- Accueil
- volume 16 (2012)
- numéro 2
- Effect of salt stress on growth and accumulation of proline and soluble sugars on plantlets of Pistacia atlantica Desf. subsp. atlantica used as rootstocks
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Effect of salt stress on growth and accumulation of proline and soluble sugars on plantlets of Pistacia atlantica Desf. subsp. atlantica used as rootstocks

Notes de la rédaction
Received on February 10, 2011; accepted on November 8, 2011
Résumé
Effet du stress salin sur la croissance, l'accumulation de la proline et des sucres solubles sur des plantules porte-greffe de Pistacia atlantica Desf. subsp. atlantica. L'effet du stress salin sur quelques paramètres physiologiques et biochimiques des plantules de Pistacia atlantica Desf. subsp. atlantica a été étudié sous conditions contrôlées. Les plantules ont été cultivées dans des pots et irriguées avec la solution nutritive de Hoagland durant 120 jours. Ensuite, les plantules ont été séparées et traitées à 100, 200 et 400 meq·l-1 de NaCl + CaCl2 pendant 10 jours. Les sels sont rajoutés à la solution nutritive de Hoagland. Il a été établi que l'application de ces concentrations de sels cause un stress aux plantules de pistachier qui s'exprime par la réduction de croissance au niveau des tiges et des racines. La teneur en proline des feuilles croît significativement avec la concentration du milieu en sels pour tous les traitements. La teneur maximale de l'acide aminé est observée sous le traitement à 400 meq·l-1 pour l'ensemble des plantules. Par ailleurs, une différence significative pour la teneur relative en eau (RWC) est enregistrée sous l'effet 400 meq·l-1 de NaCl + CaCl2 ; en revanche, les plantules stressées à 100 meq·l-1 ne manifestent aucune influence du sel sur la teneur relative en eau. L'accumulation des sucres solubles pour le lot stressé à 100 meq·l-1 est supérieure à celle du lot stressé à 200 meq·l-1. Aussi, l'effet de la concentration de 400 meq·l-1 de NaCl + CaCl2 provoque une accumulation de ces composés glucidiques. On constate qu'après sept jours de stress, la concentration en sucres solubles croît en fonction de la concentration saline. Le taux de proline et de sucres réducteurs chez le porte-greffe P. atlantica. subsp. atlantica est très élevé. Les résultats obtenus au terme de cette étude montrent que P. atlantica. subsp. atlantica peut être utilisé comme porte-greffe de Pistacia vera, car il est tolérant à la salinité.
Abstract
The effect of salt stress on several physiological and biochemical parameters of Pistacia atlantica Desf. subsp. atlantica plantlets was studied under controlled conditions in a climatic room. The plants were grown in pots and irrigated with a Hoagland nutrient solution during 120 days. Then, the plantlets were treated for 10 days with 100, 200, and 400 meq·l-1 NaCl + CaCl2, added to the Hoagland nutrient solution. The applied salts caused stress on the young Pistacia plantlets by reducing the growth of roots and shoots. The amount of free proline in leaves increased significantly with salinity under all treatments, to reach a maximum rate at the highest salinity concentration (400 meq·l-1) for all the plantlets. On the other hand, a significant difference in relative water content (RWC) was noted under the effect of 400 meq·l-1 of NaCl + CaCl2. The plantlets stressed at 100 meq·l-1 did not exhibit any influence of the salt on RWC, but their accumulation of sugars was much higher than at 200 meq·l-1. At 400 meq·l-1 the plantlets also accumulated a high content of soluble sugars, and after seven days of stress, their accumulation rose with the increasing salt concentration. The content of proline and soluble sugars in P. atlantica subsp. atlantica rootstock was very high, indicating that P. atlantica subsp. atlantica can be used as rootstock for Pistacia vera as it is more tolerant to salinity.
Table des matières
1. Introduction
1Pistacia atlantica Desf. subsp. atlantica is distributed mainly in arid and semi-arid areas in North Africa (Quezel et al., 1963). It is one of the few tall tree species that forms a population outside forests in semi-arid areas, where environmental stress, mainly salinity and drought, limits growth and productivity of most plant species (Parvaiz et al., 2008). P. atlantica subsp. atlantica can grow under the severe conditions of arid and semi-arid areas and functions as a sand stabilizer (Benhassaini et al., 2007).
2The pistachio (Pistacia vera L.) is one of the most important commercial trees grown in Iran, Turkey, Tunisia, and the USA. It seems to be among the few crops of commercial value that tolerate salinity and low quality irrigation water, and it is therefore considered as a potential crop for many arid and semi-arid regions. It thrived well in areas with salty clay soils and high salinity, while many other permanent crops grow poorly. A six-year field study on the salt tolerance of pistachios on the Westside of the San Joaquin Valley in the USA (Kallsen et al., 2009) showed that this is indeed the best nut tree to grow in saline soil. . In Algeria, pistachio plantations are established on sodic soils and irrigated with brackish to saline water. These conditions result in reduced pistachio yields. Currently, in Algerian nurseries, pistachios (P. vera) are grafted on a rootstock species of the genus Pistacia, P. atlantica subsp. atlantica, which showed no decline in growth at any salinity level. Rootstock selection is important in any pistachio orchard and must be performed at the time of orchard establishment. Selection of the most suitable and compatible rootstock in order to obtain the highest yield and quality, as well as resistance to biotic and abiotic factors has economical importance. P. atlantica subsp. atlantica rootstock was selected as it is resistant to nematodes and appropriate for growing in sandy soils, but it was also more resistant to drought stress and was thus a suitable rootstock for dry regions (Ghazvini et al., 2007; Mirzaie-Nodoushan et al., 2010).
3Salinity and its effects on biomass production have already been studied (Benmahioul et al., 2009; Karimi et al., 2009), and a parallel has been drawn between different biochemical indicators and plant tolerance. Proline accumulation in plant tissues due to salinity stress, known to serve as a compatible osmolyte (Yang et al., 2003), was reported in many studies about pistachio (Kumar et al., 2003; Koca et al., 2007; Karimi et al., 2009). Soluble sugars are also believed to accumulate in plant tissues and cells due to salinity stress. They may act as an osmotic conservation factor (Khedr et al., 2003). Salt stress, like many other abiotic stresses, inhibits plant growth. High concentrations of salts cause ion imbalance and hyperosmotic stress in plants (Zhu, 2001; Rontain et al., 2002).
4The investigation of the effects of salt on the isolated tissues, organs, cells, or calluses, may not reflect exactly what may happen in the whole plant (Benmahioul et al., 2009; Chelli-Chaabouni et al., 2010). In fact, studies involving the whole plant can provide information that cannot be obtained otherwise. Despite the concerns expressed over the negative impacts of salinity on plant cultures, to the best of the authors’ knowledge, only few reports have investigated so far the salt tolerance of Pistacia species. The early stage of development usually represents the most salt sensitive phase in woody plants (Shannon et al., 1994). For this reason young plantlets represent a proper and valuable material to detect early salt response.
5The effects of rootstocks on physiological, and biochemical characteristics have not been investigated in Algeria. In view of the limited knowledge currently available on the salt tolerance of Pistacia species in our country, the present work was carried out to assess P. atlantica subsp. atlantica rootstocks for their salt tolerance, biochemical composition, and biomass production in salinity conditions.
2. Materials and methods
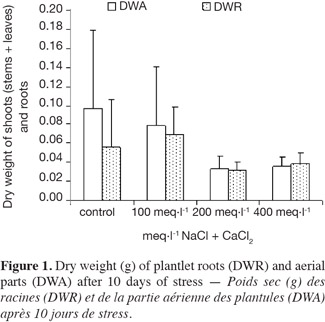
2.1. Materials and culture conditions
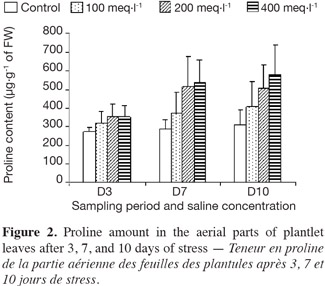
6The experiments were performed during the spring and summer seasons of 2010 at the research greenhouse of the Department of Agronomy in Tiaret University.
7Seeds of P. atlantica subsp. atlantica were collected at El-Bayadh (South-Western region of Algeria). The seeds were sterilized for 10 min in 0.1% (w/v) sodium hypochlorite and rinsed with distilled water. Then, seeds were planted in pots (25 x 40 cm) containing 6.5 kg sandy-loam soil with a pH of 7.2.
8Seedlings were grown in a greenhouse under a photoperiod of 16 h, at a day/night temperature of 28-18°C (± 2°C) and a day/night humidity of 50/60%, and were irrigated with Hoagland solution (Hoagland et al., 1938). After 120 days, the plantlets were grown individually in pots and watered with half-strength Hoagland nutrient solution. When the first leaves appeared, an experimental design with four treatments was managed as follows:
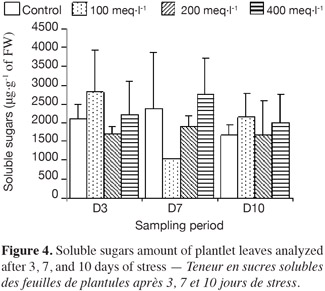
9– control plantlets, supplied with a Hoagland solution;
10– plantlets supplied with a Hoagland solution to which NaCl + CaCl2 at 100, 200, and 400 meq·l-1 were added for each salinity treatment.
11The treatment of plantlets with salts lasted for 10 days. The measurements were performed on leaves after 3, 7, and 10 days of treatment.
2.2. Relative water content (RWC)
12RWC was determined on fully expanded leaves of a similar age. Leaves were excised before dawn, weighed fresh (FW) and placed in distilled water at 2°C in the dark for 24 h to rehydrate. The following morning, leaf turgid weight (TW) was recorded. Then, leaves were dried at 80°C for 48 h, and the dry weight (DW) was determined (Barrs, 1968). The RWC was calculated as:
13RWC = [(FW - DW) / (TW - DW)] x 100.
2.3. Destructive analyses
14After 10 days of salt treatment, pistachio plantlets were harvested by extracting and washing roots from the soil. Then, the roots and aerial parts were separated, and the shoot height, root length, and biomass of shoots and roots were recorded. The shoot and root length in each sample was measured using a ruler.
15Plant material was washed thoroughly first with tap water and then twice with distilled water. Shoot and root fresh weights (FW) were then determined, while the respective dry weights (DWA) were measured after oven drying the samples at 80°C for 72 h.
2.4. Biochemical analyses
16Soluble sugars analysis. The content of soluble sugars was measured in 100 mg of dry leaves ground into a fine powder, which was soaked in 80% (v/v) ethanol for 24 h. The concentration of soluble sugars was measured following the method described by Shields and Burnet (1960). The absorbance was measured at 585 nm in a spectrophotometer (UV- Mini 1240, Shimadzu, Japan), and the values obtained were reported to a standard curve of glucose to determine the soluble sugars content (μg·g-1 of FW).
17Proline analyses. The leaf tissue proline content was measured following the method described by Troll and Lindsley (1955) and improved by Dreier and Göring (1974). Thus, 100 mg of fresh leaf material was homogenized in 2 ml of 40% methanol, and then heated in a water bath at 85°C for 60 min. The absorbance was measured at 528 nm in a spectrophotometer.
2.5. Statistics
18The data were subjected to analyses using SPSS 12.0. programme (Statistical Package for the Social Science). Comparisons between biochemical parameters were based on SPSS at 5% probability level.
19Biochemical measurements were repeated three times, and eight replications were done for the morpho-physiological parameters.
3. Results
3.1. Root length
20The length of the main root axes was not significantly affected by the salt treatment. The values obtained were, respectively, of 34.33 cm for the control, 33.62 cm at 100 meq·l-1, 29.68 cm at 200 meq·l-1, and 28.45 cm at 400 meq·l-1.
3.2. Dry weight of aerial parts and roots
21The effects of NaCl + CaCl2 on the dry weight of the aerial parts and the roots are shown in figure 1. The dry weight of the aerial parts was reduced for all P. atlantica rootstocks except for 100 meq·l-1. Conversely, NaCl + CaCl2 had no influence on the root weight, as confirmed by the statistical test (P > 0.05). The DWA/DWR ratio (DWA: Dry Weight of Aerial parts; DWR: Dry Weight of Roots) decreased with salinity.
3.3. Proline amount
22Proline accumulation was observed for all treatments. By the first three days of salt treatment, the different saline concentrations had little effect on proline accumulation in the stressed plantlets. After 7 and 10 days, and in all salt concentrations, an important increase of proline content was observed in plantlets (Figure 2). The maximum content of proline occurred at the highest salinity concentration (400 meq·l-1) for all the plantlets at both of these sampling dates.
3.4. Relative Water Content
23Figure 3 summarizes the relative water content (RWC) of leaves after 3, 7, and 10 days of treatment with 100, 200, and 400 meq·l-1 of NaCl + CaCl2.
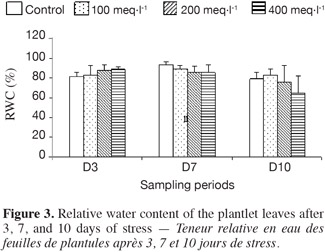
24The first 3 days of treatment induced an increase of the RWC with the rising salinity concentration. By 7 days, the RWC decreased with increasing salinity, to 89.09% of that in control leaves at 100 meq·l-1, to 85.67% at 200 meq·l-1, and to 85.29% at 400 meq·l-1. After 10 days, the leaf RWC reached 83.07% at 100 meq·l-1, compared to the control treatment and it declined further at 200 meq·l-1 (75.97%) and at 400 meq·l-1 (64.85%), when it was significantly different. The plantlets stressed at 100 meq·l-1 did not exhibit any influence of the salt on RWC.
3.5. Concentration of soluble sugars
25The accumulation of soluble sugars in the leaves of all plantlets is shown in figure 4, and the concentrations at each sampling date were significantly different between treatments. At 3 days, accumulation of sugars in the plantlets stressed at 100 meq·l-1 was much higher than in the ones stressed at 200 meq·l-1. At 400 meq·l-1 the plantlets also accumulated a high content of soluble sugars. The accumulation of soluble sugars after 7 days of stress increased as the salt concentration increased.
4. Discussion
26The effects of salinity on shoot and root weights, root length, dry mass accumulation, soluble sugars, and proline content in P. atlantica subsp. atlantica rootstocks were investigated.
27Exposure to salt may affect plant metabolism through an osmotic effect, causing water deficit, or through a specific ion effect, causing excessive ion accumulation. The RWC was closely related to the proline accumulation in leaves (Wilfried, 2005), and lowering the osmotic potential by osmolyte accumulation in response to stress improves the capacity of the cell to maintain its turgor pressure at low water potential (Yancey et al., 1982; Albouchi et al., 2003). Our results showed that applying increasing salt concentrations highly significantly increased the RWC in the first three days after the stress. The reduced shoot dry weight observed was attributed to a lower number of smaller leaves produced due to the increasing salinity in the growth solution. The ion toxic effects of salts are attributed to their excessive accumulation in plant tissues and to nutritional imbalances caused by such ions. After seven days of salt treatment there was an opposite effect: the RWC decreased with the increasing concentration of salts, and compared to the control. After ten days, the RWC was significantly higher under a 100 meq·l-1 NaCl + CaCl2 concentration, and decreased sharply for the other concentrations. We also noticed that during these 10 days of salt stress, the plantlets grew well at a concentration of 100 meq·l-1. In the past, damage to pistachio plantlets has been mainly attributed to excessive accumulation of Na+ in the leaves (Rahemi et al., 2007; Tavallali et al., 2008).
28The study of the relationship between salinity and morphological parameters showed that the recorded deficit in biomass is not the same for both parts (roots and shoots) of stressed plantlets. The aerial parts were the most affected, as confirmed statistically by a negative correlation and a highly significant relationship between the DWA and the intensity of salt stress. Conversely, the correlation between the DWR and the salt treatment, although weak, had a statistically negative trend. The salinity appears to induce a preferential allocation of biomass to roots, which increases as the stress is more intense (Boyer, 1982). We also noticed that the plantlets stressed at a concentration of 100 meq·l-1 reacted with biomass production in the aerial parts, which was of some importance but lower than in the control, while the roots produced much more biomass than the control. The DWA/DWR ratio of the Pistacia plantlets decreased when the salt concentration increased.
29The salt treatment did not induce a significant effect on root length despite the slightly oscillating values of control plantlets at the highest concentration. The mean number of roots were very similar between the control plantlets and those irrigated at 100 meq·l-1, and they decreased at the other concentrations. Roots might seem to be the most vulnerable part of the plant as they are directly exposed to salt, but they were surprisingly robust. The phenomenon is called “The enigma of roots” (Munns, 2002). As shown by Gorham et al. (1990), the growth rate of roots is not affected, their ion concentrations do not increase with time, as in leaves, and they often have a lower Na+ and Cl- concentration than shoots.
30Salinity reduces the ability of plants to take water up, and this quickly causes a reduction in growth rate, along with a suite of metabolic changes. The initial reduction in shoot growth is probably due to hormonal signals generated by the roots. Salt tolerance is usually assessed as the percentage of biomass production in saline versus control conditions over a prolonged period of time, but dramatic differences are found between plant species. For example, after some time in 200 mM NaCl, a salt-tolerant species such as sugar beet might have a reduction of only 20% in dry weight, a moderately tolerant species such as cotton might have a 60% reduction, and a sensitive species such as soybean might be dead (Greenway et al., 1980). During a short time in salinity, there will be a significant decrease in growth rate, but this decrease may be the same for species that have quite different reputations for salt tolerance (Flowers et al., 1991). This led to the consideration of time scale and of the different mechanisms that may be important in controlling growth at different periods of time for plants exposed to salinity. Munns (2002) reported that, when plants are exposed to salinity in laboratory experiments, there is a rapid and temporary drop in growth rate followed by a gradual recovery and by a new reduced rate of growth. These temporary effects are clearly due to rapid and often transient changes in plant water relations. That was the case of P. atlantica in our experiments.
31Plants accumulate compatible osmolytes such as proline and sugars when they are subjected to salinity stress, and they appear to protect plants from such stresses (Zhu, 2001). The plantlets of P. atlantica respond to elevated salt concentrations by increasing the accumulation of soluble sugars in leaves. Our results showed that the elaboration of soluble sugars is independent of increasing salt concentrations, and this was confirmed by the statistical test. Moreover, the duration of treatment had no significant influence in plants stressed at 100 meq·l-1, which accumulated more sugars than all the other treatments. The role of soluble sugars in the adaptive mechanism is controversial, and even their accumulation can be detrimental from several points of view. Soluble sugars act as osmolytes and can increase the osmotic pressure of the cell (Yancey et al., 1982). In addition, the maintenance of the soluble sugar level in leaves could be associated with decreasing growth under salinity. Azcon-Bieto (1983) reported that lower rates of carbon assimilation and a decrease in yield were associated with carbohydrate accumulation in many plant species. Singh (2004) proved that a greater accumulation of sugar lowers the osmotic potential of cells and reduces loss of turgidity in tolerant genotypes.
32Another possible role of sugar may be as a readily available energy source. Various experimental approaches have shown that sugars play a key role in this regulatory mechanism by repressing the expression of the photosynthesis genes (Koch, 1996).
33Proline is a dominant organic molecule that acts as a mediator of osmotic adjustment under salinity stress, a stabilizer of sub-cellular structures, a sink for energy, and even a stress related signal. It is also involved in cell osmoregulation and protection of proteins during dehydration, and it can act as an enzymatic regulator under stress conditions (Rontain et al., 2002). Our results showed that the different salt concentrations in plantlets caused low-intensity fluctuations in the first three days of stress; at the seventh and tenth day of stress, a significant increase in proline content in leaves was recorded.
34Proline accumulation is a consistent response of plants under stress, including salt stress (Bates et al., 1973; Delauney et al., 1993). The present study showed that the concentration of free proline in the leaves of all rootstocks increased with increasing concentrations of Sodium in the nutrient solution. The accumulation of proline was greater in leaves of all rootstocks after three days of salt stress, and it increased over time in the experiment. A positive correlation was evidenced between salt tolerance and concentration of tissues in proline. The accumulation of high amounts of proline may be due both to a higher rate of proline synthesis and to a lower magnitude of proline oxidation in salt tolerant genotypes (Kumar et al., 2003; Hokmabadi et al., 2005). Recent studies also suggested that the protective role of proline consists in protecting the protein turnover machinery against stress damage and up-regulating stress protective proteins (Khedr et al., 2003).
35The rootstock P. atlantica subsp. atlantica accumulates more proline with increasing concentration of salts, providing evidence of the effective role of this metabolite as an osmoprotector under salinity stress. These results corroborate our present study concerning the tolerance of these rootstocks to salt stress.
36In conclusion, the findings of the present study have shown that salt stress impacts negatively the growth of pistachio plantlets. However, this negative influence was significant only at concentrations exceeding 200 meq·l-1 of saline solution. Our data also demonstrated that plantlets stressed with 100 meq·l-1 exhibited a higher adaptive potential under salinity stress, as can be judged from the growth biomass accumulation and the accumulation of osmoprotectants, when compared to plantlets irrigated at 200 and 400 meq·l-1 of NaCl + CaCl2.
37In addition to the agronomic aspect, the ecological impact is also important because the P. atlantica species could be introduced in marginal areas for the stabilization of eroded land and/or land subject to salinization.
38List of abbreviations
39DW: Dry Weight
40DWA: Dry Weight of Aerial parts
41DWR: Dry Weight of Roots
42FW: Fresh Weight
43RWC: Relative Water Content
44TW: Turgid Weight
Bibliographie
Albouchi A., Bejaoui Z. & El Aouni M.H., 2003. Influence of moderate or severe water stress on the growth of Casuarina glauca Sieb. seedlings. Sécheresse, 14, 137-142.
Azcon-Bieto J., 1983. Inhibition of photosynthesis by carbohydrates in wheat leaves. Plant Physiol., 73, 681-686.
Barrs H., 1968. Determination of water deficit in plant tissues. In: Water deficit and plant growth. New York, USA: Academic Press, 235-368.
Bates L.S., Waldren R.P. & Teare I.D., 1973. Rapid determination of free proline for water-stress. Plant Soil, 39, 205-207.
Benhassaini H., Mehdadi Z., Hamel L. & Belkhodja M., 2007. Phytoécologie de Pistacia atlantica Desf. subsp. atlantica dans le Nord-Ouest algérien. Sécheresse, 18, 199-205.
Benmahioul B., Daguin F. & Harche M., 2009. Effects of salt stress on germination and in vitro growth of pistachio (Pistacia vera L.). Agron. J., 332, 752-758.
Boyer J.S., 1982. Plant productivity and environment. Science, 218, 443-448.
Chelli-Chaabouni A. et al., 2010. In vitro salinity tolerance of two pistachio rootstocks: Pistacia vera L. and P. atlantica Desf. Environ. Exp. Bot., 69, 302-312.
Delauney A.J. & Verma D.S., 1993. Proline biosynthesis and osmoregulation in plants. Plant J., 4, 215-223.
Dreier W. & Göring M., 1974. Der Einfluss hoher Salzkonzentration auf verschiedene physiologische Parameters von Maiswurzeln. Wiss. Z. Humboldt Univ. Berlin, Reihe/Math. Naturwiss., 23, 641-644.
Flowers T.J., Hajibagheri M.A. & Yeo A.R., 1991. Ion accumulation in the cell walls of rice plants growing under saline conditions: evidence for Oertli hypothesis. Plant Cell Environ., 14, 319-325.
Ghazvini Foutouhi R., Sajadian H., Hokmabadi H. & Saeed A., 2007. Effects of pistachio rootstocks on ecophysiological characteristics of commercial pistachio cultivars. Int. J. Agric. Biol., 2, 352-354.
Gorham J., Wyn Jones R.G. & Bristol A., 1990. Partial characterization of the trait for enhanced K+-Na+ discrimination in the D genome of wheat. Planta, 180, 590-597.
Greenway H. & Munns R., 1980. Mechanisms of salt stress tolerance in nonhalophytes. Annu. Rev. Plant Physiol., 31, 149-190.
Hoagland D.R. & Arnon D.I., 1938. The water-culture method for growing plants without soil. Berkeley, USA: College of Agriculture, University of California.
Hokmabadi H., Arzani K. & Grierson P.F., 2005. Growth, chemical composition, and carbon isotope discrimination of pistachio (Pistacia vera L.) rootstock seedlings in response to salinity. Aust. J. Agric. Res., 56, 135-144.
Kallsen C.E., Parfitt D.E., Maranto J. & Holtz B.A., 2009. New pistachio varieties show promise for California cultivation. California Agric., 63, 18-23.
Karimi S. et al., 2009. Effects of long-term salinity on growth and performance of two pistachio (Pistacia L.) rootstocks. Aust. J. Basic Appl. Sci., 3, 1630-1639.
Khedr A.H.A. et al., 2003. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritinum L. to salt stress. J. Exp. Bot., 54, 2553-2562.
Koca M., Bor M., Ozdemir F. & Turkan I., 2007. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content on sesame cultivars. Environ. Exp. Bot., 60, 344-351.
Koch K.E., 1996. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 47, 509-540.
Kumar S.G., Reddy A.M. & Sudhakar C., 2003. NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Sci., 165, 1245-1251.
Mirzaie-Nodoushan H. & Arefi H.M., 2010. Variability in seed blankness in Pistacia atlantica Desf. in a natural habitat. PGR Newsl. FAO-Biodivers., 127, 46-48.
Munns R., 2002. Comparative physiology of salt and water stress. Plant Cell Environ., 25, 239-250.
Parvaiz A. & Satyawati S., 2008. Salt stress and phyto-biochemical responses of plants – a review. Plant Soil Environ., 54, 89-99.
Quezel P. & Santa S., 1963. Nouvelle flore de l'Algérie et des régions désertiques méridionales. Paris : Centre National de la Recherche Scientifique.
Rahemi M. & Tavallali V., 2007. Effects of rootstock on Iranian pistachio scion cultivars. Fruits, 62, 317-323.
Rontain D., Basset G. & Hanson A.D., 2002. Metabolic engineering of osmoprotectant accumulation in plants. Metab. Eng., 4, 49-56.
Shannon M.C., Grieve C.M. & Francois L.E., 1994. Whole-plant response to salinity. In: Wilkinson R.E., ed. Plant-Environment Interactions. New York, USA: Marcel Dekker, 199-244.
Schields R. & Burnett W., 1960. Determination of protein-bound carbohydrate in serum by a modified anthrone method. Anal. Chem., 32, 885-886.
Singh A.K., 2004. The physiology of salt tolerance in four genotypes of chickpea during germination. J. Agric. Sci. Technol., 6, 87-93.
Tavallali V., Rahemi M. & Panahi B., 2008. Calcium induces salinity tolerance in pistachio rootstocks. Fruits, 63, 285-296.
Troll W. & Lindsley J., 1955. A photometric method for the determination of proline. J. Biochem., 215, 655-660.
Wilfried C., 2005. Proline as a measure of stress in tomato plants. Plant Sci., 168, 241-248.
Yancey P.H. et al., 1982. Living with water stress: evolution of osmolyte systems. Science, 217, 1214-1222.
Yang W.J. et al., 2003. Genotypic variation for glycinebetaine in sorghum. Crop Sci., 43, 162-169.
Zhu J.K., 2001. Plants salt tolerant. Trends Plant Sci., 6, 66-71.
Pour citer cet article
A propos de : Hachemi Benhassaini
Djilali Liabès University. Plant Biodiversity Laboratory. B.P. 89. Hai Larbi Benmhidi. 22000 Sidi Bel-Abbès (Algeria). E-mail: ecoreve@yahoo.fr
A propos de : Aicha Fetati
Mustapha Stambouli University. Faculty of Sciences. Route de Mamounia. BP 736. 29000 Mascara (Algeria).
A propos de : Amar Kaddour Hocine
Es-Sénia University. Faculty of Sciences. Rue des Martyrs. BP 1524. 31000 Oran (Algeria).
A propos de : Moulay Belkhodja
Es-Sénia University. Faculty of Sciences. Rue des Martyrs. BP 1524. 31000 Oran (Algeria).






