- Startpagina tijdschrift
- volume 16 (2012)
- numéro 4
- Effect of land-use types on soil enzymatic activities and chemical properties in semi-deciduous forest areas of Central-West Côte d’Ivoire
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Effect of land-use types on soil enzymatic activities and chemical properties in semi-deciduous forest areas of Central-West Côte d’Ivoire

Nota's van de redactie
Received on June 9, 2011; accepted on March 6, 2012
Résumé
Effet des types d’utilisation des terres sur les activités enzymatiques et les propriétés chimiques du sol dans les zones forestières semi-décidues du centre-ouest de la Côte d’Ivoire. Les activités enzymatiques jouent un rôle clé dans le fonctionnement biochimique des sols. Par conséquent, elles sont considérées comme des indicateurs de la qualité des sols. Cette étude a été conduite à Oumé (centre-ouest de la Côte d’Ivoire) dans le but de mesurer les activités enzymatiques impliquées dans les cycles du phosphore (phosphatases acide et alcaline), de l’azote (N-acétyl-β-D glucosaminidase) et du carbone (β-glucosidase et N-acétyl-β-D glucosaminidase). Ainsi, les sols de quatre unités agro-écologiques (forêt secondaire, plantation de cacao âgée de 20 ans, jachère à base de Chromolaena odorata de 2 ans et culture de maïs) représentatives des principaux types d’usage des sols de la zone ont été échantillonnés pour mesurer les activités enzymatiques et les caractéristiques chimiques du sol. Les résultats ont montré que les activités enzymatiques sont plus élevées dans la jachère à base de C. odorata, alors que le champ de maïs a affiché les plus faibles valeurs. De plus, le sol de la jachère a enregistré les valeurs plus élevées de C, N, bases échangeables (Mg2+, K+), CEC, mais également le plus faible rapport C:N, qui sont caractéristiques d’un sol de bonne qualité. L’analyse en composante principale a révélé une relation marquée entre la teneur en C, N et les activités enzymatiques, indiquant ainsi que ces enzymes sont appropriées pour le suivi de la qualité des sols dans la zone de forêt semi-décidue du centre-ouest de la Côte d’Ivoire.
Abstract
Enzymatic activities play a key role in the biochemical functioning of soils. As a consequence, they have been proposed as indicators of soil quality. This study was conducted at the Oumé benchmark site (Central-West, Côte d’Ivoire), and aimed at measuring the enzymatic activities involved in the phosphorus (acid phosphatase and alkaline phosphatase), nitrogen (N-acetyl-β-D glucosaminidase) and carbon (β-glucosidase and N-acetyl-β-D glucosaminidase) cycles. Soil from four main agro-ecological units (a secondary forest, a 20 year-old cocoa plantation, a 2 year-old Chromolaena odorata-based fallow and a continuous maize crop), representative of land-use systems in the area, were sampled for the measurement of enzymatic activities and chemical characteristics. Results showed that the enzymatic activity values were the highest in the fallow soil, whereas the maize crop displayed the lowest levels of enzymatic activity in soil. Moreover, soil from C. odorata fallow displayed the highest values of C, N, exchangeable bases (Mg2+, K+) contents, and CEC, and the lowest C:N ratio, which are characteristics of good quality soil. A Principal Component Analysis revealed a marked relationship between C, N and enzymatic activity levels, showing that these enzymes are suitable for monitoring soil quality in semi-deciduous forest areas in Central-West Côte d’Ivoire.
Inhoudstafel
1. Introduction
1In Central-West Côte d’Ivoire, macroeconomic constraints of the past two decades, coupled with increasing demographic pressure, have led to increased resource-use pressure and subsequent soil degradation. Indeed, forests have been overexploited due to the establishment of cocoa and coffee plantations. The resulting environmental consequences are deforestation and the invasion of landscapes by the Chromolaena odorata (L.) R.M.King & H.Rob. weed. Nowadays, this invasive weed represents one of the dominant fallow species in the area, and it is being increasingly used as an indicator by farmers in establishing their farms. Therefore, it appears that the sustainability of farming systems in such a region implies the improvement of natural fallows. It is thus obvious that changes in soil quality caused by different types of land-use must first be quantified prior to selecting the most sustainable types of use and management that will minimize soil disturbance (Acosta-Martínez et al., 2008).
2Measurement of the effect of soil disturbance on soil quality has been mainly based on the assessment of soil C and N content, which has been compared to that of a reference soil that has remained under permanent pasture or virgin vegetation, considered as a natural control (Gregorich et al., 1997). Soil properties based on biological and biochemical activities, especially those involved in energy flow and nutrient cycling, have often been shown to respond to small changes in soil, thus providing sensitive information regarding subtle alterations in soil quality (Pascual et al., 2000). Consequently, soil enzyme activities have been proposed as appropriate indicators of soil quality because of their intimate relationship with soil biology, their ease of measurement, and their rapid response to changes in soil management (Pankhurst et al., 1997). Currently, there is a widespread interest in using enzymatic activities to assess soil quality. For instance, the combination of enzymatic activities and microbial biomass measurement has been widely used over the last 10 years to study the microbiological response to agricultural management (Wik et al., 2000; Clegg et al., 2006; Bastida et al., 2007; Jangid et al., 2008; Meriles et al., 2009; Udawatta et al., 2009; Vallejo et al., 2010). However, one of the principal limitations to the use of enzymatic activities as indicators is their natural variability within and between soils (Trasar-Cepeda et al., 2000). For this reason, studies have often concluded that results obtained with one soil cannot be generalized to other soils that differ in their intrinsic properties and characteristics (Gianfreda et al., 2005; Bielińska et al., 2007). Investigations reporting the impact of land use or the disturbance of forest on soil enzyme activities and consequently on their use as indicators of soil quality are both rare and scant in Africa. However, several studies in temperate ecosystems have emphasized the potential of enzymatic activities in soil quality monitoring (Nannipieri et al., 1990; Trasar-Cepeda et al., 2008a; García-Ruiz et al., 2009). The main objective of the present study was to assess and compare the quality of soils under different land-use types including C. odorata-based fallow along a gradient of soil disturbance. Thus, soil from the main land-use types encountered in Central-West Côte d’Ivoire were sampled for the measurement of enzymatic activities, and chemical parameters.
2. Materials and methods
2.1. Site description and soil sampling
3This study was carried out with soils collected from the Oumé Region (6°31′13’’N, 5°28′59’’W) situated in Central-West Côte d’Ivoire characterized by semi-deciduous degraded forests (Chatelain et al., 2003). The average rainfall over a period of 27 years (1976-2003) ranged from 849 to 1,764 mm. The annual rainfall in 2008 was about 1,626.7 mm, while the average monthly temperature was about 26 °C. The soils in the area are ferrasols (FAO, 2006) of homogeneous distribution across the landscape, with, however, differences related to topography (Assié et al., 2008). The surface organic layer (20-30 cm) is friable (resistance to penetration varies between 200 and 1,000 kPa) and well mineralized. Four main agro-ecological units (a secondary forest, a 20 year-old cocoa plantation, a 2 year-old C. odorata based fallow and a maize crop), representative of the land-use types in the study area, were investigated. The fallowing followed two successive maize cultivations. In the maize field, maize was found to be growing (twice per year) for the third consecutive year with no soil amendments. Soils were sampled in November 2008. In each agro-ecological unit, five sampling points were randomly allocated at a distance of 30 m. At each of the sampling points, soil sub-samples were collected from the top 10 cm at five distinct points using an auger. The sub-samples were then mixed to obtain a composite sample, which was fresh-sieved (< 2 mm) on the same day. For determination of enzymatic activities, composite samples were stored at 4 °C until analysis (less than one week). For chemical analysis, the samples were air-dried for one week.
2.2. Chemical analyses
4Soil total N was extracted according to the methods used by Nelson et al. (1980) and was determined using a Technicon Autoanalyzer (Technicon Industrial Systems, 1977). Carbon was determined using a modified Anne method (Nelson et al., 1982). Available phosphorus was extracted according to the Bray-1 procedure (Olsen et al., 1982) and was determined using the same Technicon Autoanalyzer. Exchangeable bases (Ca2+, Mg2+, K+, Na+) were extracted with acetate ammonium (1N, pH = 7). These bases and the cation exchange capacity (CEC) were determined using Atomic Absorption Spectrometry techniques (Thomas, 1982). Soil pH (pHwater) was measured in a soil:water suspension ratio of 1/2.5.
2.3. Enzymatic activities
5Acid and alkaline phosphatases (Pac and Pal), β-glucosidase (β-Glu) and N-acetyl-β-D-glucosaminidase (NAG) activities were assayed. These enzymes were chosen based on their importance in nutrient cycles and organic matter decomposition. Thus, Pac and Pal were chosen because of their role in releasing inorganic phosphorus in the P cycle. β-Glu was considered for its critical role in releasing low molecular weight sugars, which provide a source of energy for decomposers (microorganisms). NAG hydrolyzes N-acetyl-β-D-glucosamine residues from chitooligosaccharides in soils. This biocatalyst is one of the enzymes that play a major role in N mineralization in soils.
6Pac and Pal activities were assayed according to the modified method of Tabatabai et al. (1969). A 0.1 g of soil sample was incubated for 1 h at 37 °C with acetate (pH 5.6) or Tris-HCl (pH 8) buffers and p-Nitrophenyl phosphate (pNPP; 10 mM). The reaction was stopped by adding 1 ml of 0.5 M CaCl2 and 4 ml of 0.5 M NaOH, and the mixture was immediately centrifuged for 2 min at 12,000 g. The amount of p-Nitrophenol released from pNPP was measured in the supernatant at 410 nm. Enzymatic activity was expressed in micromoles of p-Nitrophenol per g dry soil per hour.
7β-Glu and NAG activities were assayed for 0.2 g of soil sample according to the methods of Eivazi et al. (1988) and of Parham et al. (2000), respectively. Enzymatic activity was also expressed in micromoles of p-Nitrophenol per g dry soil per hour.
2.4. Statistical analyses
8Significant differences in soil chemical properties and enzymatic activities between types of land use were evaluated using a one-way ANOVA at 5%. These statistical analyses were performed using the STATISTICA ver. 7.0 program (Stat Soft, Tulsa, USA).
9A Principal Component Analysis (PCA) was used to determine the relationships between enzymatic activities and soil chemical properties on the one hand, and on the other hand, to discover which enzymatic activities were sensitive to land-use changes. The ADE-4 software (Thioulouse et al., 1997) was used for this purpose.
3. Results
3.1. Chemical parameters
10The soil pH values ranged between 6.14 ± 0.09 (forest) and 6.51 ± 0.05 (C. odorata fallow) with no significant variations, showing that the soils were slightly acid.
11As for soil nutrient status, results revealed a significant difference in C and N contents between land-use types in the first 10 cm of soil. Chromolaena odorata fallow displayed the highest values of C (15.56 ± 0.43 g·kg-1) and N (1.68 ± 0.08 g·kg-1) contents (Table 1).
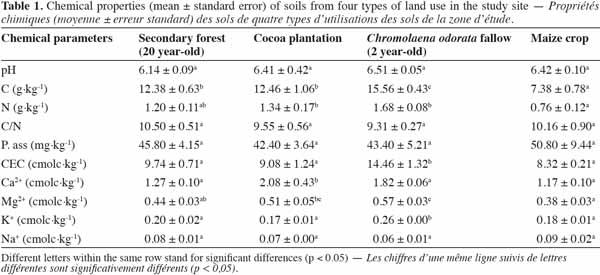
12Furthermore, there was no significant difference between the secondary forest and C. odorata fallow, as far as N content was concerned. Likewise, the C:N ratios did not vary significantly, although the lowest value was recorded under C. odorata fallow (9.31 ± 0.27).
13Available phosphorus values ranged from 42.40 ± 3.64 to 50.80 ± 9.44 mg·kg-1 but did not show any significant difference between the land-use types (Table 1).
3.2. Cation exchange capacity
14Cation exchange capacity (CEC) and exchangeable bases varied significantly between the land-use types (Anova 1, p = 0.0017). The highest value (14.46 ± 1.32 cmolc·kg-1) was found under C. odorata, while the other land-use types displayed similar values (Table 1).
3.3. Enzymatic activities
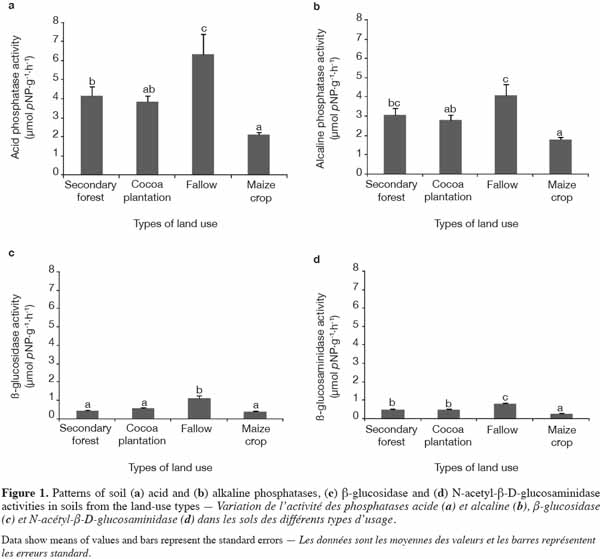
15All enzymatic activities assayed were well expressed in all the land-use types investigated. Levels of Pac and Pal activity, in particular, were higher than those of NAG and β-Glu and there were significant differences between land-use types in enzymatic activity levels. Indeed, cumulative enzymatic activities were higher in C. odorata fallow than in the other types of land use (Figure 1). Levels of Pac activity varied significantly (p = 0.002) between land-use types. The value of Pac activity recorded in soil under C. odorata (6.32 μmol pNP·g-1 dry soil·h-1) was the highest compared with other land-use types (Figure 1a). Although the level of Pal activity was lower compared with Pac, it was also found to be sensitive (p = 0.004) to land-use change (Figure 1b). Apart from C. odorata fallow, which displayed the highest value (1.108 μmol pNP·g-1 dry soil·h-1), the levels of β-Glu activity were similar under secondary forest, cocoa plantation, and maize crop conditions (Figure 1c). Of the enzymatic activities assayed, NAG presented the lowest value. Nevertheless, the activity of this enzyme was significantly affected (p < 0.0001) by land-use types (Figure 1d). Here again, the highest (0.81 µmol pNP·g-1 dry soil·h-1) value of activity was recorded under C. odorata fallow.
3.4. Factors determining discrimination between land-use types
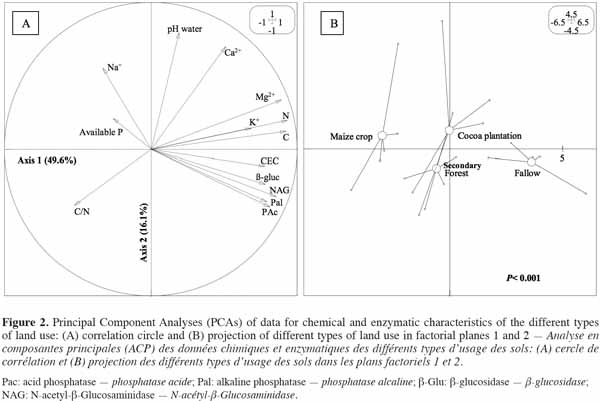
16A PCA was performed on a correlation matrix of the data obtained on enzymatic activities and soil chemical parameters. The correlation circle mainly revealed a strong relationship between enzymatic activities and C, N and CEC, with the two first axes accounting for 65.7% of the total inertia (Figure 2a). Land-use types were significantly separated (p < 0.001) along the first axis (49.6%) on the basis of bio-chemical parameters and three groups emerged:
17– maize crop,
18– secondary forest and cocoa plantation,
19– Chromolaena odorata fallow (Figure 2b).
20This result showed that soil from C. odorata fallow with high levels of enzymatic activity and high organic C and total N contents was of good quality, in contrast to maize crop soil, which was characterized by the lowest levels of enzymatic activity. Cocoa plantation and secondary forest soils, characterized by moderate soil chemical properties and enzymatic activities, were found to have an intermediate value of soil quality.
4. Discussion
21With regard to the chemical soil parameters, the present study showed that C. odorata fallow exhibited higher values, except for available phosphorus. These values were intermediate in both cocoa and secondary forest, and lower under maize crop. The higher values obtained in C. odorata-based fallow could be explained by the regular litter fall that occurs under this vegetation, in addition to better decomposability, due to the good quality of organic residues. For example, data from the literature have reported a higher N and P contents in C. odorata leaf litter, 12 mg·kg-1 and 1.1 mg·kg-1, respectively (Koné, 2009) in comparison with cocoa leaf litter, with content levels of 9.7 mg·kg-1 and 0.6 mg·kg-1, respectively (Ofori-Fripomg et al., 1999). This result could also reflect the probable more rapid turnover of C. odorata (fine) roots, as these are less lignified than those of cocoa or of secondary forest plant species. These two nutrients, N and P, together with other parameters, such as lignin content, are known to be important in determining the decomposition rate of plant materials (Loranger et al., 2002). As both cocoa plantation and secondary forest are perennial ecosystems, the accumulation of low decomposition-rate litter should have resulted in a greater accumulation of organic matter and exchangeable bases in the soil. However, this was not apparent, probably because a large proportion of these nutrients was still being sequestered in the vegetation (Hartemink, 2005). Lower contents in soil organic matter and exchangeable bases under maize cropping, relative to perennial systems were observed by Seyfried et al. (1991). Comparing two different cropping systems, namely mixed perennial (composed of timber species, cocoa and plantain) and annual maize cropping, the authors reported that losses of exchangeable bases and NO3- in the maize system were by far greater than in the perennial systems studied. Such losses, which contribute to a decrease in soil nutrients, may be explained by land disturbance characterized by recurrent cropping and weeding coupled with exposure of soil to sunlight. It has been well established that land disturbance can negatively affect soil fertility by breaking down soil aggregates, accelerating organic matter decomposition and hence, subjecting nutrients to a greater risk of runoff and/or leaching (Hooker et al., 2005).
22Knowledge of soil enzyme activity also indicates the potential of soil to support some of the basic processes necessary for maintaining soil fertility (Hernández et al., 1997). Therefore, we investigated enzymatic activities in addition to soil chemical properties. Enzyme activity profiles can reflect an essential part of soil functional diversity, which is controlled by the genetic diversity of soil microorganisms, plants and soil animals in close relation to environmental effects and ecological interactions (Nannipieri et al., 2002; Hamido et al., 2009). Among the enzymatic activities explored, Pal, Pac and β-Glu are considered as the most commonly used specific parameters to assess changes in soil quality (Gil-Stores et al., 2005; Trasar-Cepeda et al., 2008b). In our results, Pac and Pal displayed the highest levels of activity in the soils investigated. Acosta-Martinez et al. (2007) obtained similar results when evaluating the impact of soil use on the activity of certain hydrolytic enzymes. Interestingly, in the present study, all the enzymatic activities were consistent with soil chemical parameters and they varied together in the same trend. Gao et al. (2010) showed that absolute enzymatic activities varied under different land uses (showing a small decrease or an increase) depending on the types of land use or management and the type of enzyme. It was also noteworthy in the present study that except for C. odorata fallow (2 year-old), enzymatic activities generally decreased with increasing anthropogenic activities. According to Tondoh et al. (2006), the land-use intensification index, which reflects the recurrence of human intervention in a given area, allows the ranking of land-use types as follows: secondary forest and cocoa plantations (0.1) < fallow (0.3) < crop fields (0.4). If we consider that an increase in anthropogenic activities contributes much more to the degradation of natural resources, this observation is of great importance to the extent that it highlights the probable role of C. odorata fallow in soil fertility improvement. Koné (2009) reported that C. odorata produced quality-litter, which is thus more easily accessible to decomposers compared to the leaf litter of cocoa and secondary forest plant species, which mainly consists of hard leaves. Kanmegne et al. (1999) showed that C. odorata residues decompose quickly and lead to an improvement in soil properties. The better availability of soil C and N under C. odorata is probably due to enzyme activities, as our data seem to show a positive correlation between enzymatic activities and soil C, and a negative correlation between enzymatic activities and the C:N ratio. Numerous studies have established a relationship between soil extracellular enzyme activities and the availability of substrates, and C and N mineralization (Waldrop et al., 2004; Geisseler et al., 2009; Hamido et al., 2009; Xueyong et al., 2009). Indeed, extracellular enzyme synthesis has been found to be induced by the presence of their substrates (Suto et al., 2001; Duo-Chuan, 2006). However, substrate induction is not the only factor regulating extracellular enzyme production. High levels of end products or molecules containing the target nutrient, for example N, repress enzyme production, while low levels of end products or target nutrients de-repress enzyme production (Geisseler et al., 2009). Therefore, the activity of extracellular enzymes may yield information about the temporal availability of specific organic compounds and thus of the availability of C and N. As the correlation circle of our PCA revealed a relationship between C, N and enzymatic activity levels, we can conclude that these enzymes are suitable for monitoring soil quality in semi-deciduous forest areas in Central-West Côte d’Ivoire.
5. Conclusion
23Among the four agro-ecological units assessed for soil quality, soil from C. odorata fallow displayed higher levels of enzymatic activity and chemical properties than other land-use types. Thus C. odorata-based fallow has the potential for improving soil quality in the short term.
24Acknowledgements
25This work was supported by the CSM-BGBD Project (Conservation and Sustainable Management Below-Ground Biodiversity) funded by GEF/UNEP in Côte d’Ivoire. The authors are grateful to Professor Tano Yao, the Project Coordinator, for enabling the implementation of this study, to Professor Lucien P. Kouamé and Professor Irié A. Zoro Bi for their assistance.
Bibliographie
Acosta-Martinez V., Cruz L., Sotomayor-Ramirez D. & Pérez-Alegria L., 2007. Enzyme activities as affected by soil properties and land use in a tropical watershed. Appl. Soil Ecol., 35, 35-45.
Acosta-Martínez V., Acosta-Mercado D., Sotomayor-Ramírez D. & Cruz-Rodríguez L., 2008. Microbial communities and enzymatic activities under different management in semi-arid soils. Appl. Soil Ecol., 38, 249-260.
Assié K.H., Angui K.T.P. & Tamia J.A., 2008. Effets de la mise en culture et des contraintes naturelles sur quelques propriétés physiques d’un sol ferralitique au centre-ouest de la Côte d’Ivoire : conséquences sur la dégradation des sols. Eur. J. Sci. Res., 23, 149-166.
Bastida F., Moreno J.L., Hernández T. & García C., 2007. The long-term effect of the management of a forest soil on its carbon content, microbial biomass and activity under a semi-arid climate. Appl. Soil Ecol., 37, 53-62.
Bielińska E.J. & Pranagal J., 2007. Enzymatic activity of soil contaminated with triazine herbicides. Pol. J. Environ. Stud., 16, 295-300.
Chatelain C., Dao H., Gautier L. & Spichiger R., 2003. Forest cover changes in Côte d’Ivoire and Upper Guinea. In: Poorter L., Bongers F., Kouamé F.N. & Hawthorne W.D., eds. Biodiversity of West Africa forests. An ecological atlas of woody plant species. Wallingford, UK: CABI, 15-31.
Clegg C.D. et al., 2006. Impact of cattle grazing and inorganic fertiliser additions to managed grassland on the microbial community compositions of soils. Soil Biol. Biochem., 31, 73-82.
Duo-Chuan L., 2006. Review of fungal chitinases. Mycopathologia, 161, 345-360.
Eivazi F. & Tabatabai M.A., 1988. Glucosidases and galactosidases in soil. Soil Biol. Biochem., 20, 601-606.
FAO, 2006. World reference base for soil resources 206. A framework for international classification, correlation and communication. World soil resource report, 103. Roma: FAO.
Gao Y. et al., 2010. Spatial characteristics of soil enzyme activities and microbial community structure under different land uses in Choming Island, China: geostatistical modeling and PCR-RAPD method. Sci. Total Environ., 408, 3251-3260.
García-Ruiz R. et al., 2009. Soil enzymes, nematode community and selected physico-chemical properties as soil quality indicators in organic and conventional olive oil farming: influence of seasonality and site features. Appl. Soil Ecol., 41, 305-314.
Geisseler D. & Horwath W.R., 2009. Relation between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia, 53, 87-98.
Gianfreda L. et al., 2005. Soil enzyme activities as affected by anthropogenic alterations: intensive agricultural practices and organic pollution. Sci. Total Environ., 341, 265-279.
Gil-Stores F., Trasar-Cepeda C., Leirós M.C. & Seoane S., 2005. Different approaches to evaluate soil quality using biochemical properties. Soil Biol. Biochem., 37, 877-887.
Gregorich E.G. et al., 1997. Biological attributes of soil quality. In: Gregorich E.G. & Carter M.R., eds. Soil quality for crop production and ecosystem health. Amsterdam: Elsevier, 81-113.
Hamido S.A. & Kpomblekou-A K., 2009. Cover crop and tillage effect on soil enzyme activities following tomato. Soil Tillage Res., 105, 269-274.
Hartemink A.E., 2005. Nutrient stocks, nutrient cycling, and soil changes in cocoa systems: a review. Adv. Agron., 86, 227-253.
Hernández T., Garcia C. & Reinhardt I., 1997. Short-term effect of wildfire on the chemical, biochemical and microbiological properties of Mediterranean pine forest soils. Biol. Fertil. Soils, 25, 109-116.
Hooker B.A., Morris T.F., Peters R. & Cardon Z.G., 2005. Long-term effects of tillage and corn stalk return on soil carbon dynamics. Soil Sci. Soc. Am. J., 69, 188-196.
Jangid K. et al., 2008. Relative impact of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem., 40, 2843-2853.
Kanmegne J., Duguma B., Henrot J. & Isirimah N.O., 1999. Soil fertility enhancement by planted tree-fallow species in the humid lowlands of Cameroon. Agrofor. Syst., 46, 239-249.
Koné W.A., 2009. Qualité des sols en zone de savane humide de Côte d’Ivoire : utilisation des légumineuses herbacées comme alternative pour une valorisation des terres marginales et une agriculture durable. Thèse de doctorat : Université d’Abobo-Adjamé, Abidjan (Côte d’Ivoire).
Loranger G., Ponge J.-F., Imbert D. & Lavelle P., 2002. Leaf decomposition in two semi-evergreen tropical forests: influence of litter quality. Biol. Fertil. Soils, 35, 247-252.
Meriles J.M. et al., 2009. Soil microbial communities under different soybean cropping systems: characterization of microbial population dynamics, soil microbial activity, microbial biomass, and fatty acid profiles. Soil Tillage Res., 103, 271-281.
Nannipieri P., Greco S. & Ceccanti B., 1990. Ecological significance of the biological activity in soil. In: Bollag J.M. & Stotzky G., eds. Soil biochemistry. Vol. 6. New York, USA: Marcel Dekker, 293-355.
Nannipieri P., Kandeler E. & Ruggiero P., 2002. Enzyme activities and microbiological and biochemical processes in soil. In: Burn R.G. & Dick R., eds. Enzymes in the environment. New York, USA: Marcel Dekker, 1-33.
Nelson D.W. & Sommers L.E., 1980. Total nitrogen analysis for soil and plant tissues. J. Assoc. Off. Anal. Chem., 63, 770-778.
Nelson D.W. & Sommers L.E., 1982. Total carbon, organic carbon, and organic matter. In: Page A.L., Miller R.H. & Keeney D.R., eds. Methods of soil analysis. Part 2. 2nd ed. Agronomy Monograph 9. Madison, WI, USA: American Society of Agronomy, 539-579.
Ofori-Fripomg K. & Rowel D.L., 1999. The decomposition of cocoa leaves and their effect on phosphorus dynamics in tropical soil. Eur. J. Soil Sci., 50, 165-172.
Olsen S.R. & Sommers L.E., 1982. Phosphorus. In: Page A.L., Miller R.H. & Keeny D.R., eds. Methods of soil analysis. Part 2. 2nd ed. Agronomy Monograph 9. Madison, WI, USA: American Society of Agronomy, 403-430.
Pankhurst C.E., Doube B.M. & Gupta V.V.S.R., 1997. Biological indicators of soil health: synthesis. In: Pankhurst C.E., Doube B.M. & Gupta V.V.S.R., eds. Biological indicators of soil health. Wallingford, UK: CAB International, 419-435.
Parham J.A. & Deng S.P., 2000. Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol. Biochem., 32, 1183-1190.
Pascual J.A. et al., 2000. Soil microbial activity as a biomarker of degradation and remediation processes. Soil Biol. Biochem., 32, 1877-1883.
Seyfried M.S. & Rao P.S.C., 1991. Nutrient leaching loss from two contrasting cropping systems in the humid tropics. Trop. Agric., 68, 9-18.
Suto M. & Tomita F., 2001. Induction and catabolite repression mechanisms of cellulose in fungi. J. Biosci. Bioeng., 92, 305-311.
Tabatabai M.A. & Bremner J.M., 1969. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem., 1, 301-307.
Technicon Industrial Systems, 1977. Individual/simultaneous determination of nitrogen and/or phosphorus in BD acid digests. Tarrytown, NY, USA: Technicon Industrial Systems.
Thioulouse J., Chessel G., Dolédec S. & Olivier J.M., 1997. ADE-4: a multivariate analysis and graphical display software. Stat. Comput., 7, 75-83.
Thomas G.W., 1982. Exchangeable cations. In: Page A.L., Miller R.H. & Keeney D.R., eds. Methods of soil analysis. Part 2. 2nd ed. Agronomy Monograph 9. Madison, WI, USA: American Society of Agronomy, 159-164.
Tondoh E.J., Monin L.M., Tiho S. & Csuzdi C., 2006. Can earthworms be used as bio-indicators of land-use perturbations in semi-deciduous forest? Biol. Fertil. Soils, 43, 585-592.
Trasar-Cepeda C., Leiros M.C., Seoane S. & Gil-Sotres F., 2000. Limitations of soil enzymes as indicators of soil pollution. Soil Biol. Biochem., 32, 1867-1875.
Trasar-Cepeda C., Leirós M.C. & Gil-Sotres F., 2008a. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem., 40, 2146-2155.
Trasar-Cepeda C., Leirós M.C., Seoane S. & Gil-Sotres F., 2008b. Biochemical properties of soils under crop rotation. Appl. Soil Ecol., 39, 133-143.
Udawatta R.P., Kremer R.J., Garett H.E. & Anderson S.H., 2009. Soil enzyme activities and physical properties in watershed managed under agroforestry and row-crop systems. Agric. Ecosyst. Environ., 131, 98-104.
Vallejo V.E., Roldan F. & Dick R.P., 2010. Soil enzymatic activities and microbial biomass in an integrated agroforestery chronosequence compared to monoculture and a native forest of Colombia. Biol. Fertil. Soils, 46, 577-587.
Waldrop M.P., Zack D.R. & Sinsabaugh R.L., 2004. Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol. Biochem., 36, 1443-1451.
Wik B., Tiessen H. & Menezes R., 2000. Land quality changes following the conversion of natural vegetation into silvopastoral systems in semi-arid NE Brazil. Plant Soil, 222, 59-70.
Xueyong P., Ning W., Qing L. & Weikai B., 2009. The relation among soil microorganism, enzyme activity and soil nutrients under subalpine coniferous forest in Western Sichuan. Acta Ecol. Sinica, 29, 286-292.
Om dit artikel te citeren:
Over : Jean T. Gonnety
Université d’Abobo-Adjamé. Laboratoire de Biocatalyse et Bioprocédés. UFR des Sciences et Technologie des Aliments. 02 BP 801. Abidjan 02 (Côte d’Ivoire). E-mail: netygone@yahoo.fr
Over : Embi F. L. Assémien
Université d’Abobo-Adjamé. Laboratoire de Biocatalyse et Bioprocédés. UFR des Sciences et Technologie des Aliments. 02 BP 801. Abidjan 02 (Côte d’Ivoire).
Over : Arnauth M. Guéi
Université d’Abobo-Adjamé. UFR des Sciences de la Nature. Centre de Recherche en Écologie. 02 BP 801. Abidjan 02 (Côte d’Ivoire).
Over : Aya A. N’Dri
Université d’Abobo-Adjamé. Laboratoire de Biocatalyse et Bioprocédés. UFR des Sciences et Technologie des Aliments. 02 BP 801. Abidjan 02 (Côte d’Ivoire).
Over : Yves Djina
Université d’Abobo-Adjamé. Laboratoire de Biocatalyse et Bioprocédés. UFR des Sciences et Technologie des Aliments. 02 BP 801. Abidjan 02 (Côte d’Ivoire).
Over : Armand W. Koné
Université d’Abobo-Adjamé. UFR des Sciences de la Nature. Centre de Recherche en Écologie. 02 BP 801. Abidjan 02 (Côte d’Ivoire).
Over : Jérôme E. Tondoh
International Center for Tropical Agriculture (CIAT). IER, CRRA de Sotuba. Laboratoire Sol-Eau-Plante. c/o ICRAF Sahel. BPE 5118. Bamako (Mali).






