of the phytoestrogens: a review of the evidence (reprinted from Maturitas, 2002, 42, 173-185). Maturitas, 61, 214-226.
Apers S., Vlietinck A. & Pieters L., 2003. Lignans and neolignans as lead compounds. Phytochem. Rev., 2, 201-217.
Attoumbré J. et al., 2010. Development of antibodies against secoisolanciresinol - Application to the immunolocalization of lignans in Linum usitatissimum seeds. Phytochemistry, 71, 1979-1987.
Bakke J.E. & Klosterman H.J., 1956. A new diglucoside from flaxseed. Proc. Nat. Acad. Sci. USA, 10, 18-22.
Bartkiene E. et al., 2011. Enterolignans enterolactone and enterodiol formation from their precursors by the action of intestinal microflora and their relationship with non-starch polysaccharides in various berries and vegetables. Food Sci. Technol., 44, 48-53.
Beejmohun V. et al., 2007. Coniferin dimerisation in lignan biosynthesis in flax cells. Phytochemistry, 68, 2744-2752.
Boccardo F., Puntoni M., Guglielmini P. & Rubagotti A., 2006. Enterolactone as a risk factor for breast cancer: a review of the published evidence. Clin. Chim. Acta, 365, 58-67.
Bonzanini F. et al., 2009. Identification and distribution of lignans in Punica granatum L. fruit endocarp, pulp, seeds, wood knots and commercial juices by GC-MS. Food Chem., 117, 745-749.
Bozan B. & Temelli F., 2002. Supercritical CO2 extraction of flaxseed. JAOCS, 79, 231-235.
Cacace J.E. & Mazza G., 2006. Pressurized low polarity water extraction of lignans from whole flaxseed. J. Food Eng., 77, 1087-1095.
Cai Y.Z. et al., 2006. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci., 78, 2872-2888.
Cassidy A., Hanley B. & Lamuela-Raventos R.M., 2000. Isoflavones, lignans and stilbenes - origins, metabolism and potential importance to human health. J. Sci. Food Agric., 80, 1044-1062.
Chu A. et al., 1993. Stereospecificity of (+)-pinoresinol and (+)-lariciresinol reductases from Forsythia intermedia. J. Biol. Chem., 268, 27026-27033.
Davin L.B. & Lewis N.G., 2005. Dirigent phenoxy radical coupling: advances and challenges. Curr. Opin. Biotechnol., 16, 398-406.
Del Signore G. & Mathias Berner O., 2006. Recent developments in the asymmetric synthesis of lignans. Stud. Nat. Products Chem., 33, 541-600.
Dinkova-Kostova A.T. et al., 1996. (+)-Pinoresinol/(+)-lariciresinol reductase from Forsythia intermedia - Protein purification, cDNA cloning, heterologous expression and comparison to isoflavone reductase. J. Biol. Chem., 271, 29473-29482.
Duncan A.M., Phipps W.R. & Kurzer M.S., 2003. Phyto-oestrogens. Best Pract. Res. Clin. Endocrinol. Metab., 17, 253-271.
Eich E. et al., 1996. (-)-Arctigenin as a lead structure for inhibitors of human immunodeficiency virus type-1 integrase. J. Med. Chem., 39, 86-95.
Eklund P., Sillanpaa R. & Sjoholm R., 2002. Synthetic transformation of hydroxymatairesinol from Norway spruce (Picea abies) to 7-hydroxysecoisolariciresinol, (+)-lariciresinol and (+)-cyclolariciresinol. J. Chem. Soc. Perkin Trans. 1, 1906-1910.
Eliasson C., Kamal-Eldin A., Andersson R. & Aman P., 2003. High-performance liquid chromatographic analysis of secoisolariciresinol diglucoside and hydroxycinnamic acid glucosides in flaxseed by alkaline extraction. J. Chromatogr. A, 1012, 151-159.
Fischer J., Reynolds A.J., Sharp L.A. & Sherburn M.S., 2004. Radical carboxyarylation approach to lignans. Total synthesis of (-)-arctigenin (Ia), (-)-matairesinol (IIa), and related natural products. Org. Lett., 6(9), 1345-1348
Ford J.D. et al., 2001. Biosynthetic pathway to the cancer chemopreventive secoisolariciresinol diglucoside-hydroxymethyl glutaryl ester-linked lignan oligomers in flax (Linum usitatissimum) seed. J. Nat. Prod., 64, 1388-1397.
Fritsche J., Angoelal R. & Dachtler M., 2002. On-line liquid-chromatography-nuclear magnetic resonance spectroscopy-mass spectrometry coupling for the separation and characterization of secoisolariciresinol diglucoside isomers in flaxseed. J. Chromatogr. A, 972, 195-203.
Fryatt T. & Botting N.P., 2005. The synthesis of multiply 13C-labelled plant and mammalian lignans as internal standards for LC-MS and GC-MS analysis. J. Labelled Compd. Radiopharm., 48, 951-969.
Gottlieb O.R., 1978. Neolignans. Fortschr. Chem. Org. Naturstoff., 35, 1-72.
Haworth R.D., 1936. Natural resins. Annu. Rep. Prog. Chem., 33, 266-279.
Hosseinian F.S. & Beta T., 2009a. Patented techniques for the extraction and isolation of secoisolariciresinol diglucoside from flaxseed. Recent Patents Food Nutr. Agric., 1, 25-31.
Hosseinian F.S. & Mazza G., 2009b. Triticale bran and straw: potential new sources of phenolic acids, proanthocyanidins, and lignans. J. Funct. Foods, 1, 57-64.
Hu C., Yuan Y.V. & Kitts D.D., 2007. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol., 45, 2219-2227.
Itoh T., Takagi Y. & Tsukube H., 1997. Synthesis of chiral building blocks for organic synthesis via lipase-catalyzed reaction: new method of enhancing enzymatic reaction enantioselectivity. J. Mol. Catal. B: Enzym., 3, 259-270.
Kitts D.D., Yuan Y.V., Wijewickreme A.N. & Thompson L.U., 1999. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell. Biochem., 202, 91-100.
Lainé E., Hano C. & Lamblin F., 2007. Les lignanes phytoestrogènes du lin sont-ils des bienfaiteurs méconnus ? Phytothérapie, 5, 121-128.
Mäkelä T.H., Wahala K.T. & Hase T.A., 2000. Synthesis of enterolactone and enterodiol precursors as potential inhibitors of human estrogen synthetase (aromatase). Steroids, 65, 437-441.
Mazur W. & Adlercreutz H., 1998. Natural and anthropogenic environmental estrogens: the scientific basis for risk assessment. Naturally occurring oestrogens in food. Pure Appl. Chem., 9, 1759-1776.
Mesa-Siverio D. et al., 2008. Structure and estrogenic activity of new lignans from Iranthera lancifolia. Bioorg. Med. Chem., 16, 3387-3394.
Milder I.E.J. et al., 2005. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Brit. J. Nutr., 93, 393-402.
Morreel K. et al., 2010. Mass spectrometry-based fragmentation as an identification tool in lignomics. Anal. Chem., 82, 8095-8105.
Moss G.P., 2000. Nomenclature of lignans and neolignans (IUPAC Recommendations 2000). Pure Appl. Chem., 72, 1493-1523.
Nemes S.M. & Orsat V., 2011. Microwave-assisted extraction of secoisolariciresinol diglucoside. Method development. Food Bioprocess. Technol., 4, 1219-1227.
Okunishi T., Umezawa T. & Shimada M., 2004. Semi-micro chiral HPLC analysis of lignans. J. Wood Sci., 50, 93-96.
Pelter A., Ward R.S., Jones D.M. & Maddocks P., 1992. Asymmetric syntheses of lignans of the dibenzylbutyrolactone, dibenzylbutanediol, aryltetralin and dibenzocyclooctadiene series. Tetrahedron: Asymmetry, 3, 239-242.
Peñalvo J.L. et al., 2004. Plant lignans in soy-based health supplements. J. Agric. Food Chem., 52, 4133-4138.
Raffaelli B., Hoikkala A., Leppala E. & Wahala K., 2002. Enterolignans. J. Chromatogr. B, 777, 29-43.
Schwartz H. & Sontag G., 2006. Determination of secoisolariciresinol, lariciresinol and isolariciresinol in plant foods by high performance liquid chromatography coupled with coulometric electrode array detection. J. Chromatogr. B, 838, 78-85.
Sefkow M., Raschke M. & Steiner C., 2003. Enantioselective synthesis and biological evaluation of a-hydroxylated lactone lignans. Pure Appl. Chem., 75(2-3), 273-278.
Thompson L.U., Rickard S.E., Orcheson L.J. & Seidl M., 1996. Flaxseed and its lignan and oil components reduce mammary tumor growth at a late stage of carcinogenesis. Carcinogenesis, 17, 1373-1376.
Touré A. & Xu X.M., 2010. Flaxseed lignans: source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr. Rev. Food Sci. Food Saf., 9, 261-269.
Umezawa T., 2003. Diversity in lignan biosynthesis. Phytochem. Rev., 2, 371-390.
Umezawa T., Davin L.B. & Lewis N.G., 1991. Formation of lignans (-)-secoisolariciresinol and (-)-matairesinol with Forsythia intermedia cell-free extracts. J. Biol. Chem., 266, 10210-10217.
van Oeveren A., Jansen J.F.G.A. & Feringa B.L., 1994. Enantioselective synthesis of natural dibenzylbutyrolactone lignans (-)-enterolactone, (-)-hinokinin, (-)-pluviatolide, (-)-enterodiol, and furofuran lignan (-)-eudesmin via tandem conjugate addition to gamma-alkoxybutenolides. J. Org. Chem., 59(20), 5999-6007.
Wang C.C., Prasain J.K. & Barnes S., 2002. Review of the methods used in the determination of phytoestrogens. J. Chromatogr. B, 777, 3-28.
Wang C.Z. et al., 2010. Production of enterodiol from defatted flaxseeds through biotransformation by human intestinal bacteria. BMC Microbiol., 10.
Wang L.Q., 2002. Mammalian phytoestrogens: enterodiol and enterolactone. J. Chromatogr. B, 777, 289-309.
Wang L.Q. et al., 2000. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem. Pharm. Bull., 48, 1606-1610.
Wang Q. et al., 2006. An efficient method for the synthesis of lignans. Tetrahedron, 62, 6107-6112.
Ward R.S. & Hughes D.D., 2001. Oxidative cyclisation of 3,4-dibenzyltetrahydrofurans using ruthenium tetra(trifluoroacetate). Tetrahedron, 57, 2057-2064.
Westcott N.D. & Muir A.D., 1998. Process for extracting lignans from flaxseed. United States Patent 5705618.
Westcott N.D. & Muir A.D., 2003. Flaxseed lignan in disease prevention and health promotion. Phytochem. Rev., 2, 401-417.
Willfor S.M., Smeds A.I. & Holmbom B.R., 2006. Chromatographic analysis of lignans. J. Chromatogr. A, 1112, 64-77.
Om dit artikel te citeren:
Over : Pauline Sainvitu
Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Katherine Nott
Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium) – Univ. Liege - Gembloux Agro-Bio Tech. Department of General and Organic Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Gaëtan Richard
Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium) – Univ. Liege - Gembloux Agro-Bio Tech. Department of General and Organic Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Christophe Blecker
Univ. Liege - Gembloux Agro-Bio Tech. Department of Food Science and Formulation. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Christine Jérôme
Univ. Liege. Center for Education and Research on Macromolecules. Chemistry Institute. Sart-Tilman, B6a. B-4000 Liege (Belgium).
Over : Jean-Paul Wathelet
Univ. Liege - Gembloux Agro-Bio Tech. Department of General and Organic Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Michel Paquot
Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Over : Magali Deleu
Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: magali.deleu@ulg.ac.be






