- Accueil
- volume 16 (2012)
- numéro 2
- Kinetics of the hydrolysis of polysaccharide galacturonic acid and neutral sugars chains from flaxseed mucilage
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Kinetics of the hydrolysis of polysaccharide galacturonic acid and neutral sugars chains from flaxseed mucilage

Notes de la rédaction
Received on February 25, 2011; accepted on November 17, 2011
Résumé
Cinétique d’hydrolyse des chaines de sucres neutres et d’acide galacturonique des polysaccharides du mucilage de graines de lin. Différentes voies d’hydrolyse (chimique et enzymatique) des polysaccharides du mucilage de graines de lin ont été réalisées avec H2SO4, HCl et le TFA à des concentrations d'acide (0,2, 1 et 2 M) et à des températures (80 et 100 °C) différentes. Les hydrolyses enzymatiques chimiques combinées à l'hydrolyse enzymatique ont également été étudiées. Les meilleures teneurs en monosaccharides de graines de lin sont obtenues à des conditions d'hydrolyse acide (2 M H2SO4, 4 h, 100 °C). La voie enzymatique (Pectinex™ Ultra SP) limite la destruction du sucre lors de l'hydrolyse, mais elle est également insuffisante pour la dépolymérisation complète. La combinaison des deux traitements, à savoir l'hydrolyse chimique modérée (0,2 M H2SO4, 80 °C, 48 h) et l'hydrolyse enzymatique, n'est pas plus efficace par rapport à l'hydrolyse chimique dans des conditions drastiques (2 M H2SO4 à 100 °C). La forte interaction entre les fractions acide et neutre du mucilage de lin est suggérée comme responsable de sa dépolymérisation partielle. Un traitement physique avant l’hydrolyse pourrait être nécessaire pour une dépolymérisation complète du mucilage de lin.
Abstract
Different hydrolysis procedures of flaxseed polysaccharides (chemical and enzymatic) were carried out with H2SO4, HCl and TFA at different acid concentrations (0.2, 1 and 2 M) and temperatures (80 and 100°C). Enzymatic and combined chemical and enzymatic hydrolyses of polysaccharide from flaxseed mucilage were also studied. Acid hydrolysis conditions (2 M H2SO4, 4 h, 100°C) are required to quantify total monosaccharide content of flaxseed mucilage. The enzymatic pathway (Pectinex™ Ultra SP) limits sugar destruction during hydrolysis, but it is also insufficient for complete depolymerization. The combination of the two treatments, i.e. moderate chemical hydrolysis (0.2 M H2SO4, 80°C, 48 h) combined with enzymatic hydrolysis is not more effective compared to chemical hydrolysis in drastic conditions (2 M H2SO4 at 100°C). The strong interaction between the neutral and acid fractions of flaxseed mucilage may hinder total release of sugar residues. Physical treatment prior to the hydrolysis could be necessary to achieve complete depolymerisation of flaxseed mucilage.
Table des matières
1. Introduction
1Flaxseed (Linum usitatissimum L.) is known as a functional food source due to their high content in oil rich in α-linolenic acid and dietary fibre (Oomah, 2003), and their medicinal properties like anti-inflammatory and anti-tumor effects (Tolkachev et al., 2000). Flaxseed contains approximately 40% lipid, 25% proteins, and 30% fibre. Soluble mucilage represents 3.5-10% of fibre fraction, and is known to have potential benefits against diabetes and cardiovascular diseases (Rickard et al., 1997). Recently, flaxseed mucilage has received attention as a source of biological active oligosaccharides (Guilloux et al., 2009). Mucilage is often used as a stabilizer in beverage, and is patented as a texture ingredient in dairy desserts (Qin et al., 2005; Anttila et al., 2008). A possible synergistic effect with proteins on their functionalities could also be valuable in foods (Rabetafika et al., 2011).
2Flax mucilage is located in the epidermal cell layer of the seed coat and its extraction can be achieved by aqueous method from the seeds followed by a drying process after which a low viscosity mucilage is obtained (Attström et al., 1993; Kankaanpaa-Antilla, 1999; Oomah et al., 2001). It can also be obtained by extraction from the hull (Cui et al., 1996a; Myllymäki, 2002). The water soluble mucilage is constituted by a polysaccharidic mixture, containing a neutral (75%) and two acidic fractions (25%) (Muralikrishna et al., 1987; Cui et al., 1994; Fedeniuk et al., 1994; Warrand et al., 2003). In addition, it contains traces of pectin and glucan.
3The neutral polysaccharides or arabinoxylans are composed of linear β-(1→4)-xylopyranose backbone substituted with arabinofuranose side chains attached via α-(1→3) and/or α-(1→2) linkages (Izydorczyk et al., 1995; Naran et al., 2008). This fraction contains L-arabinose, D-xylose and D-galactose. The acidic polysaccharide fraction is essentially composed of rhamnogalacturonan, and contains L-rhamnose, L-fucose, L-galactose and D-galacturonic acid (Muralikrishna et al., 1987).
4According to Cui et al. (1994), rheological properties of flaxseed mucilage depend on their chemical structure and composition. Some variability in the composition of flaxseed mucilage is however observed depending on the species as well as the cultivars and the seasons (Oomah et al., 1998; Cui et al., 1996b; Kadivar, 2001; Oomah, 2003). Moreover, the analysis method may reveal variety in the same species. Various quantification methods of flaxseed sugars are reported in the literature (Bhatty et al., 1990; Cui et al., 1996a; Warrand et al., 2003). Neutral and acid sugars can be quantified by colorimetric assays after reaction with, respectively, resorcinol-sulphuric acid and meta-hydroxydiphenyl. This method is however not specific due to the interference with impurities in the analyzed sample. Chromatographic methods such as gas chromatography with flame ionization detector (GC-FID) and high performance anion exchange chromatography coupled with pulsed amperometric detector (HPAEC-PAD) are more accurate but need to form volatile derivative in the case of gas chromatography analysis. Prior to these analyses, samples have to be hydrolyzed without destruction of sugar residues. Recovery of total hemicellulose's sugars is also an important factor in the context of biorefineries for bioethanol and other added value chemicals production (Carvalheiro et al., 2008).
5Several procedures are available for hydrolysis of the mucilage to uronic acid and neutral monosaccharide components. Methods involving acids (Cui et al., 1996a; Wanasundara et al., 1997; Oomah et al., 1998; Oomah et al., 2001; Myllymäki, 2002; Warrand et al., 2005; Guilloux et al., 2009) have been reported. The differences observed in the results suggest that hydrolytic conditions and techniques should be investigated.
6Moreover, studies on pectin polysaccharides showed different types of hydrolysis namely chemical hydrolysis (Leitao et al., 1995; Marga et al., 1995), enzymatic hydrolysis and combined chemical and enzymatic hydrolysis (Garna et al., 2004; Garna et al., 2006). Acid hydrolysis of pectin to galacturonic acid requires a prolonged treatment with acid to achieve complete hydrolysis and, once released, the galacturonic acid is subjected to degradation, forming lactones in irreproducible amounts (Blake et al., 1968). The conditions recommended for acid hydrolysis have been found to be far from ideal for the quantitative analysis of the urinate content in acidic polysaccharides (Leitao et al., 1995). Enzymatic pectin hydrolysis is a better technique for hydrolysis of this polymer without degradation. However, this procedure needs different types of enzyme activities such as pectolytic, hemicellulolytic and carbohydratases for an efficient degradation of pectin (Matsuhashi et al., 1992; Leitao et al., 1995). It is reported that the structure of the acid fraction in flaxseed mucilage (Figure 1) is similar to that of pectin (Naran et al., 2008).
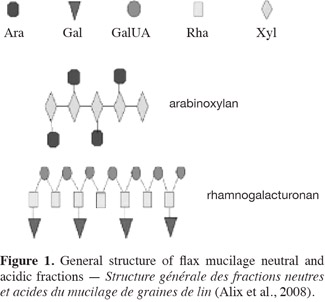
7On the basis of studies on neutral and acidic polysaccharides, it would be interesting to investigate other types of hydrolysis such as enzymatic hydrolysis and combined chemical and enzymatic hydrolysis.
8The purpose of the present work is to investigate the kinetic of the liberation and degradation of galacturonic acid and neutral sugar chains as a function of hydrolysis parameters namely acid concentration, nature and temperature of reactions in order to achieve complete characterization of flaxseed polysaccharide fraction.
9In this context, three patterns of hydrolysis have been carried out in order to obtain the complete release of galacturonic acid and neutral sugars from flaxseed mucilage: acid or enzymatic or combined chemical and enzymatic hydrolysis.
2. Materials and methods
2.1. Flaxseed mucilage production
10Flaxseed (Linum usitatissimum L.) cultivar ‘Bethune’ was obtained from SA Vandeputte (Mouscron, Belgium). Mucilage was supplied by the research team of the PROFLAX WAGRALIM project.
11Mucilage was produced from flaxseed by aqueous extraction (1/10: w/v) at 100°C under high agitation during 3 min, and after filtration through a metallic sieve (1 mm). The mucilage thus obtained was, further, freeze-dried prior to analysis.
2.2. Standard solutions
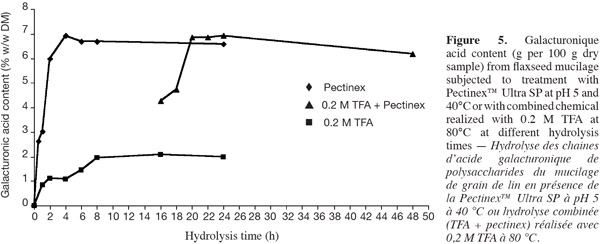
12The determination of galacturonic acid was done using a standard solution of 1 mM galacturonic acid and 1 mM glucuronic acid. They were prepared from a 10 mM stock solution. This was also used to determine the relative response factors.
13The determination of neutral sugars was done using 2-desoxy-D-glucose (purity > 99.5%, from Sigma Chemical Co., St Louis, MO) as internal standard. Other standard solutions included L-arabinose, D-galactose (Aldrich, Germany), L-rhamnose (Sigma, Germany), D-mannose, D-xylose (Fluka, Czech Republic) and D-glucose (Merck, Germany). Standard solutions were prepared as recommended by the manufacturer.
2.3. Enzymes
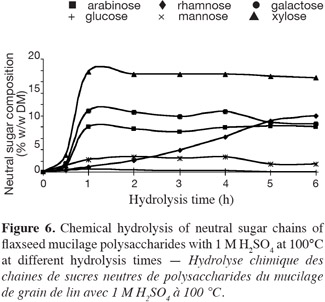
14The enzyme used, Pectinex™ Ultra SP is a commercial liquid preparation obtained from Novo Nordisk (Copenhagen, Denmark). This enzyme preparation is produced from a selected strain of Aspergillus niger.
2.4. Chemical hydrolysis
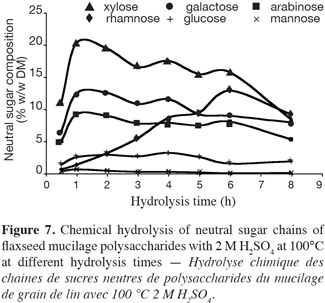
15H2SO4 hydrolysis. Twenty-five milligrams of mucilage were hydrolyzed (at different time points) with 0.2, 1 and 2 M of H2SO4 (2.5 ml) at 80 or 100°C. After the hydrolysis, the reaction medium was neutralized with NH4OH (14 M) and 2 ml of glucuronic acid (10 mM) was added as internal standard. This solution is adjusted to pH 7 and diluted to 25 ml. Aliquot of hydrolysate was filtered through a 0.45 µm filter membrane before injection in HPAEC-PAD.
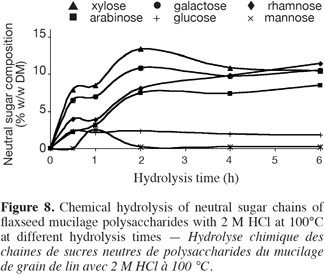
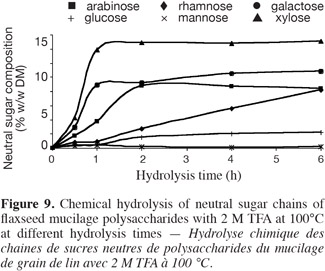
16TFA hydrolysis. Twenty-five milligrams of mucilage was hydrolyzed with 2.5 ml of 2 M TFA at 100°C; then, the hydrolysate was treated as in the case of H2SO4 hydrolysis.
2.5. Enzymatic hydrolysis
17A volume of 10 ml of mucilage solution (2 g·l-1) was mixed with 10 ml of Pectinex™ Ultra SP (Novo Nordisk, Denmark) diluted 500-fold in 20 mM sodium acetate buffer (pH 4.5-4.6) containing 0.2 mM glucuronic acid as internal standard. The mixture was incubated at 40°C for 24 h. During the hydrolysis, aliquots of hydrolysate were taken at time interval (0 to 24 h) and heated at 100°C for 3 min to inactivate the enzymes and filtered through a 0.45 µm filter membrane before injection in HPAEC-PAD.
2.6. Combined chemical and enzymatic hydrolysis
18Twenty-five milligrams of mucilage were subjected to treatment with 2.5 ml of 0.2 M TFA and H2SO4 during 16 to 72 h at 80°C. The final pH was adjusted at 5 and the hydrolysate was diluted to 25 ml. Twenty milliliters of this solution was mixed with 20 ml of Pectinex™ Ultra SP enzyme diluted 500-fold in 20 mM pH 5 sodium acetate buffer and containing glucuronic acid (2 mM) as internal standard. The mixture was incubated at 40°C for 4 h and treated as described above.
2.7. Moisture
19The moisture content was determined by drying at 105°C for 24 h. All values were calculated on a dry-weight basis.
2.8. Analytical method
20Galacturonic acid was determined by HPAEC-PAD, while the neutral sugars were acetylated, and analyzed by gas chromatography (GC).
21HPAEC-PAD: The separation of galacturonic acid was done by high-performance anion-exchange chromatography hyphenated to a pulsed amperometric detector (HPAEC-PAD) (Garna et al., 2006). Hydrolysates (25 µl) were injected on a Dionex DX-500 chromatographic system (Dionex Corp., Sunnyvale, CA) using a CarboPac PA100 column (4 × 250 mm) in combination with a CarboPac PA100 guard column (4 × 50 mm). The mobile phase consisted of sodium hydroxide (100 mM) elution in isocratic mode, followed by a linear gradient with a solution containing both sodium hydroxide (100 mM) and sodium acetate (150 mM). The gradient ended by washing with sodium hydroxide 500 mM. Then, the column was conditioned with sodium hydroxide 100 mM. All eluents were pumped at a flow rate of 1 ml·min-1 at 30°C.
22Gas Chromatography analysis of neutral sugars was carried out using a Hewlett Packard HP 6890 Series GC system, fitted with a hydrogen flame ionization detector. The monosaccharides liberated by chemical hydrolysis were converted to alditol acetates according to the procedure of Blakeney et al. (1983). Alditol acetates derivatives were separated in a high performance capillary column HP1 (length 30 m, internal diameter 0.5 mm, film thickness 0.25 μm). Injector (splitless mode) and detector temperatures were 250 and 300°C, respectively. Oven temperature initially at 120°C was programmed to rise linearly at 4°C·min-1 until 220°C after the separation of sugar and subsequently to reach 290°C at a rate of 35°C·min-1 in order to condition the column. Carrier gas was helium.
2.9. Statistical analysis
23All experiments were done in duplicate. To maintain quality control during chemical analyses, the error between duplicate samples was determined. If the error between duplicate samples was greater than 5%, the analysis was repeated.
3. Results and discussion
3.1. Chemical hydrolysis of galacturonic acid chains of flax mucilage
24The different results obtained by the chemical hydrolysis of galacturonic acid chains of flax mucilage with different acid concentrations at temperature 80 and 100°C are shown (Figure 2 to Figure 4).
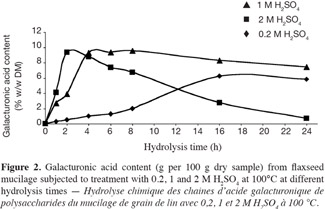
25Chemical hydrolysis with 0.2, 1 and 2 M sulphuric acid at 100°C. The results presented in figure 2 show that recoveries of galacturonic acid after treatment with 0.2, 1 and 2 M sulphuric acid at 100°C are the highest obtained after 16, 8 and 2 h, respectively. The galacturonic acid of flax mucilage within these conditions represented 6%, 10% and 10% by weight of total dry matter (w/w DM), respectively. For long-time hydrolysis, the destruction rate of free galacturonic acid is greater than the release rate from the polysaccharide. Garna et al. (2006) showed similar effect on the liberation of pectin galacturonic acid under the same conditions of hydrolysis. The release of galacturonic acids was considerably accelerated with greater concentration of sulphuric acid.
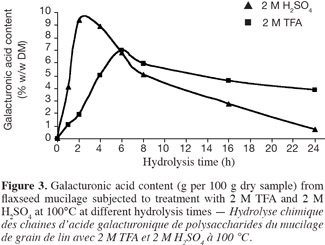
26Chemical hydrolysis with 2 M TFA at 100°C. The trifluoroacetic acid (2 M) was also used at 100°C for the hydrolysis of flax mucilage polysaccharides. As presented in figure 3, it is clear that the type of acid quantitatively affects the end product. The highest percentage was obtained when the mucilage was treated with H2SO4. Ten percent and 7 % (w/w DM) of the galacturonic acid was quantified, respectively, after 2 h hydrolysis with 2 M H2SO4 and after 6 h hydrolysis with 2 M TFA. It was however observed that trifluoroacetic acid causes less damage to galacturonic acid than H2SO4. At the same concentration of H+ (or same pH), H2SO4 1 M was also more efficient to release acid galacturonic compared to TFA 2 M regarding the highest content of acid galacturonic (10% versus 7% w/w DM). Guilloux et al. (2009) used trifluoroacetic acid in drastic conditions (100°C, 4 h, 4 M) and obtained lower recoveries (6% w/w DM) of the galacturonic acid compared to our results. This may be due to differences in sugar composition of flaxseed (Wannerberger et al., 1991).
27Furthermore, hydrolysis of polysaccharides by sulphuric acid has been described as a superior hydrolysis procedure for carbohydrates (Garleb et al., 1989) because it allows almost a total hydrolysis of these polymers. However, the removal of sulfate ions after hydrolysis is difficult. It is inadequate in the analysis of sugars by HPAEC analysis (Salvador et al., 2000).
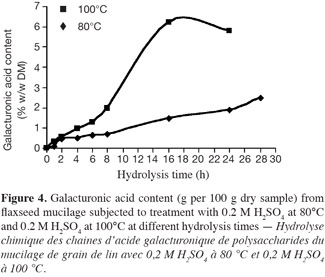
28Chemical hydrolysis with 0.2 M sulphuric acid at 80°C. The chemical hydrolysis of polysaccharides of flax mucilage with 0.2 M H2SO4 at 80°C is characterized by the liberation of galacturonic acid without any degradation for the first 28 h (Figure 4). However, only 2.5% (w/w DM) of galacturonic acids of flax mucilage polysaccharide were obtained in these conditions. Compared with hydrolysis in drastic conditions at 100°C, lower contents are obtained, which is due to the higher resistance of the galacturonic linkages under milder hydrolysis conditions (Biermann, 1988; De Ruiter et al., 1992).
3.2. Enzymatic hydrolysis of galacturonic acid chains of flax mucilage
29Enzymatic hydrolysis of polysaccharide galacturonic acid chains using Pectinex Ultra SP was studied. This mixture contains multiple enzyme activities mainly polygalacturonases and hemicellulases (Wanasundara et al., 1997). According to figure 5, up to 7% (w/w DM) of the galacturonic acids were released after 4 h of hydrolysis. Compared to the results obtained with 2 M TFA for 4 h at 2 M (acid that causes less damages), it is clear that the enzymatic hydrolysis produces better results. In contrast, under 2 M H2SO4 conditions the galacturonic-acid bond is broken more efficiently. Several authors have recommended enzymatic approach to estimate total content of galacturonic acid from pectin chain (Leitao et al., 1995; Rumpunen et al., 2002; Garna et al., 2004). Garna et al. (2004) obtained a high yield (66%) of galacturonic acid chain for apple pectin with enzymatic hydrolysis compared to 40% obtained with chemical hydrolysis.
3.3. Combined chemical and enzymatic hydrolysis galacturonic acid chains of flax mucilage
30Previous results show that the destruction rate of free galacturonic acid is greater than the release rate from the polysaccharide with drastic conditions of hydrolysis while mild chemical hydrolysis is insufficient for complete depolymerization. In addition, the trifluoroacetic acid causes less damage to galacturonic acid than H2SO4.
31For this reason, the chemical hydrolysis with 0.2 M TFA at 80°C was followed by an enzymatic hydrolysis with Pectinex™ Ultra SP enzymatic extracts. This enables the assurance to obtain a complete hydrolysis of galacturonic acid. The results of this combination are presented in figure 5 and are compared with those obtained by the two treatments separately. According to this figure, the galacturonic acid content is comparable to that obtained by enzymatic hydrolysis. However, H2SO4 hydrolysis gives the higher galacturonic acid content (Figure 2). According to the literature, the D-galacturonic acid is strongly attached to the rhamnosyl O-3 position (Naran et al., 2008). Thus, drastic hydrolysis conditions are required to release the acid galacturonic residues.
3.4. Chemical hydrolysis of neutral sugar chains of flax mucilage
32Chemical hydrolysis with 1 M sulphuric acid at 100°C. The results obtained after the chemical hydrolysis of flax mucilage polysaccharide neutral sugar chains using 1 M H2SO4 at 100°C are shown in figure 6. The released sugar concentrations increased till a maximal value before decreasing.
33It was observed that the release of polysaccharide sugars was not realized at the same rate for each carbohydrate; the different optima differ from one sugar to another. The maximum concentrations obtained after 1 h of hydrolysis were as follows: xylose (17.7% w/w DM), galactose (8.1% w/w DM) and arabinose (10.8% w/w DM). The xylose was first liberated followed by galactose then arabinose. The L-rhamnose linked to the D-galacturonic acid was the last sugar released during the hydrolysis. The reason for the longer time of liberation of rhamnose is the multiple bonds between this sugar and galacturonic acid (De Ruiter et al., 1992). This observation was confirmed by a previous study (Anderson et al., 1947).
34Chemical hydrolysis with 2 M sulphuric acid at 100°C. The chemical hydrolysis of acidic fraction using 2 M H2SO4 at 100°C (Figure 7) shows the same kinetics as with 1 M H2SO4. The liberation and degradation of sugar was faster than in the first case of hydrolysis (1 M H2SO4 at 100°C). Xylose (20% w/w DM), galactose (12% w/w DM), and arabinose (9% w/w DM) have a maximum hydrolysis after 1 h contrary to rhamnose (6 h).
35Chemical hydrolysis with 2 M HCl and 2 M TFA at 100°C. Other acids such as hydrochloric acid (2 M) and trifluoroacetic acid (2 M) were used at 100°C for the hydrolysis of flax mucilage polysaccharide. The kinetics of sugar release with HCl 2 M (Figure 8) were less efficient compared to those of H2SO4 (1 M and 2 M) hydrolysis (Figures 6 and 7). Whereas, the results obtained with TFA 2 M (Figure 9) were similar to those obtained with 1 M H2SO4 hydrolysis (Figure 6). Hydrolysis with H2SO4 2 M was the most effective. However it was found that the trifluoroacetic acid caused less damage to sugars than H2SO4 or HCl especially in the case of rhamnose. The results obtained with the chemical hydrolysis with 2 M TFA and 100°C differ from those obtained by Guilloux et al. (2009) in more drastic conditions (100°C, 4 h, 4 M). In a previous research the same kinetics of the hydrolysis of polysaccharide neutral sugars chains were obtained with H2SO4 2 M, TFA 2 M and 2 M HCl at 80°C (results not shown).
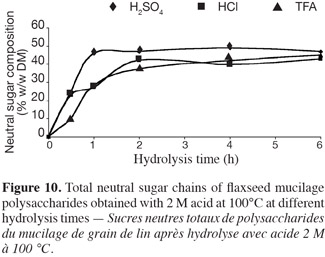
36Total neutral sugar with 2 M H2SO4, 2 M HCl and 2 M TFA at 100°C. The content of certain neutral sugars increased during hydrolysis while other monosaccharides were also destroyed. Thus, the effect of the acid type (H2SO4, HCl and TFA) was also assessed; the results are presented in figure 10. The hydrolysis with 2 M H2SO4 was more effective compared to the two other acid types. In the case of HCl and H2SO4 hydrolysis, the acid type influenced the hydrolysis kinetics of mucilage polysaccharides. The kinetic pattern of sugar hydrolysis with TFA 2 M were similar to that of H2SO4 1 M. The difference between the results obtained with TFA 2 M and H2SO4 2 M may be explained by the concentration of the catalytically active protons. It was also observed that the maximum of total neutral monosaccharide content was reached after 4 h hydrolysis. Thus for routine assays and to compare different samples, the following hydrolysis conditions may be used: 2 M H2SO4, 4 h, at 100°C.
4. Conclusion
37The knowledge of the optimal conditions for hydrolysis of the flax mucilage is of utility for its valorization. Studying the kinetics of the total hydrolysis of the flax mucilage has not been reported before and from the results obtained, it can be concluded that chemical hydrolysis of flax mucilage with strong acid (H2SO4) at high temperature (100°C) seems to combine two simultaneous phenomena: firstly, the release of sugars as a product of hydrolysis of the flax mucilage and, secondly, their degradation under action of the acid and the heat. The use of trifluoroacetic acid for hydrolysis of flax mucilage causes less damage to sugars than that of H2SO4 and HCl. However, the concentrations of the sugars obtained with mild hydrolysis are less than those reached after drastic hydrolysis. The enzymatic pathway limits sugar destruction during hydrolysis, the combination of the two treatments, i.e. moderate chemical hydrolysis (0,2 M H2SO4, 80°C, 48 h) combined with enzymatic hydrolysis is not more effective compared to chemical hydrolysis in drastic conditions (2 M H2SO4 at 100°C). It would be interesting to investigate other types of enzymes capable of releasing more galacturonic acid than Pectinex™ Ultra SP used in this study. However, the following hydrolysis conditions (H2SO4 2 M, 4 h, 100°C) are required when quantifying total monosaccharide contents of flaxseed mucilage. The difficulty of quantifying total monosaccharides in flaxseed mucilage arises from the strong interaction between its neutral and acid fractions. Physical treatment prior to the hydrolysis could be necessary to achieve complete depolymerisation of flaxseed mucilage. In practice, knowledge of optimal condition of hemicellulose hydrolysis pretreatment is also an important economic factor in sugar biorefinery platforms.
38Acknowledgements
39This research was supported by the University of Liege, Gembloux Agro-Bio Tech through a post-doctoral fellowship awarded to Dr Thomas Happi Emaga.
Bibliographie
Anderson E. & Lowe H.J., 1947. The composition of flaxseed mucilage. J. Biol. Chem., 168, 289-297.
Alix S., Marais S., Morwan C. & Lebrun L., 2008. Biocomposite materials from flax plants: preparation and properties. Composites Part A, 39, 1793-1801.
Anttila M. et al., 2008. Improving of texture of dairy products. WO/2008/000913, Jan. 3, 2008.
Attström R., Glantz P., Hatansson H. & Larsson K., 1993. Saliva substitute. US patent 5260282, Nov. 9, 1993.
Bhatty R. & Cherdkiatgumchai P., 1990. Compositional analysis of laboratory-prepared and commercial samples of linseed meal and of hull isolated from flax. J. Am. Oil Chem. Soc., 67, 79-84.
Biermann C.J., 1988. Hydrolysis and other cleavages of glycosidic linkages in polysaccharides. Adv. Carbohydr. Chem. Biochem., 46, 251-271.
Blake J.D. & Richards G.N., 1968. Problems of lactonization in the analysis of uronic acids. Carbohydr. Res., 8, 275-281.
Blakeney A.B., Harris P.J., Henry R.J. & Stone B.A., 1983. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res., 113, 291-299.
Carvalheiro F., Duarte L. & Girio F.M., 2008. Hemicellulose biorefineries: a review on biomass pretreatments. J. Sci. Ind. Res., 67, 849-864.
Cui W. et al., 1994. Chemical structure, molecular size distributions, and rheological properties of flaxseed gum. J. Agric. Food Chem., 42, 1891-1895.
Cui W., Kenaschuk E. & Mazza G., 1996a. Influence of genotype on chemical composition and rheological properties of flaxseed gums. Food Hydrocoll., 10, 221-227.
Cui W. & Mazza G., 1996b. Physicochemical characteristics of flaxseed gum. Food Res. Int., 29, 397-402.
De Ruiter G.A., Schols H.A., Voragen A.G. & Rombouts F.M., 1992. Carbohydrate analysis of water-soluble uronic acid containing polysaccharides with high-performance anion-exchange chromatography using methanolysis combined with TFA hydrolysis is superior to four other methods. Anal. Biochem., 207, 176-185.
Fedeniuk R.K. & Biliaderis C.G., 1994. Composition and physicochemical properties of linseed mucilage. J. Agric. Food Chem., 42, 240-247.
Garleb K.A., Bourquin L.D. & Fahey G.C., 1989. Neutral monosaccharide composition of various fibrous substrates: a comparison of hydrolytic procedures and use of anion-exchange high-performance liquid chromatography with pulsed amperometric detection of monosaccharides. J. Agric. Food Chem., 37, 1287-1293.
Garna H., Mabon N., Wathelet B. & Paquot M., 2004. New method for two-step hydrolysis and chromatographic analysis of pectin neutral sugar chains. J. Agric. Food Chem., 52, 4652-4659.
Garna H. et al., 2006. Kinetic of the hydrolysis of pectin galacturonic acid chains and quantification by ionic chromatography. Food Chem., 96, 477-484.
Guilloux K. et al., 2009. Production of arabinoxylan-oligosaccharides from flaxseed (Linum usitatissimum). J. Agric. Food Chem., 57, 11308-11313.
Izydorczyk M. & Biliaderis C., 1995. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr. Polym., 28, 33-48.
Kadivar M., 2001. Studies on integrated processes for the recovery of mucilage, hull, oil and protein from solin (Low Linolenic acid flax). Thèse de doctorat : Université de Saskatchewan (Canada).
Kankaanpaa-Antilla B., 1999. Flax preparation, its use and production. US Patent 5925401, July 20, 1999, http://www.freepatentsonline.com/5925401.html (July 20, 2010).
Leitao M.C.A., Alarcao Silva M.L., Januario M.I.N. & Azinheira H.G., 1995. Galacturonic acid in pectic substances of sunflower head residues: quantitative determination by HPLC. Carbohydr. Polym., 26, 165-169.
Marga F., Freyssac V. & Morvan H., 1995. Rapid gas liquid chromatography microanalysis of carbohydrates in woody plant tissues. J. Trace Microprobe Tech., 13, 473-478.
Matsuhashi S., Inoue S.I. & Hatanaka C., 1992. Simultaneous measurement of the galacturonate and neutral sugar contents of pectic substances by an enzymatic-HPLC method. Biosci. Biotechnol. Biochem., 56, 1053-1057.
Muralikrishna G., Salimath P.V. & Tharanathan R.N., 1987. Structural features of an arabinoxylan and rhamnogalacturonan derivative from linseed mucilage. Carbohydr. Res., 161, 265-271.
Myllymäki O., 2002. Processing of flaxseed. US Patent 6440479 B1. Aug. 27, 2002.
Naran R., Chen G. & Carpita N., 2008. Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiol., 148, 132-141.
Oomah B.D., 2003. Chapter 20: processing of flaxseed fiber, oil, protein, and lignan. In: Thomson L. & Cunnane S., eds. Flaxseed in human nutrition. Champaign, IL, USA: AOCS Press, 363-386.
Oomah B.D. & Mazza G., 1998. Compositional changes during commercial processing of flaxseed. Ind. Crop. Prod., 9, 29-37.
Oomah B.D. & Mazza G., 2001. Optimization of a spray drying process for flaxseed gum. Int. J. Food Sci. Tech., 36, 135-143.
Qin L., Xu S.-Y. & Zhang W.-B., 2005. Effect of enzymatic hydrolysis on the yield of cloudy carrot juice and the effects of hydrocolloids on color and cloud stability during ambient storage. J. Sci. Food Agric., 85, 505-512.
Rabetafika H.N. et al., 2011. Flaxseed proteins: food uses and health benefits. Int. J. Food Sci. Tech., 46, 221-228.
Rickard S.E. & Thompson L.U., 1997. Health effects of flaxseed mucilage, lignans. Int. News Fats Oils Rel. Mat., 8, 860-865.
Rumpunen K., Thomas L., Badilas N. & Thibault J.-F., 2002. Validation of a combined enzymatic and HPLC method for screening of pectins in fruits of Japanese quince (Chaenomeles japonica). Lebensm. Wiss. Technol., 35, 490-496.
Salvador L.D. et al., 2000. Monosaccharide composition of sweetpotato fiber and cell wall polysaccharides from sweetpotato, cassava and potato analysed by the high-performance anion exchange chromatography with pulsed amperometric detection method. J. Agric. Food Chem., 48, 3448-3454.
Tolkachev O.N. & Zhuchenko A.A., 2000. Biologically active substances of flax: medicinal and nutritional properties (a review). Pharm. Chem. J., 34, 360-366.
Wanasundara P. & Shahidi F., 1997. Removal of flaxseed mucilage by chemical and enzymatic treatments. Food Chem., 59, 47-55.
Wannerberger K., Nylander T. & Nyman M., 1991. Rheological and chemical properties of mucilage in different varieties from linseed (Linum usitatissimum). Acta Agric. Scand., 41, 311-319.
Warrand J. et al., 2003. Large-scale purification of water-soluble polysaccharides from flaxseed mucilage, and isolation of a new anionic polymer. Chromatographia, 58, 331-335.
Warrand J. et al., 2005. Flax (Linum usitatissimum) seed cake: a potential source of high molecular weight arabinoxylans? J. Agric. Food Chem., 53, 1449-1452.
Pour citer cet article
A propos de : Thomas Happi Emaga
Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: guythappi@yahoo.fr – African Research Centre on Bananas and Plantains (CARBAP). PO Box 832. CAM-Douala (Cameroon).
A propos de : Nadia Rabetafika
Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium) – Univ. Liege - Gembloux Agro-Bio Tech. Department of Food Technology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Christophe S. Blecker
Univ. Liege - Gembloux Agro-Bio Tech. Department of Food Technology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
A propos de : Michel Paquot
Univ. Liege - Gembloux Agro-Bio Tech. Department of Industrial Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






