- Accueil
- volume 16 (2012)
- numéro 4
- Ecology and management of Pericopsis elata (Harms) Meeuwen (Fabaceae) populations: a review
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Ecology and management of Pericopsis elata (Harms) Meeuwen (Fabaceae) populations: a review

Notes de la rédaction
Received on February 29, 2012; accepted on August 29, 2012
Résumé
Synthèse bibliographique : écologie et gestion des populations de Pericopsis elata (Harms) Meeuwen (Fabaceae). Pericopsis elata (Fabaceae) est un arbre de haute valeur commerciale des forêts denses humides semi-sempervirentes africaines. Son aire de distribution, aujourd’hui en régression, couvrait autrefois plusieurs régions disjointes allant de la Côte d’Ivoire à la République Démocratique du Congo. Cette espèce est exploitée depuis la deuxième moitié du 20e siècle pour son bois précieux. Elle souffre aujourd’hui d’un manque de régénération. De ce fait, elle est inscrite en annexe II de la CITES et est considérée « en danger » par l’IUCN. Comme d’autres essences exigeantes en lumière et présentant un déficit de régénération, elle pourrait s’être établie à la faveur d’importantes perturbations qu’ont connues les forêts du Bassin du Congo durant les derniers millénaires. Alors que le commerce international et les utilisations industrielles de son bois sont bien documentés, les données décrivant l’écologie de cette espèce sont, selon la region, partielles, contradictoires, voire inexistantes (e.g., son diamètre de fructification régulière). En outre, seules des bribes d’information sont connues quant à sa sylviculture et aucune solution crédible n’est actuellement apportée pour pallier la carence en régénération naturelle. Enfin, les connaissances sur sa génétique n’en sont encore qu’à leurs balbutiements, tandis que seules de vagues hypothèses ont été émises quant à l’historique de ses populations. Ces derniers constats mettent en lumière la nécessité de nouvelles recherches scientifiques dans ces domaines. Ces études devraient être à même d’apporter les informations manquantes qui permettraient de statuer sans ambigüité sur les menaces qui pèsent potentiellement sur l’espèce. Enfin, ces recherches devraient aboutir à des mesures de gestion efficaces, pragmatiques et peu couteuses, conditions pour garantir la survie de l’espèce sur le long terme dans un contexte d’exploitation forestière.
Abstract
Pericopsis elata (Fabaceae) is a valuable timber species occurring in moist semi-deciduous African forests. While it is at present substantially reduced, the tree’s natural distribution previously covered several distinct areas from Côte d’Ivoire to the Democratic Republic of Congo. This species has been logged since the second half of the 20th century. Because it suffers from a lack of regeneration, P. elata is now included in CITES Appendix II and is recorded as “Endangered A1cd” on the IUCN Red List. As with other long-lived light-demanding species, the survival of P. elata may have been favored by important disturbances that occurred in the Congo Basin during the last millennia. While both international trade and industrial uses of the wood of P. elata are well documented, information about its ecology are very sparse or contradictory, and even absent in some cases (e.g., regarding its effective flowering diameter). Furthermore, data describing the management of P. elata are scarce, including potential solutions to compensate for the deficit of natural regeneration. Along the same lines, genetic studies still remain at an early stage and only vague hypotheses have been offered to explain the origins of the tree’s populations. We emphasize the need for new research on those topics. Further studies would be useful in deciding whether P. elata populations can continue to be logged without the species being threatened with extinction. Finally, such research needs to target effective and inexpensive management procedures that could secure the future of the species in a logging context.
Table des matières
1. Introduction
1Formerly considered as pristine, Central African rainforests have undergone important changes during the last millennia. Indeed, recent studies (e.g., Bayon et al., 2012) have come to the conclusion that this development could have been caused by climatic variations as well as past anthropogenic activities. Nowadays, these forests are logged to provide the international timber trade with valuable wood. In the Congo Basin, national regulations impose the implementation of management plans based on relatively strict standards. Among other management tasks, logging companies are obliged to conduct specific botanical inventories. Covering large forested areas, such inventories also provide relevant information regarding the population dynamics of timber species. Several timber species are suffering from a lack of regeneration within their natural distribution areas. How these aging timber populations originated is still in question (see for instance van Gemerden et al., 2003; Willis et al., 2004). Nomadic human populations, mainly through slash and burn practices, may have played an important role in the establishment of these species (Brncic et al., 2007; Bayon et al., 2012). Pericopsis elata (Harms) Meeuwen (Fabaceae), a tall tree occurring in the semi-deciduous African forest, is an example of a long-lived light-demanding commercial species, together with Terminalia superba Engl. & Diels and Triplochiton scleroxylon K.Schum. The main goal of this paper is to draw up an inventory of existing information in relation to P. elata, which is a potentially threatened species (IUCN, 2001; Abensperg-Traun, 2009). Particular attention is paid to the study of the tree’s autecology and to the understanding of its population history, important information for everyone interested in securing its future.
2. General and botanical descriptions
2.1. General description of Pericopsis elata
2Pericopsis elata is a medium-sized to large tree of up to 130 cm in diameter and 40-50(60) m in height. The tree is mainly known under the local names of afrormosia (DRC, Congo, trade name most commonly used), assamela (Cameroon, Côte d’Ivoire) and kokrodua (Ghana). Howland (1979) and Dahms (1999) have cited other local names including ole and oleo pardo (Congo), bohalala and ole (DRC), ejen and obang (Cameroon), mohole (Ghana), and ayin, aneran and elo uta (Nigeria). Also commercialized as African teak or gold teak (because of the color of its dry heartwood), this high commercial value timber species is restricted to moist semi-deciduous African forests (West and Central Africa). Pericopsis elata is valued for the high quality of its wood, and its exploitation started more than 50 years ago, mainly in Ghana and Côte d’Ivoire (Dickson et al., 2005).
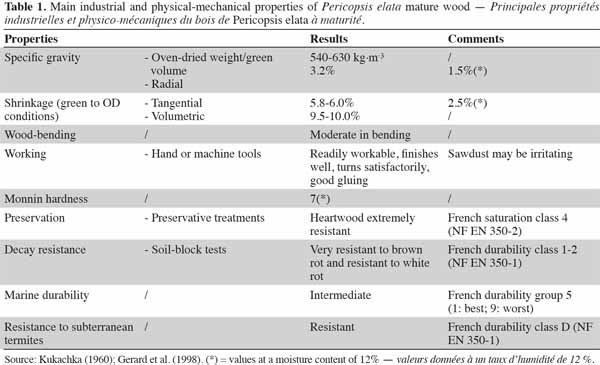
3Main characteristics of the wood and most common uses. Pericopsis elata heartwood combines very good technical properties such as dimensional stability and good natural durability. The tree also offers good resistance against termites and its wood density is quite high. Heartwood is resistant to pressure impregnation (Table 1). Growth rings are present and the species has a straight but often interlocked grain. Its wood is considered to be an excellent substitute for teak (Tectona grandis L.f.) in all respects (Kukachka, 1960). Calcium deposits have been reported by some authors, with minor or no consequences for technical applications (e.g., Dahms, 1999). On average, the sapwood is rather narrow (3 cm; Toussaint et al., 1953). Because of its relative ease of working, traditional uses of wood derived from P. elata include boards (coffins, furniture) and tool handles. The principal industrial uses are flooring, furniture, window/door frames, decorative veneer and shipbuilding (Kukachka, 1960).
4Finally, with regard to agroforestry systems, Anglaaere (2005) identified P. elata as being among the shade tree species preferred by farmers in cocoa plantations in Ghana (species declared as “good for cocoa”). Nevertheless, when some P. elata seedlings were utilized during on-farm trials, they showed a very poor ability to survive and those that did survive demonstrated low initial growth rates (Anglaaere, 2005).
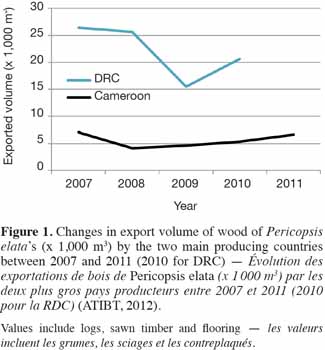
5International timber trade. The earliest shipments of P. elata timber are thought to have started in 1947-1948 from Ghana to England, as the main wood characteristics of the species were still unknown before World War II (Howland, 1979; Dahms, 1999). Since the beginning of the 1990s, Cameroon and DRC have been the main exporters of P. elata (ATIBT, 2012). The drop in the quantity of the timber exported by DRC in 2009 (Figure 1) may be explained by difficulties encountered in the implementation of sustainable management procedures. In 2009, Belgium and France imported ca. 60 and 30% of the total volume of sawn wood produced in Cameroon, respectively. After ca. 60 years of lumbering, the price paid for the wood of P. elata became one of the highest on the world tropical timber market (800-1,000 €.m-3 FOB in January 2012).
2.2. Main botanical aspects of Pericopsis elata
6The information given in this section are derived from a selection of reference documents (Louis et al., 1943; Toussaint et al., 1953; Bonnier, 1957; Taylor, 1960; de la Mensbruge, 1966; Allen et al., 1981; Vivien et al., 2011; Hawthorne et al., 2006; Anglaaere, 2008; Kouadio, 2009).
7Leaves, leaflets and canopy architecture. Pericopsis elata has a pinnate leaf (petiole and rachis of 12-20 cm in length) with 7-11 alternate glabrous ovate leaflets (petiolule of 3-7 mm in length). Each leaflet is 3-9.5 and 2-4 cm in length and width, respectively. Leaflets increase in length along the leaf (Figure 2). Their venation is not very clear (the veins are very fine) and the underside is more or less glaucous. The rachis, with linear persistent filiform-subulate stipels, is more or less glabrous and is 2-5 mm in length. The foliage is light, supported by first horizontal then drooping spreading branches (Figure 3).
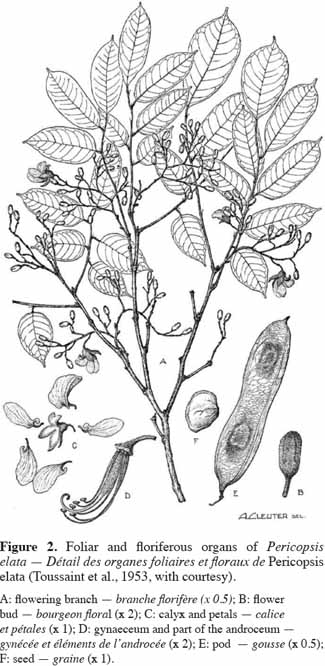

8Flowers. Pericopsis elata has bisexual flowers held in panicles with pubescent to glabrous peduncles and a rachis of 2-6(12) cm in length. White flowers (1.5 cm long, figure 4) have ovate bracts, each of 1.5 mm in length, a 5-toothed pubescent calyx (9-12 mm long) with the upper 2 closely joined, ten glabrous stamens with filaments of 9-19 mm in length, and a silky-silver or gold compressed ovary of 5-6 mm in length and 1.5 mm in width, containing 3-5 ovules.

9Fruits. The fruit of P. elata is an indehiscent flat pendulous wind-dispersed pod with a reticulate ornamentation and two faint longitudinal ridges. Length and width range from 7 to 17 and from 2.5 to 3 cm, respectively. Bourland et al. (2010) have computed an average weight of 131.6 ± 10.1 cg per pod. This oblong fruit presents pointed ends and a highly reticulate surface. The fruit holds 1-4(5) seeds, with their position being easily visible from the outside. These seeds are flat with a width varying from 1 to 1.5 cm.
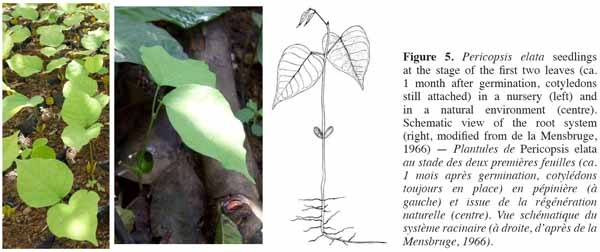
10Germination and seedlings. Epigeal germination generally occurs 5 to 10(15) day after sowing. If sowed within the first 10-20 days, viable seed germination rates are on average ca. 85-95%. Seedlings are characterized by (4.5)6.5(9) cm long hypocotyls and somewhat fleshy opposite oval cotyledons (12 mm long and 13 mm large; figure 5 left and centre). The first two leaves are stipulated, unifoliate and opposite or subopposite. The third and fourth leaves alternate with 2-3 leaflets (Figure 5 right). Young trees frequently show a bushy pattern of growth characteristic of the species.
11Roots, bole and bark. Pericopsis elata has nitrogen-fixing nodules. The stem is often uneven and unbuttressed, although in some places it is slightly fluted. The bark has a light color. At the pole stage, the bark peels off in irregular slender scales to leave reddish patches, which make the species easy to recognize. The bark thickness ranges from 0.5 to cm. The outer layer of the slash is green, while the remaining layer is yellow, turning darker upon exposure. The diameter at breast height can reach up to 130 cm.
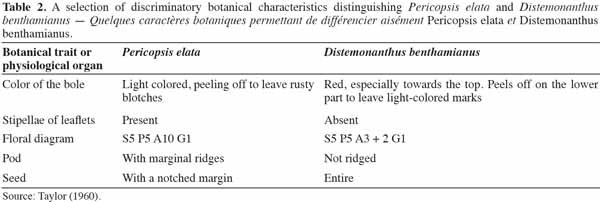
12It is interesting to note that many authors insist on alleged similarities between P. elata and Distemonanthus benthamianus Baill., mainly because of the red color of the bark and ecological similarities between the two species. Table 2 presents some discriminatory characteristics of both species.
2.3. Taxonomy
13From Ormosia to Pericopsis. The species P. elata was originally included in the genus Ormosia Jacks. Harms (1913) created the genus Afrormosia to separate the African (“Afr”, with flat indehiscent pods) species from the Asian and American ones (with inflated dehiscent pods). While Louis (1943) provided additional descriptions of the species, Knaap-van Meeuwen (1962) reduced Afrormosia to Pericopsis. Based on the anatomy of the wood of P. elata, Normand et al. (1976) reached conclusions similar to those of Knaap-van Meeuwen in differentiating African species from the Asian and American ones.
14Pericopsis Thwaites (Fabaceae, Papilionoideae) now comprises five species, four of which naturally occur in Africa (the remaining one in Asia).
15Pericopsis laxiflora (Benth.) Meeuwen vs. P. elata. Harms (1913) stated that the main differences between Afrormosia elata (forest tree) and Afrormosia laxiflora (savanna tree/shrub) lay in the size of the leaves and of the rachi, with additional differences to be found in some traits of fertile branches (the former species presenting more pubescence). The author suggested that some specimens identified at the beginning of the 20th century as A. laxiflora (1) in a Ghanaian mixed deciduous forest and (2) in a Cameroonian forest should probably be considered as A. elata. The potential confusion between the two species is similar to the one observed in the early 20th century between Lophira alata Banks ex C.F.Gaertn. (forest species) and Lophira procera A.Chev. (savanna species, see Sabatié, 1994). Studying both P. elata and P. laxiflora in Cameroon, Sabatié (1994) noted that the natural distribution areas of both species were located at separated opposite sides of the country. This separation fits a North-South rain gradient, with P. laxiflora occurring in much drier environments than the taller P. elata. The author defined the two trees as allopatric/vicariate species, and thus suggested that they would share the same genotype. However, this is not necessarily the case.
3. Distribution and origin of Pericopsis elata populations
3.1. Abiotic requirements and current geographical distribution
16Rainfall and soil water regime. Pericopsis elata is restricted to the 1,250-1,500 mm rainfall zone in Ghana (Swaine, 1996) and to 1,000 to 1,500 mm throughout its natural range. The species is tolerant to a wide range of water regimes ranging from well drained soils to seasonally waterlogged ones (Swaine, 1996; Anglaaere, 2008). No data are available regarding the potential pedological requirements of the species.
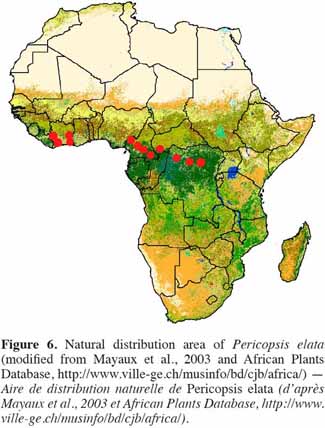
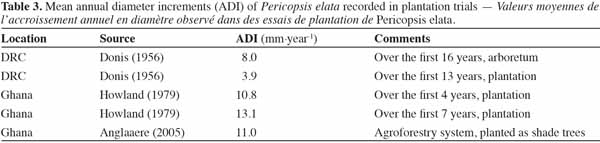
17Natural distribution pattern. Pericopsis elata is characteristic of the semi-deciduous forest (Swaine, 1996). The tree is native to countries of Central and West Africa, occurring mainly in Ghana (West), Cameroon, Congo and DRC (Howland, 1979; Hall et al., 1981; Hawthorne, 1995; Dickson et al., 2005; Hawthorne et al., 2006; Vivien et al., 2011). The species was detected in Nigeria at the beginning of the 20th century (Howland, 1979; Dahms, 1999). It has also been observed in Côte d’Ivoire (contiguously to Ghana) and the Central African Republic (CAR), where only a few specimens have been reported (Figure 6).
18This disrupted large-scale distribution pattern includes a series of possibly genetically isolated sub-populations. Thus, Howland (1979) suggested that further studies should be conducted in the following five major provenances (with a decreasing gradient of average annual rainfall from 1 to 5:
191. Côte d’Ivoire/Ghana;
202. Western Nigeria;
213. Eastern Nigeria;
224. Eastern Cameroon/Congo;
235. DRC.
24Samples from each provenance (urgently 1, 2 and 3 as they are dramatically reduced) should be collected for further genetic investigations. According to Dahms (1999), the quality of the wood of P. elata is not homogeneous throughout its distribution pattern. The author goes on to suggest that even though the provenance of the wood of P. elata has not substantially affected either its price or its industrial applications so far, provenance does make a difference to the qualities of wood itself. For example, the wood of P. elata from Côte d’Ivoire/Ghana would differ from the wood from DRC, particularly in terms of its calcium deposit levels. This remark strengthens the need to conduct further investigations regarding the existence of such provenances as well as their origins.
25Gregariousness of the species. When analyzed on a smaller scale, P. elata is defined as a gregarious species (Sabatié, 1994; Hawthorne, 1995; Boyemba, 2011), meaning large numbers of trees generally and naturally occur within spatially restricted areas. This ecological characteristic has also been observed for other long-lived light-demanding commercial species, such as T. scleroxylon. As a colonizer, the gregariousness of T. scleroxylon would result from its ability to invade bare areas resulting from extensive forest disturbance caused by anthropogenic activities (Hall et al., 1979). A similar reasoning could be held for P. elata, the two species sharing important traits (wind dispersed diaspores, tall-growing and light-demanding trees, etc.).
3.2. Assumptions regarding the origins of Pericopsis elata populations
26Recent references (van Gemerden et al., 2003; Willis et al., 2004; Brncic et al., 2007; Bayon et al., 2012; Neumann et al., 2012) provide circumstantial evidence for the fact that Central African rainforests should no longer be considered as pristine. For some of these studies, anthracological and/or archaeological surveys have shed new light on the possible link between the dramatic changes these forests have undergone since 2500 BP (Before Present) and large-scale settlements of farming populations of Bantu origin in that area. The fact that anthropogenic activities may have strengthened the effects of already occurring climate variations is now widely accepted (Brncic et al., 2007; Dube, 2009; Greve et al., 2011). For instance, the presence of pearl millet in the excavation pits of sites where the weather is no longer suitable for its cultivation has been confirmed by pollen profiles (Neumann et al., 2012). This kind of cultivation, which still occurs today in this part of Africa, creates openings which size is estimated at between 3,300 and 7,300 m² (de Wachter, 2001; Nyemeck Binam et al., 2004), substantially more than the lower limit of 1,000 m² suggested by Boyemba (2011) for spontaneous regeneration of the species. In a forest concession under low-impact logging management where P. elata occurs, Kouadio et al. (2009) estimated that a logging gap area of ca. 265 m² is practiced. This would help to explain why low impact logging, as applied today in many parts of the distribution area of the species, does not create openings large enough to favor the natural regeneration of the species.
27To complete this review of the potential positive influence of slash and burn practices on P. elata regeneration, it is interesting to note that the mature trees of the species are difficult to fell with traditional methods because of their high wood density. As P. elata adult trees have a lower than average sensitivity to fire (Dupuy, 1998), it is thus not surprising that some of them are preserved within or close to cropping areas in shifting cultivation systems (CTFT, 1956), where they may act as seed trees.
4. Ecology of natural populations
4.1. Population structure
28Since juvenile and old stems are said to be rare throughout its natural distribution area, the typical population structure of P. elata follows a bell-shaped curve in Cameroon (Betti, 2007; Kouadio, 2009), Ghana (Swaine et al., 1988), and in some places in DRC (Boyemba, 2011). This Gaussian-type distribution suits light-demanding species, which were once favored by past large openings.
4.2. Phenology
29Three documents describing the phenological traits of P. elata are available for the region of Yangambi (DRC; Vangu-Lutete, 1985; Pieters, 1994; Boyemba, 2011). Only a few references briefly refer to the phenology of the species in Ghana (Taylor, 1960). Apart from the preliminary results of Bourland et al. (2010), no other information are available for Cameroon. Finally, Howland (1979) gives some sparse phenological data for the species, without giving population locations.
30Leaf shedding and flushing. Classified as a deciduous species by Vivien et al. (2011), leaf shedding occurs during the main dry season (January-February in the Yangambi region). Agyeman et al. (1999) consider the tree as an evergreen species in the Ghanaian context.
31Flowering. In DRC and Ghana, flowering occurs from February to (May-)June, with a maximal frequency being reached in March. In Cameroon, flowering starts in March-April (Bourland et al, 2010).
32Fruiting. In the Yangambi region, the seed rain occurs either from June to September (Boyemba, 2011) or from November to March (Howland, 1979). Such a difference in the fruit maturation process, observed for the same species growing in a similar (if not identical) environment, is difficult to explain. In Ghana, the P. elata pods do not become ripe until August to November. According to Boyemba (2011), 70% of the fruits are disseminated within the first 50 m away from the canopy, with only 3% reaching a distance of 100 m. The author also suggests that mast fruiting would occur every 2-3 years, while Pieters (1994) suggests every 2-4 years in the same ecological conditions. Vangu-Lutete (1985) showed that dissemination of ripe fruit lasts ca. 2 weeks.
33In their study of P. elata in Cameroon, Bourland et al. (2010) noted that minimum fertile and effective fruiting diameters were 32 and 35 cm (> 30 cm for Boyemba, 2011), respectively. They also found that the fruit ripened over 7 months.
34During germination experiments conducted under the forest canopy, Pieters (1994) observed that some of the pods he had dropped on the ground had disappeared. He suggested that rodents might have fed on the pods. However, no clear evidence or other mentions of such removal/destruction by animals can be found in the literature for P. elata.
4.3. Germination processes, natural regeneration and light requirements
35Pieters (1994) reported that P. elata germination occurs naturally in the Yangambi region in February and March, which suggests a seed rain from November to March, as stated by Howland (1979). In his conclusions, Pieters (1994) stated with certainty that the untouched forest microclimate (close to the ground, with a very low level of light exposure and a high moisture content) is the most favorable for the germination of these species, while the environment created after clear felling is extremely unfavorable. After germination in an untouched forest, he noted a high mortality rate among young seedlings, especially during the first weeks after germination. This mortality rate fell sharply 14 months after germination but remained higher in the darkest spots of his study site. Pieters found that the development of seedlings was strongly inhibited at that stage and that the removal of lianas and understorey trees had only a limited positive effect. The most favorable environment for height growth was achieved when all of the vegetation, apart from the possible seed trees, was removed one year after germination. Pieters’ (1994) experiments confirmed the findings of Ampofo et al. (1972; seedling growth occurred best under a relative light intensity of 18%), showing that too much light inhibits height increase during the first months after germination. In the darkest spots of his study site, Pieters (1994) recorded that seedlings had a reduced number of leaves and were weak, but that they suffered little from insect attack. In contrast, in artificially opened environments, the author reported that the top shoots of seedlings were regularly nipped out by insects (leading to a bushy aspect of the young crown) and that there were high rates of defoliation by caterpillars. The assumption was that those attacks were positively linked to the importance of the silvicultural intervention in that area (but that the attacks did not necessarily induce the death of seedlings).
36Taylor (1960) obtained similar results in Ghana, showing that P. elata is tolerant to a certain amount of shade in its early youth, but shortly afterwards becomes a light-demander. In the same Ghanaian context, P. elata is classified among either pioneer or non-pioneer light-demanders by various authors (Ampofo et al., 1972; Swaine et al., 1988; Hawthorne, 1995; Kyereh et al., 1999).
4.4. Growth and natural mortality
37Growth. Growth monitoring of P. elata has been carried out in natural stands as well as plantation trials. Table 3 summarizes the data available for planted stands in Cameroon, DRC and Ghana. The ADI ranges from 3.9 to 15.2 mm per year in enrichment plantings; the variation might be explained by differences in growth conditions. Increments obtained in line plantings in Cameroon (in similar environmental conditions) under the canopy forest and in wide open areas provide among the lowest and the highest increments, respectively. These findings emphasize high light requirements of the species at the seedling and sapling stages. Regarding growth performance at the adult stage, Schmitz (1962), Vangu-Lutete (1974) and Boyemba (2011) reported ADIs in Yangambi mixed moist forest of 6.8 (11 trees), 4.5 (101 trees) and 4.2 ± 1.4 mm per year (422 trees), respectively. In natural stands in Cameroon, Kouadio (2009) and Bourland et al. (2010) found similar results (ADI of 3.2 ± 0.4 mm per year). Except for Schmitz’s finding (6.8 mm), which should be treated with caution (study restricted to 11 trees, based on tree ring analysis), ADI values range from 3.2 to 4.5 mm per year. This variability could originate in differences in soil fertility rather than other abiotic parameters (rainfall quantity and seasonality, temperature and light exposure are fairly similar between both sites).
38Mortality. In the Yangambi forest, Vangu-Lutete (1974) computed over a period of 30 years annual mortality rates of 0.73 and 0.85% for stems with a dbh > 12.8 cm and for all 137 stems of his study site, respectively. In the same forest, Boyemba (2011) recorded over a much shorter period of time (but with 422 trees) an annualized mortality rate of 0.6%. No other data are currently available regarding this important forest management parameter.
5. Forest management
5.1. Main identified pests, nursery-raising, plantations and other silvicultural interventions
39Identified pests. A checklist of Scolytidae (Coleoptera) pests affecting P. elata is given by Brigham Young University (1992). Among the 26 insects listed, two are widely distributed, notably in West Africa: Xyleborus ferrugineus Wood & Bright (ambrosia beetle) and Doliopygus conradti Wood & Bright, both of which normally breed in dead/dying trees (Howland, 1979; Wagner et al., 2008).
40In their study of P. elata in Cameroon, Bourland et al. (2010) preliminary observations of the seed rain showed that the proportion of seeds eaten by insect larvae significantly depended on the remoteness of the seed tree from other individuals of the same species (extreme rates ranging from 10 to 95% for totally isolated to clustered trees, respectively). In their study of forest insects of Ghana, Wagner et al. (2008) noted that Laspeyresia sp. nr. tricentra Meyr. may be implicated in seed feeding. Taylor (1960) suggested that instead of lack of light being a causal factor, seed damage caused by insects could cause the paucity of natural regeneration. Pieters (1994) described similar important seed losses after insect attacks in DRC populations.
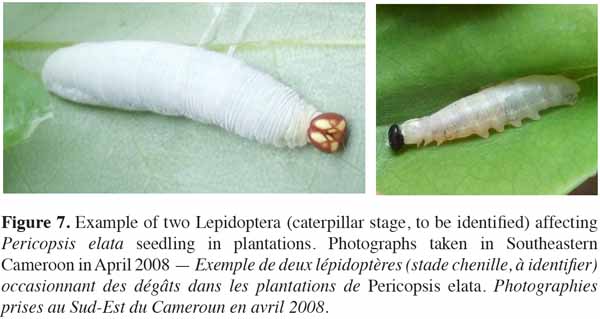
41Regarding pests potentially affecting seedling mortality and growth in Ghana (both in nursery and natural environments), Lemmens et al. (2010) and Wagner et al. (2008) showed that the leaf-tying moth Lamprosema lateritialis Hampson (Lepidoptera: Pyralidae) posed the most serious threat. According to Wagner et al. (2008), repeated defoliations by caterpillars cause up to 31% of the mortality rate of 6-month-old seedlings. The authors showed that within their average larval life span (21 days), each caterpillar consumes the equivalent of 2-3 pinnate leaves. Since an average egg batch may contain up to 200 eggs (laid on the upper surface of leaflets), caterpillars emerging from those eggs would be able to totally defoliate several 6(7)-month-old seedlings. As a pest management method for application in nurseries, the authors recommend a mechanical removal of eggs during normal morning watering. In Cameroon, other Lepidoptera may impact seedling growth and/or survival rates in plantations (see photographs in figure 7).
42In Ghana, Apate indistincta Murr (Coleoptera: Bostrichidae; wood borer of living trees) has been reported as attacking the seedlings of P. elata (Atuahene, 1976). In his conclusions, the author of that study recommends removal of infested individuals so as to control this pest in the nursery. Apart from damage caused by insects, damage has also been reported as being caused by gorillas feeding on young leaves and fruit (Tropicos.org. Missouri Botanical Garden. 11 May 2012 <http://www.tropicos.org/Specimen/78675>). This is likely to have been noted during the fruit ripening period in an environment where P. elata is scarce.
43Handling of nursery-raised seedlings. Together with relatively high increments reached in open areas, the ease of handling P. elata seedlings in the nursery makes the tree a most promising plantation species (see Howland, 1979). Pieters (1994) noted a germination rate of 87% in a controlled artificial environment in Yangambi. In a nursery in Cameroon, Kouadio (2009) recorded germination rates of between 44 and 74% (reached on average after 10 days) when seeds were sowed immediately after collection. Provided basic good management procedures are applied in the nursery (including pest control) and seeds are stored for no longer than 2 months before sowing, Kouadio (2009) showed that vigorous seedlings of ca. 50-60 cm tall can be obtained after 9-12 months without fertilization.
44Plantations and other silvicultural interventions. Despite the fact that P. elata can easily be propagated from seed, the species is not being planted on a large scale. Nevertheless, some experiments have been conducted on a smaller scale, mainly in Ghana and DRC.
45Working on methods to compensate for sparse recruitment in the Yangambi area, Pieters (1994) stated that no specific initial intervention should be applied, since germination occurs best under an intact forest cover. The author recommends the cutting of lianas and saplings of undesired species for the first weeks after the tree’s germination. In the meantime, a progressive release within the upper canopy cover should be promoted to avoid a massive loss of seedlings. Pieters (1994) recommends that, after this initial germination, seedlings should be freed twice during the first year and then the area weeded once a year. Although this technique led to positive results in the context of Pieters’ study, its routine application is unlikely. This approach would probably be too expensive and time-demanding for most forest managers (logging companies in Central Africa). In addition, as also pointed out by Kouadio (2009), temporary storage of fruits before sowing is one of the main causes of poor regeneration success (Pieters, 1994).
46In Cameroon, Kouadio (2009) investigated the possibility of rehabilitating logging gaps by applying the direct sowing method. The germination rate was found to be 44.9 ± 7.7% for ca. 1,000 sowed seeds in 11 gaps. After 30 months (with no intervention), 68.3 ± 9.4% of the seedlings had survived but growth (in height) was quite limited compared to other tested species (e.g., Baillonella toxisperma Pierre). Such experiments conducted in logging gaps require further investigation, for instance through the planting of nursery-raised seedlings (Kouadio et al., 2009) instead of direct sowing.
5.2. Implications of national regulations for forest management
47Regulations governing logging have implications for the management of P. elata populations. In the Congo Basin, logging companies are obliged to implement management plans based on specific inventories, a minimum logging cycle (e.g., of 30 years in Cameroon) and the calculation of commercial species recovery rates (sensu Durrieu de Madron et al., 1998) over this period (as well as meeting other requirements). A minimum recovery rate of 50% of the initial stock (in terms of number of trees) is generally suggested. If required, the MLD may be increased. In Cameroon, exportation of P. elata logs is prohibited in order to promote local processing and the MLD was lowered from 100 to 90 cm in June 2010. This MLD value remains higher than the one used in other central African countries (Table 4).

5.3. Conservation status
48Presently, stocks of P. elata are reported to be dramatically reduced, specifically in Ghana, Côte d’Ivoire, Nigeria and CAR (Hawthorne, 1995; Dickson et al., 2005). With the exception of Ghana, the species may even be close to disappearance in those countries (especially Côte d’Ivoire and CAR). Consequently, and because of its continuing exploitation in Cameroon, Congo and DRC, P. elata is now included in CITES Appendix II (Abensperg-Traun, 2009), and is recorded as “Endangered A1cd” on the IUCN Red List (IUCN, 2001). According to Abensperg-Traun (2009), national legislation in those countries is generally able to meet all (Cameroon and DRC) or parts of (Congo) the requirements for the implementation of CITES. In addition, being included in CITES Appendix II means that export and import licenses are necessary for logs and sawn wood of the species. These licenses are controlled by the scientific authorities of the CITES administration for the exporting and importing countries concerned.
6. Conclusion and research perspectives
49In most of the literature reviewed in the present study, regeneration of P. elata is reported as being remarkably rare, despite abundant seed production wherever it is studied in its distribution area. It is probable that the species will soon disappear from CAR, Côte d’Ivoire and Nigeria because of a combination of a lack of regeneration and the current increasing human pressure (mainly intense logging activities and agriculture). Pericopsis elata populations in Ghana are said to have been dramatically reduced over time (Dickson et al., 2005).
50Despite being listed both in CITES Appendix II and on the IUCN Red List, important information on P. elata ecology are still lacking (Howland, 1979; Anglaaere, 2008). Indeed, too few data are available on phenological patterns and fertility, making it difficult, for example, to analyze the impact of logging on seed tree populations. Lemmens et al. (2010) identified growth, genetic selection for plantations and resistance to L. lateritialis as the main research issues still to be addressed. In addition, almost nothing is known about the history of P. elata populations in general and specifically why regeneration is dramatically lacking in its natural distribution area. Consequently, more research is needed before a definitive decision can be made to allow harvesting of P. elata, in order to ensure that this action does not threaten the species with extinction. Recent developments in genetics (Micheneau et al., 2011) should prove useful in investigating Sabatié’s (1994) assumption that phenotypic differences between P. elata and P. laxiflora are induced by environmental changes. As highlighted by Daïnou et al. (2012) for another timber species, such a study would also investigate the genetic variation and spatial genetic structure of P. elata. These genetic studies (especially the analysis of the origin of phylogeographic patterns for the species), together with work involving archaeological and anthracological aspects, could help us to understand the origins of P. elata natural populations as well as their evolution. Finally, plantation trials need to be conducted to identify affordable and effective enrichment methods that could be routinely applied by logging companies (including pest identification and control techniques).
51Abbreviations
52ADI: mean annual diameter increment
53ATIBT: Association Technique Internationale des Bois Tropicaux
54CAR: the Central African Republic
55CITES: Convention on International Trade in Endangered Species of Wild Fauna and Flora
56CTFT: Centre Technique Forestier Tropical
57DRC: the Democratic Republic of Congo
58FOB: free-on-board
59IUCN: International Union for Conservation of Nature
60MLD: minimum logging diameter (official value)
61Acknowledgements
62Financial support was provided by Gembloux Agro-Bio Tech mainly through the self-funded project TROPDIV-PPR10,000 and by the Belgian Fund for Scientific Research (FRFC grants n°2.4576.07 and 2.4577.10). We are indebted to the Pallisco company (Cameroon), the NGO Nature+ and Gembloux Agro-Bio Tech for their logistical support and technical assistance during Mr Bourland’s PhD field work. We are grateful to Mrs Federspiel and Messrs. Rougeron, Douaud, Brostaux, Limbourg, Vaianopoulos, Vermeulen, Ayol, Djompandé, Gassang, Nana and Zok. Special thanks go to Mrs Hernandez-Schiller for German to English translations.
Bibliographie
Abensperg-Traun M., 2009. CITES, sustainable use of wild species and incentive-driven conservation in developing countries, with an emphasis on Southern Africa. Biol. Conserv., 142, 948-963.
Agyeman V.K., Swaine M.D. & Thompson J., 1999. Responses of tropical forest tree seedlings to irradiance and the derivation of a light response index. J. Ecol., 87(5), 815-827.
Allen O.N. & Allen E.K., 1981. The Leguminosae. A source book of characteristics, uses and nodulation. London, UK: Macmillan.
Ampofo S.T. & Lawson G.W., 1972. Growth of seedlings of Afrormosia elata Harms in relation to light intensity. J. Appl. Ecol., 9(1), 301-306.
Anglaaere L.C.N., 2005. Improving the sustainability of cocoa farms in Ghana through utilization of native forest trees in agroforestry systems. PhD thesis: University of Wales, Bangor (UK).
Anglaaere L.C.N., 2008. Pericopsis elata (Harms) Meeuwen. In: Louppe D., Oteng Amoako A.A. & Brink M., eds. Ressources végétales de l’Afrique tropicale. Bois d’œuvre 1. Wageningen, The Netherlands: Fondation PROTA, 478-482.
ATIBT, 2012. La lettre de l’ATIBT n° 34. Paris: ATIBT.
Atuahene S.K.N., 1976. Incidence of Apate spp. (Coleoptera: Bostrychidae) on young forest plantations species in Ghana. Ghana For. J., 23, 29-35.
Bayon G. et al., 2012. Intensifying weathering and land use in Iron Age Central Africa. Science, 335, 1219-1222.
Betti J.L., 2007. Exploitation and exportation of Pericopsis elata (Fabaceae) in Cameroon. Yaoundé: Ministère des Forêts et de la Faune.
Bonnier C., 1957. Symbiose Rhizobium-légumineuses en région équatoriale. Séries Scientifique 72. Bruxelles: INEAC.
Bourland N., Kouadio Y.L., Colinet G. & Doucet J.-L., 2010. Pericopsis elata (Harms) Meeuwen in the southeastern part of Cameroon: ecological and pedological approaches to improve the management of an endangered commercial timber species. Int. For. Rev., 12(5), 111.
Boyemba F., 2011. Écologie de Pericopsis elata (Harms) Van Meeuwen (Fabaceae), arbre de forêt tropicale africaine à répartition agrégée. PhD thesis: Université Libre de Bruxelles (Belgique).
Brigham Young University, 1992. A catalogue of Scolytidae and Platypodidae (Coleoptera). Great Basin naturalist memoirs n°13. Part 2, Volumes A & B, 604 & 1244.
Brncic T.M., Willis K.J., Harris D.J. & Washington R., 2007. Culture or climate? The relative influences of past processes on the composition of the lowland Congo rainforest. Philos. Trans. R. Soc. London, Sér. B, 362(1478), 229-242.
CTFT, 1956. Fiche botanique, forestière, industrielle et commerciale: Asamela. Bois For. Trop., 50, 17-20.
Dahms K.-G., 1999. Afrikanische Exporthölzer. Leinfelden-Echterdingen, Germany: DRW-Verlag Weinbrenner GmbH & Co. KG.
Daïnou K., Sinsin B., Doucet J.-L. & Mahy G., 2012. Identité et écologie des espèces forestières commerciales d’Afrique Centrale : le cas de Milicia spp. Biotechnol. Agron. Soc. Environ., 16, 229-241.
de la Mensbruge G., 1966. La germination et les plantules des essences arborées de la forêt dense humide de la Côte d’Ivoire. Publication n° 26. Nogent-sur-Marne, France : CTFT.
de Wachter P., 2001. L’agriculture itinérante sur brûlis, base de l’économie Badjoué. In : Delvingt W., ed. La forêt des hommes. Gembloux, Belgique : Les Presses agronomiques de Gembloux, 15-42.
Dickson B. et al., 2005. An assessment of the conservation status, management and regulation of the trade in Pericopsis elata. Cambridge, UK: Fauna & Flora International.
Donis C., 1956. La forêt dense congolaise et l’état actuel de sa sylviculture. Bull. Agric. Congo Belg., 47(2), 261-289.
Dube O.P., 2009. Linking fire and climate: interactions with land use, vegetation, and soil. Curr. Opin. Environ. Sustainability, 1, 161-169.
Dupuy B., 1998. Bases pour une sylviculture en forêt dense tropicale humide africaine. Série FORAFRI, Doc. 4. Montpellier, France : CIRAD-Forêt.
Durrieu de Madron L. et al., 1998. Le projet d’aménagement Pilote intégré de Dimako (Cameroun). Série FORAFRI, Doc. 7. Montpellier, France : CIRAD-Forêt.
Gérard J. et al., 1998. Synthèse sur les caractéristiques technologiques de référence des principaux bois commerciaux africains. Série FORAFRI, Doc. 11. Montpellier, France : CIRAD-Forêt.
Greve M., Lykke A.M., Blach-Overgaard A. & Svenning J.-C., 2011. Environmental and anthropogenic determinants of vegetation distribution across Africa. Global Ecol. Biogeogr., 20, 661-674.
Harms H., 1913. Leguminosae africanae. In: Engler A., ed. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. Leipzig, Germany: Verlag von Wilhelm Engelmann, 430-432.
Hall J.B. & Bada S.O., 1979. The distribution and ecology of obeche (Triplochiton Scleroxylon). J. Ecol., 67, 543-564.
Hall J.B. & Swaine M.D., 1981. Distribution and ecology of vascular plants in a tropical rainforest. Forest Vegetation in Ghana. The Hague: W. Junk.
Hawthorne W.D., 1995. Ecological profiles of Ghanaian forest trees. Tropical Forestry Papers 29. Oxford, UK: Oxford Forestry Institute.
Hawthorne W.D. & Jongkind C., 2006. Woody plants of Western African forests. A guide to the forest trees, shrubs and lianes from Senegal to Ghana. Kew, UK: Royal Botanic Gardens.
Howland P.P., 1979. Pericopsis elata (Afrormosia). CFI Occasional Papers 9. Oxford, UK: University of Oxford.
IUCN, 2001. IUCN Red List of threatened species. Gland, Switzerland: IUCN Species Survival Commission.
Knaap-van Meeuwen M.S., 1962. Reduction of Afrormosia to Pericopsis (Papilionaceae). Bull. Jard. Bot. Nat. Belg., 32(2), 213-219.
Kouadio Y.L., 2009. Mesures sylvicoles en vue d'améliorer la gestion des populations d'essences forestières commerciales de l'Est du Cameroun. Thèse de doctorat : Faculté universitaire des Sciences agronomiques de Gembloux (Belgique).
Kouadio Y.L. & Doucet J.-L., 2009. Étude du comportement de Baillonella toxisperma Pierre (moabi) dans les trouées d’abattage enrichies. Biotechnol. Agron. Soc. Environ., 13(2), 317-324.
Kukachka B.F., 1960. Kokrodua – Afrormosia elata Harms, Leguminosae (Papilionaceae). Foreign Wood Series 1978. Washington: U.S. Department of Agriculture, Forest Service.
Kyereh B., Swaine M.D. & Thompson J., 1999. Effect of light on the germination of forest trees in Ghana. J. Ecol., 87(5), 772-783.
Lemmens R.H.M.J., Omino E.A. & Bosch C.H., 2010. Timbers of Tropical Africa. Conclusions and recommendations based on PROTA 7(1): “Timbers 1”. 2009. Nairobi: Fondation PROTA.
Louis J., 1943. Contribution à l'étude du genre Afrormosia au Congo belge. Bull. Jard. Bot. de l'État à Bruxelles, 17(1), 109-116.
Louis J. & Fouarge J., 1943. Essences forestières et bois du Congo. Coll. in-4°, fasc. 2. Bruxelles : INEAC.
Mayaux P. et al., 2003. A land cover map of Africa. Carte de l'occupation du sol de l'Afrique. Luxembourg : European Commission.
Micheneau C. et al., 2011. Development and characterization of microsatellite loci in Pericopsis elata (Fabaceae) using a cost-efficient approach. Am. J. Bot., 98(10), e268-270.
Neumann K. et al., 2012. First farmers in the Central African rainforest: a review from southern Cameroon. Quat. Int., 249, 53-62.
Normand D. & Paquis J., 1976. Manuel d’identification des bois commerciaux. Vol. 2: Afrique guinéo-congolaise. Nogent-sur-Marne, France : CTFT.
Nyemeck Binam J. et al., 2004. Factors affecting the technical efficiency among smallholder farmers in the slash and burn agriculture zone of Cameroon. Food Pol., 29, 531-545.
Pieters A., 1994. Natural regeneration in the equatorial forest of the Yangambi Region, applied to Afrormosia elata Harms. Leuven, Belgium: A. Pieters and F. Pauwels.
Sabatié B., 1994. Biosystématique et vicariance dans la flore camerounaise. Bull. Jard. Bot. Nat. Belg., 63, 125-170.
Schmitz A., 1962. Établissement d’une courbe de répartition par âge d’une essence caducifoliée (Application à l’Afrormosia elata Harms). Bull. Soc. R. For. Belg., 12, 517-550.
Swaine M.D., 1996. Rainfall and soil fertility as factors limiting forest species distributions in Ghana. J. Ecol., 84(3), 419-428.
Swaine M.D. & Whitmore T.C., 1988. On the definition of ecological species groups in tropical rain forests. Vegetatio, 75(1-2), 81-86.
Taylor C. J., 1960. Synecology and silviculture in Ghana. London: Thomas Nelson and Sons Ltd.
Toussaint L., Wilczek R., Gillett J.B. & Boutique R., 1953. Flore du Congo Belge et du Ruanda-Urundi: Papilionaceae (Sophoreae, Genisteae, Trifolieae et Loteae). Vol. IV. Bruxelles : INEAC.
Van Gemerden B.S., Olff H., Bongers F. & Parren P.E., 2003. The pristine rain forest? Remnants of historical human impacts on current tree species composition and diversity. J. Biogeogr., 30(9), 1381-1390.
Vangu-Lutete C., 1974. Accroissement en circonférence de l’Afrormosia elata Harms (Syn. Pericopsis elata Harms) dans une forêt naturelle de Yangambi. Thèse de doctorat : Université Nationale du Zaïre (Yangambi, DRC).
Vangu-Lutete C., 1985. Rythme phénologique de l’Afrormosia elata Harms dans la région de Yangambi. Scientia, 1, 31-43.
Vivien J. & Faure J.J., 2011. Arbres des forêts denses d'Afrique centrale. Clohars-Carnoët, France : Éditions Nguila-Kerou.
Wagner M.R., Cobbinah J.R. & Bosu P.P., 2008. Forest entomology in West Tropical Africa: forest insects of Ghana. Dordrecht, The Netherlands: Springer.
Willis K.J. & Brncic T.M., 2004. How “virgin” is the virgin rainforest? Science, 304, 402-403.
Pour citer cet article
A propos de : Nils Bourland
Univ. Liège - Gembloux Agro-Bio Tech. Unit of Forest and Nature Management. Laboratory of Tropical and Subtropical Forestry. B-5030 Gembloux (Belgium). E-mail: nils.bourland@aigx.be
A propos de : Yao Lambert Kouadio
Univ. Liège - Gembloux Agro-Bio Tech. Unit of Forest and Nature Management. Laboratory of Tropical and Subtropical Forestry. B-5030 Gembloux (Belgium) – Abobo Adjamé University. Unit of Nature Sciences. 01 BP 4403. CI-Abidjan (Côte d’Ivoire).
A propos de : Fousséni Fétéké
Univ. Liège - Gembloux Agro-Bio Tech. Unit of Forest and Nature Management. Laboratory of Tropical and Subtropical Forestry. B-5030 Gembloux (Belgium) – Pallisco SARL. Avenue des Cocotiers, 478. BP 394. CAM-Douala (Cameroon).
A propos de : Philippe Lejeune
Univ. Liège - Gembloux Agro-Bio Tech. Unit of Forest and Nature Management. B-5030 Gembloux (Belgium)
A propos de : Jean-Louis Doucet
Univ. Liège - Gembloux Agro-Bio Tech. Unit of Forest and Nature Management. Laboratory of Tropical and Subtropical Forestry. B-5030 Gembloux (Belgium).






