- Startpagina tijdschrift
- volume 16 (2012)
- numéro 4
- Characteristics of African traditional beers brewed with sorghum malt: a review
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Characteristics of African traditional beers brewed with sorghum malt: a review

Nota's van de redactie
Received on September 13, 2011; accepted on August 6, 2012
Résumé
Caractéristiques des bières traditionnelles africaines brassées avec le malt de sorgho (synthèse bibliographique). Les bières traditionnelles à base de sorgho sont produites dans plusieurs pays d’Afrique, mais les processus de fabrication varient en fonction de leur localisation géographique. Elles sont très riches en calories, en vitamines du groupe B comprenant la thiamine, l’acide folique, la riboflavine et l’acide nicotinique, et en acides aminés essentiels tels que la lysine. Cependant, les bières africaines à base de sorgho sont moins attrayantes que les bières occidentales en raison de leur qualité hygiénique, de la variation de leurs caractéristiques organoleptiques et de leur courte durée de conservation. Les recherches sur les caractéristiques microbiologiques, biochimiques et technologiques des bières traditionnelles africaines ont été effectuées et documentées dans plusieurs pays d’Afrique. L’objectif de cette revue bibliographique sur les bières traditionnelles à base de sorgho est de récapituler le processus de fabrication et les caractéristiques des bières traditionnelles africaines (ikigage, merissa, doro, dolo, pito, amgba et tchoukoutou) à base de sorgho, tout en précisant les différences majeures avec la bière à base de malt d’orge. Cette revue s’adapte également au progrès accompli dans l’amélioration des bières traditionnelles africaines à base de sorgho.
Abstract
Traditional sorghum beers are produced in several countries of Africa, but variations in the manufacturing process may occur depending on the geographic localization. These beers are very rich in calories, B-group vitamins including thiamine, folic acid, riboflavin and nicotinic acid, and essential amino acids such as lysine. However, the traditional sorghum beer is less attractive than Western beers because of its poorer hygienic quality, organoleptic variations and shorter shelf life. Research into the microbiological and biochemical characteristics of traditional sorghum beers as well as their technologies have been performed and documented in several African countries. This review aims to summarize the production processes and compositional characteristics of African traditional sorghum beers (ikigage, merissa, doro, dolo, pito, amgba and tchoukoutou). It also highlights the major differences between these traditional beers and barley malt beer, consumed worldwide, and suggests adaptations that could be made to improve the production process of traditional sorghum beer.
Inhoudstafel
1. Introduction
1Sorghum, unlike barley, is very well adapted to the semi-arid and sub-tropical conditions prevailing over most of the African continent (Agu et al., 1998). Like barley, sorghum belongs to the grass family of Gramineae. In Africa, sorghum grain is the major cereal crop used to produce the traditional “opaque” beers (Novellie, 1976; Asiedu, 1991). However, only certain sorghum varieties (e.g. red grain) are specifically used to produce sorghum beers. These beers are known as ikigage in Rwanda (Lyumugabe et al., 2010), tchoukoutou in Benin and Togo (Kayodé et al., 2005), dolo in Burkina- Faso (Dicko et al., 2006), pito or burkutu in Nigeria and Ghana (Ekundayo, 1969; Faparusi et al., 1973), amgba in Cameroon (Chevassus-Agnes et al., 1979), doro or chibuku in Zimbabwe (Chamunorwa et al., 2002), merissa in Sudan (Dirar, 1978), mtama in Tanzania (Tisekwa, 1989), bili bili in Chad (Maoura et al., 2005) and kaffir in South Africa (Novellie et al., 1986).
2The manufacturing processes of African traditional sorghum beer essentially involves malting, drying, milling, souring, boiling, mashing and alcoholic fermentation, but variations may occur depending on the geographic localization (Haggblade et al., 2004). These types of beer differ from European (lager) types in the fact that lactic fermentation also occurs during sorghum beer processing. In addition, African traditional sorghum beer is consumed while it is still fermenting, and the drink contains large amounts of fragments of insoluble materials (Rooney et al., 1991). These fragments are mainly starch residues and dextrins that are not digested during mashing and fermentation (Glennie et al., 1986). Sorghum beers bear very little resemblance in appearance to Western beer made with barley. However, some studies have suggested that the use of sorghum malt (instead of barley malt) in lager-beer brewing is unlikely to succeed because of some inherent problems (enzymes, starch characteristics, polyphenols) associated with sorghum (Aisien, 1982; Palmer, 1991; Bajomo et al., 1994).
3Several studies into the microbiological and biochemical characteristics of traditional sorghum beers as well as their technologies have been carried out and documented in different African countries (Novellie, 1962; Ekundayo, 1969; Faparusi et al., 1973; Dirar, 1978; Tisekwa, 1989; Chamunorwa et al., 2002; Maoura et al., 2005). A very varied yeast and lactic bacteria acid flora has been found in African sorghum beers, although Saccharomyces cerevisiae and heterofermentative Lactobacillus usually predominate (Novellie, 1976; Sefa-dedeh et al., 1999; Chamunorwa et al., 2002; Maoura et al., 2005; Kayodé et al., 2007a; Lyumugabe et al., 2010). Traditional African sorghum beers are very rich in calories, B-group vitamins including thiamine, folic acid, riboflavin, and nicotinic acid, and essential amino acids such as lysin (Chevassus-Agnes et al., 1979). The beers are consumed at various festivals and African ceremonies (e.g., marriage, birth, baptism, the handing over of a dowry, etc.) and constitute a source of economic return for the female beer producers. However, in the majority of African countries, traditional sorghum beers are less attractive than Western beers brewed with barley malt because of their poor hygienic quality, low ethanol content, organoleptic variation and unsatisfactory conservation (Novellie et al., 1986; Tisekwa, 1989; Sanni et al., 1999; Lyumugabe et al., 2010). This review aims to summarize the production processes and characteristics of African traditional sorghum beers. It also highlights the major differences between these traditional beers and the familiar barley malt beer, consumed worldwide, and suggests adaptations that could be made to improve the production processes of traditional sorghum beer.
2. Malting
4Malting is the germination of cereal grain in moist air under controlled conditions, the primary objective being to promote the development of hydrolytic enzymes, which are not present in the ungerminated grain. The malting process essentially involves steeping, germinating and limiting cereal seedling growth, once enzymes have been produced for the degradation of starch and proteins in the cereal grain, but before the exhaustion of the polysaccharide.
2.1. Steeping
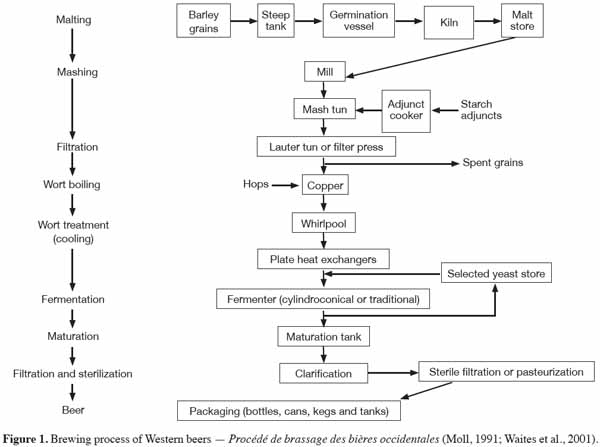
5The steeping or soaking of cereal grain in water is widely acknowledged as the most critical stage of the malting process (French et al., 1990; Dewar et al., 1997). This is a consequence of the importance of initiating germination such that modification of the endosperm structure will progress at a rate producing malt of the desired quality. During the Western beers brewing process (Figure 1), malting begins with the soaking of the barley in water for 2 days at 10-16 °C in order to increase the moisture content to around 45% (Moll, 1991; Waites et al., 2001). Periodically, the water is temporarily drained off and aeration is provided, thus preventing anaerobic conditions that can cause grain embryo damage. In Africa, the traditional sorghum malting process also starts with the soaking of the sorghum grain in water for 10 to 24 h at ambient temperature (Maoura et al., 2009; Lyumugabe et al., 2010), but, in this case, the water is not renewed or aired. The steep-out moisture content of sorghum grain is affected by both steeping time and temperature (Dewar et al., 1997). However, the steeping period at a given time varies according to the sorghum cultivar. A variation in moisture content of 32.4 to 43.4% has been observed after steeping 26 sorghum cultivars for 24 h (Kumar et al., 1992). The steep moisture increases as the steeping temperature rises from 10 to 30 °C, for any given period (Novellie, 1962). The effect of steeping conditions has been extensively investigated in an attempt to increase sorghum malt amylase activity. In 1962, Novellie reported that steeping time had little effect on the final diastatic power of sorghum malt. Steep moisture of 42 to 48%, attained after steeping sorghum for 18 to 22 h at 30 °C, is optimal for enzymatic activity (Morall et al., 1986). An increase in steep moisture with a steeping time of between 12 and 20 h at 30 °C is accompanied by a corresponding increase in reducing sugar content, and in cold-and hot water extracts (Owuama et al., 1994). The level of increase in these features of the sorghum malt appears to be directly proportional to its diastatic power (Pathirana et al., 1983). A steeping regime, and in particular the use of air-rests and a final warm water (40 °C) steeping period has been shown to enhance sorghum malt quality, including β-amylase activity (Ezeogu et al., 1995). A later study specifically confirmed the importance of the effect of air-rests on the level of sorghum malt β-amylase activity (Okungbowa et al., 2002). It is likely that the presence of oxygen leads to a more rapid increase in seedling metabolic activity (Dewar et al., 1997). The diastatic power of malt has also been shown to increase with steeping temperature (up 30 °C) and with the level of free amino nitrogen (FAN) (Dewar et al., 1997). Steeping the sorghum grain in dilute alkaline liquor (0.1% of Ca(OH)2, KOH or NaOH) has been shown to significantly enhance the diastatic activity of sorghum malt especially β-amylase activity (Okolo et al., 1996; Okungbowa et al., 2002).
2.2. Germination
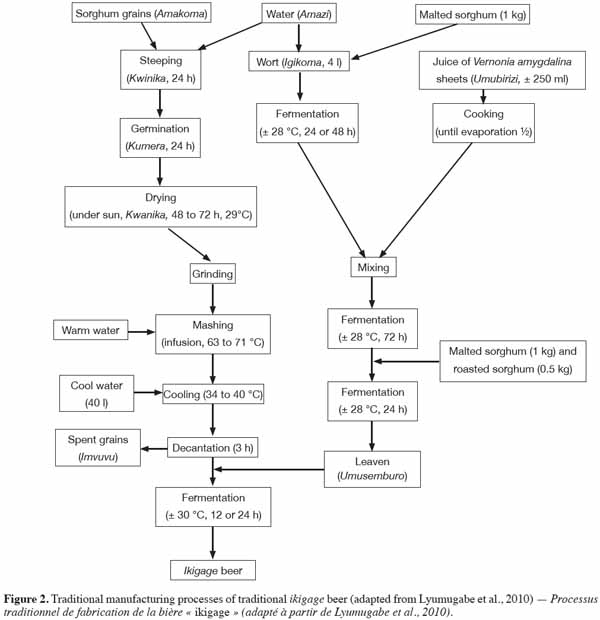
6After steeping, the sorghum grains are then spread out on a germination device (e.g. green plantain leaves or a plastic sheet) to form a layer (2 to 3 cm in thickness) and the grains are kept covered for 2-3 days at ambient temperature (Chevassus-Agnes et al., 1979; Lyumugabe et al., 2010). The layer of grains is sometimes turned over twice a day and the initial moisture level is maintained by spraying with water. This technique is similar to the old germination process that was previously used to produce Western beers, where barley grains were spread out on malting floors to a depth of 10-20 cm for 3-5 days at 16-19 °C (Moll, 1991). However, Western breweries now use various mechanized systems, which have grain beds of about 1 m in depth. These grains beds are aerated with moist cool air and turned mechanically every 8-12 h to aid respiration by the grain and to prevent the build-up of heat; otherwise the grain embryo may become damaged.
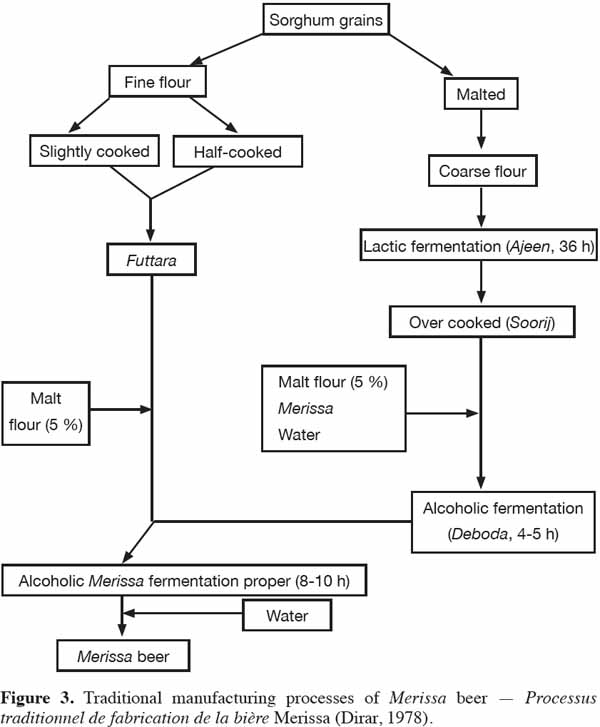
7Germination involves the outgrowth of the plumule and radicle of the seedling until suitable enzymes (e.g. starch degrading enzymes and proteases) have been produced for the malt (Palmer, 1989). During germination, the hormone gibberellic acid (GA), at low concentration (0.1-0.2 ppm), induces the barley aleurone layer to produce endosperm-degrading enzymes such as α-amylase, protease, pentosanases and endo-beta-glucanase (Palmer, 1989), but this hormone plays no such role in enzyme development in sorghum (Aisien et al., 1983; Palmer, 1989). In sorghum, α-amylase and carboxypeptidases are produced by the scutellum, while endo-β-glucanase, limit dextrinase and endo-protease develop in the starchy endosperm. These contrasts with malting barley where α-amylase, endo-protease, limit dextrinase and endo-β-glucanase develop in the aleurone layer, while carboxypeptidases and β-amylase are found in the starchy endosperm (Aisien et al., 1983; Palmer, 1989). Phosphate, an important mineral found in barley aleurone tissue and in the sorghum embryo (Palmer et al., 1989), may account for differences in the enzyme-producing potentials of the barley aleurone and the sorghum embryo (Agu et al., 1998). The endosperm of malted sorghum retains starch compaction and is not as friable as the barley malt grain. Malting (respiration and root) loss of sorghum malts is about 20%, while the malting loss of barley malt is about 7% after 6 d of growth at 25 °C and 16 °C (Palmer et al., 1989).
8Another important physiological difference between sorghum and barley malts is that malted sorghum grains contain low levels of endo-β-glucanase and β-amylase (Aisien et al., 1983). In their experiments, Beta et al. (1995) found that levels of α-amylase activity (25-183 U·g-1) and β-amylase activity (11-41 SDU·g-1) in sorghum malt varied, depending on the sorghum variety. Recently, a comparative study of white sorghum varieties indicated that the F-2-20 varieties from Senegal have an α-amylase activity of 312, 6 U·g-1 and a β-amylase activity of 62, 7 U·g-1 (Khady et al., 2010). However, compared to barley malt, whose β-amylase activity is 400 U·g-1 (Taylor et al., 1993), sorghum malt is not adapted to be used in an efficient brewing industry. Several assumptions explain the weakness of β-amylase activity in sorghum malt. Uriyo et al. (1999) explained this weakness in terms of an interaction between β-amylase and polyphenols during the extraction process, whereas Dufour et al. (1992) showed that β-amylase activity remains weak, even in sorghum varieties with low polyphenol content. Other authors claim that inhibitors apart from polyphenols would be present, causing partial solubilization of β-amylase or a weak output during malting (Palmer et al., 1989; Agu et al., 1997a). According to Taylor et al. (1993), ungerminated sorghum also does not exhibit β-amylase activity. This is fundamentally different from barley, where the ungerminated grain does exhibit β-amylase activity. It appears that tropical cereal grains such as pearl millet, sorghum and maize possess only the “ubiquitous” form of β-amylase, whereas temperate Triticeae cereals such as barley, wheat and rye also possess the “endosperm specific” form of the enzyme, which is present in these grains at seed maturity (Zeigler, 1999).
9Germination of sorghum grains at a temperature of between 25 and 30 °C is recommended for the development of optimum amylase and diastatic power in sorghum malt (Novellie, 1962; Okafor et al., 1980). At 30 °C, 3 to 7 days of sorghum grain germination produces well modified malts with a high diastatic power, and an increase level of hot-water extract, sugar content and free amino nitrogen (Morrall et al., 1986; Lasekan et al., 1995), but the optimal germination time and temperature vary with the sorghum variety (Novellie, 1962; Okafor et al., 1980; Demuyakor et al., 1992). Activity of α-amylase and β-amylase has been shown to develop to a greater extend in the yellow and red sorghum varieties than in white sorghum varieties when germinated at 30 °C (Agu et al., 1997b). However, germinating sorghum at the relatively high temperature of 35 °C or at lower temperatures of between 15 and 20 °C, slows down amylase formation and consequently reduces diastatic power (Morrall et al., 1986). Moreover, germinating sorghum grains heavily infected with moulds produce a malt with low amylase activity (Agu et al., 1999). Microbial infection of Nigerian sorghum grain has been shown to be caused by the presence of Aspergillus sp., Penicillium sp., Neurospora sp., Rhizopus sp., Fusarium sp., Curvularia sp. and Dreschlera sp. (Boboye et al., 1994). Formaldehyde (0.1%) can be added to the steep to retard fungal activity (Palmer et al., 1989). As a result of fungal grain infection, some African traditional opaque beers have been reported to contain different amounts of aflatoxins (Nikander et al., 1991). Recently, studies carried out by Matumba et al. (2011) indicate the presence of aflatoxin (6,6 to 54,6 µg·kg-1) in a sorghum malt from Malawi.
10Maltase, or α-glucosidase, which catalyses the hydrolysis of maltose into glucose, is present in ungerminated sorghum and does not increase significantly during malting. Sorghum maltase is a very heavy molecular weight enzyme, whose solubility characteristics differ from those of barley. Sorghum α-glucosidase is soluble in water, but is also active in its insoluble state and adheres strongly to insoluble solids (Novellie, 1982; Taylor et al., 1994). The development of α-glucosidase in sorghum is influenced by length of germination period and temperature (Agu et al., 1997a). In barley, α-glucosidase levels are generally lower than those of sorghum malt, especially at 30 °C and at day 5 of germination (Agu et al., 1997c). The sorghum malt with the highest maltase activity, however, produces the lowest glucose levels in wort, suggesting that maltase is not the dominant enzyme producing sugar during the mashing of sorghum malts (Agu et al., 1997a).
2.3. Kilning
11Kilning involves the drying of the green malt in a kiln or oven at a relatively high temperature until the rootlets become friable or brittle. Kilning has the objective of stopping embryo growth and enzyme activity, while minimizing enzyme denaturation, and the process develops flavor and color (melanoidin compounds). In the African traditional sorghum beer brewing process, the germinated sorghum grains are dried under the sun and are stored under protection during the night to avoid rehydration. Generally, this drying step takes 2-3 days depending on sunlight intensity. However, in the Western beer brewing process, the germinated barley grains are kilned via a two-stage process. The grain are first of all dried at 50-60 °C and are then cured at 80-110 °C (Moll, 1991).
12As part of the sorghum malting process, Novellie (1962) advocated the kilning of the malt up to 50 °C. However, whilst kilning periods at 80 °C may enhance the flavor of the malt, such a temperature may damage the enzymatic activity of the malt (Aisien et al., 1987) and reduce volatile compounds. Kilning malts in two stages, initially to 55 °C and subsequently to 65 °C, has been shown to produce good malts with a considerable reduction in moisture and a higher sugar content than kilning at a single temperature of 65 °C (Owuama et al., 1994). Thus, the two-stage kilning process allows for the greater survival of hydrolytic enzymes and for the malt to acquire its characteristic flavor.
3. Mashing
13The objectives of mashing are to form and extract into solution, fermentable sugars, amino acids, vitamins, etc., from malt. Malt normally provides most of the potential fermentable materials and sufficient enzymes to generate a well balanced fermentation medium. African sorghum beer is unique as a fermented beverage in requiring starch, not only as a source of sugar, but as a thickening and suspending agent. Gelatinized starch gives the beer its characteristic creamy body and keeps in suspension the particles of grain and malt that are essential constituents of beer.
14One of the problems involved in sorghum beer brewing is the efficient conversion of the starch extracts into fermentable sugars for yeast (Saccharomyces cerevisiae). Regarding the relative amounts of fermentable sugars in sorghum and barley worts, the major difference has been found to reside in the glucose content (Palmer, 1989; Dufour et al., 1992). While some studies have found barley malt worts to contain more maltose than glucose (Briggs et al., 1981; Dufour et al., 1992), others have reported that sorghum malt worts contain similar levels of glucose and maltose (Taylor, 1992). The difference observed in the proportions of glucose and maltose sugars in sorghum and barley malt worts has been attributed to the low levels of β-amylase in sorghum malt (Palmer, 1989). Other authors (Taylor et al., 1994) have attributed the high level of glucose found in sorghum malt wort to the catalytic activity of α-glucosidase, from the maltase family, in hydrolysing maltose into glucose in sorghum malt wort. However, Agu et al. (1997c) showed that there is no direct relationship between the α-glucosidase levels in sorghum or barley malt and the maltose to glucose ratios found in their worts. It is worth noting that, in that study, barley malt developed a higher level of α-glucosidase than did sorghum malt, but that it produced less glucose and several times more maltose in the wort than it was the case in sorghum wort. The main reason for the limitation of maltose production in sorghum malt wort is likely to be inadequate gelatinization of sorghum starch rather than inadequate levels of hydrolytic enzymes (Dufour et al., 1992; Agu et al., 1997c). Results obtained by Agu et al. (1997b) showed that different sorghum varieties malted and mashed under similar conditions presented wide variations in their sugar profiles due to seasonal and processing differences. For example, the authors showed that, when malt is produced at 30 °C, the white (ISCV400) and yellow (SS20) sorghum varieties produce high glucose and maltose levels, while SS9 (red variety) and SS16 (white variety) produce low glucose and higher maltose levels. However, these last two varieties produce higher levels of glucose and maltose when malt is produced at 20 °C. However, the variations caused by seed variety and malting temperature do not alter the greater influence exerted by starch gelatinization on the sugar profile of sorghum worts than on the sugar profile of barley worts (Agu et al., 1998).
15Generally, sorghum starch gelatinization temperatures (67-81 °C) are far higher (Akingbala et al., 1982; Beta et al., 2001) than the range quoted for barley starch of 51-60 °C (Lineback, 1984). Furthermore, these temperatures increase the thermal deactivation of the sorghum malt enzyme (Guerra et al., 2009). Consequently, the simultaneous gelatinization and hydrolysis of starch, which occurs during mashing of the barley malt, is problematical in the case of sorghum malt. Although short mashing (gelatinization) periods at 75 °C followed by a conversion (saccharification) period at 65 °C would improve the development of extracts from sorghum malt, unacceptable extract loss would still occur because of enzyme inactivation and inadequate gelatinization (Palmer et al., 1989). Indeed, relatively high levels of starch extract comparable to those of barley malts have been obtained by using a non-conventional mashing procedure. The procedure involves, decanting active enzyme wort after mashing sorghum malt at 45 °C for 30 min, and gelatinizing the starchy grist residue at 80 to 100 °C before mixing with the wort, to achieve a saccharifying temperature of 63-65 °C (Palmer, 1989; Igyor et al., 2001). The viscosities of sorghum malt worts have been shown to be similar to those of barley malts (Igyor et al., 2001), but the fermentable extracts of these sorghum worts have been shown to be still lower than those of barley malt (Palmer, 1989). These results suggest that small quantities of β-amylase in sorghum wort also affect saccharification.
16The drawbacks highlighted above in using sorghum malt in beer brewing led to the approach proposing the use of mixtures of malted barley (30-40%) with sorghum (60-70%) during mashing (Okafor et al., 1980; Goode et al., 2003), or the addition of exogenous enzymes to the unmalted sorghum (Dale et al., 1989; Bajomo et al., 1994). In this last case, the addition of external enzymes is associated with processing difficulties such as α-amino nitrogen (FAN) depletion (Dale et al., 1989). The advantage of including a percentage of malted sorghum as a source of endogenous proteases has also been reported. The addition of malted sorghum avoids the need to add these enzymes, thereby avoiding the poor foam retention associated with commercial proteolytic enzymes (Agu et al., 1998). However, these optimal solutions for reducing the levels of non-fermentable sugars in sorghum worts are inappropriate in an African traditional brewing context because the tropical climate is not conducive to barley cultivation, and commercial enzymes are very expensive. Nevertheless, a 20% (w/v) sweet potato flour substitution for sorghum malt has been shown to increase the level of β-amylase in sorghum wort (Etim et al., 1992). Pearl millet (Pennisetum glaucum) malt also appears to have some advantages compared to sorghum as it has a higher β-amylase activity and higher FAN levels (Pelembe et al., 2004). In Rwandan traditional sorghum beer brewing, the association of sorghum malt and Eleusine coracana (uburo) or the addition of banana juice (umutobe) during sorghum malt mashing increases the fermentable sugars in sorghum wort (Lyumugabe et al., 2010). As β-amylase is the enzyme responsible for the hydrolysis of starch into maltose, the high level of activity of this enzyme in Eleusine coracana malt compared with sorghum malt (Taylor, 2009) could explain the increase in fermentable sugars in this wort. However, brewing processes using a mixture of sorghum with local cultures have not been extensively investigated.
17Generally, after mashing, the mash is filtered before boiling. During the African traditional beer brewing process, filtration is achieved by simple decantation (Lyumugabe et al., 2010) or via a rudimentary press filter made of a nylon cloth stretched over a bowl and raked with a wooden stick (Maoura et al., 2009). In comparison with barley, sorghum malt mashes filter poorly (Aisien et al., 1987). This is clearly related to differences in the qualities of the endosperm cell walls of sorghum and barley. Unlike in barley, the endosperm cell walls in sorghum are not substantially broken down during malting (Glennie, 1984). The cell walls themselves are rich in water-unextractable glucoronoarabinoxylans (Verbruggen, 1996) and sorghum malt appears to be deficient in the wall degrading enzyme endo-β-glucanase (Aisien et al., 1983). This seems to pose a serious filtration problem for sorghum malt mashes, and the addition of exogenous hemicellulolytic enzymes is probably only a short-term solution.
18Boiling of wort is performed for several reasons, in particular to bring about the denaturation of malt enzymes and any enzymes supplements, and the sterilization of the wort. Although this stage exists in the brewing process of many African traditional sorghum beers (e.g. dolo, tchoukoutou, amgba) (Chevassus-Agnes et al., 1979; Dicko et al., 2006; Kayodé et al., 2007b), it is absent from the brewing process of traditional sorghum beers (e.g. ikigage, mtama, impeke) from East African countries (Tisekwa, 1989; Nzigamasabo et al., 2009; Lyumugabe et al., 2010). In the European beer brewing process, barley wort obtained from the mash is transferred to a “copper” (“kettle”) for boiling, along with dried hops or hop extracts. Hops are the flower cones of the female hop vine (Humulus lupulus), and they contain α and β acids, primarily humulones and lupulones. These give to beer its bitter flavor, after isomerization of α-acids into iso-α acids during boiling, and they also help inhibit certain beer spoilage bacteria and maintain foam stability. African traditional sorghum beers are generally unhopped. However, several studies have reported the possibility of using African plants (e.g. Vernonia amygdalina, Gongronema latifolium, Garcinia kola) instead of hops in African sorghum beers (Ogundiwin et al., 1991; Okoh et al., 1995; Ajebesone et al., 2004; Okoro et al., 2007; Adenuga et al., 2010) because the hop plant is a temperate crop and cannot be successfully grown in Africa tropical countries. Vernonia amygdalina, known as “bitter leaf”, can be used instead, because it resembles hops in its antimicrobial properties (Mboto et al., 2009; Oboh et al., 2009) and bitter flavor (Ajebesone et al., 2004; Adenuga et al., 2010). Furthermore the addition of extract of V. amygdalina leaves to sorghum wort increases the levels of amino acids, mainly isoleucine, leucine and histidine (Lasekan et al., 1999). However, further research needs to be directed in particular towards the contribution of this plant to the organoleptic properties of sorghum beer.
4. Fermentation
19Fermentation is the important step by which yeast converts the sugars in the wort into ethyl alcohol. In Western breweries, the fermentation process is started by selected yeast strains (S. cerevisae or S. carlsbergensis) and the fermentation time ranges between 8-15 days at 10-16 °C (Moll, 1991; Waites et al., 2001). In the case of African traditional sorghum beers, sorghum wort is inoculated with a traditional leaven, and fermentation time varies between 10 and 24 h in ambient temperature.
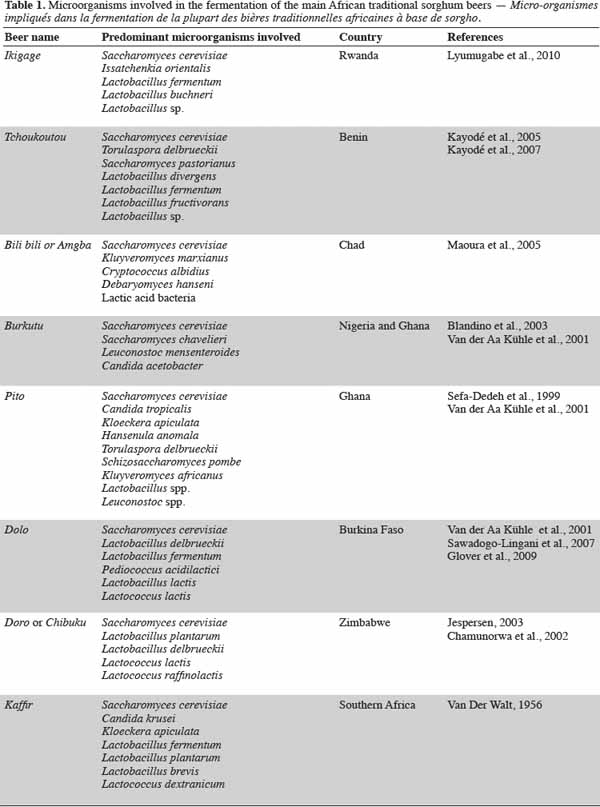
20African traditional leaven is a result of the spontaneous fermentation of sorghum malt wort (Kayodé et al., 2005; Lyumugabe et al., 2010). The manufacturing methods of this leaven are diverse in Africa and depend on built-in ingredients. Table 1 shows the types of microorganisms involved in spontaneous fermentation in traditional sorghum beer brewing. Very varied yeasts and bacteria flora have been found in African sorghum beers, although S. cerevisiae and Lactobacillus sp. usually predominate (Novellie, 1976; Maoura et al., 2005; Kayodé et al., 2007a; Lyumugabe et al., 2010). Unlike European beer made with barley, African sorghum beers are typical examples of lactic fermentation followed by alcoholic fermentation in which initially, lactic acid bacteria (LAB), and later yeasts, play the dominant role (Novellie, 1982; Holzapfel, 1997; Kayodé et al., 2005; Maoura et al., 2009). Due to their higher growth rate, bacteria typically dominate the early stages of fermentation. A symbiotic relationship could explain the simultaneous presence of yeasts and LAB. LAB create an acid environment favorable to the proliferation of yeasts. These yeasts produce vitamins and increase other factors, such as amino acids, to aid the growth of LAB.
21Unlike European beers, where the desired flavor is often critically affected by wild yeasts and other microorganisms, African beers may display a wide variation in tastes and aromas while still being acceptable to the consumer. As in the case of the Belgian beer, Lambic, African sorghum beers are the product of more or less spontaneous fermentation, in that pitching is not practiced. On the other hand, African sorghum beers differ from Lambic in that the Belgian beer is subjected to a very long post-fermentation period during which yeasts of the genus Brettanomyces are responsible for creating the typical bouquet of that beer (Van der Walt, 1956).
5. Types of African traditional sorghum beer brewing
22Generally, African traditional sorghum beers are brewed with pigmented sorghum varieties (red or brown). The white varieties are always mixed with red sorghum because consumers prefer to drink colored beers which they believe to be healthy (Kayodé et al., 2005). These African sorghum beers are not a clear, sparkling liquid, but opaque with suspended solids (5-7%). The beers have a rather low alcohol content (2-4.5% v/v), a pH of between 3.3 and 4 and a lactic acid rate of about 0.26%. Their color varies from a pale buff to a pinky brown according the ingredients used. Usually, African sorghum beers have a touch of fruitiness added to their fermentation odor. They are beer is consumed in an actively fermenting state and therefore their shelf life is a quite short (24 h-72 h) (Novellie et al., 1986; Tisekwa, 1989; Maoura et al., 2009; Lyumugabe et al., 2010). However, African traditional sorghum beers vary in their denomination and their production processes, according to their geographic localization.
5.1. Ikigage of Rwanda
23Ikigage or amarwa is a traditional alcoholic beverage manufactured in Rwanda with malted sorghum (Figure 2). The traditional process of ikigage manufacture has been described by Lyumugabe et al. (2010). After washing, red sorghum grains are immersed in water (kwinika) for 24 h. The grains are then drained in a bag with a stone top for 24 h so that the process of germination is completed and grain rootlets appear (kumera). The grains are spread out on a cloth in a wet place. Ash is spread over the cloth and leaves of the eucalyptus or banana tree are laid on top of the ash. The sorghum grains are then spread out on the leaves, to encourage germination. The grains are dried under the sun for at least two days at 29 °C. When the grains are semi-dry, the rootlets are removed (kuyavunga). The semi-dry malt grains are ground or crushed. Brewers heat water (20 l) to boiling and add approximately 2 kg of ground malt grains in order to gelatinize the starch. This solution is then mixed with ground malt (16 kg) in a large container. The mixing temperature is typically between 63 °C and 71 °C. Following the infusion process, cool water is added (40 l) to bring the temperature back to between 34 °C and 40 °C. In some cases, brewers leave a decanter of this mixture to rest for approximately 3 h in order to eliminate the draffs (imvuzo). After cooling, the traditional leaven (umusemburo) is inoculated in order to start the fermentation process. The fermentation container is covered with leaves of the banana tree, and then by a cloth and a lid. After 12 to 24 h of fermentation, ikigage is ready for consumption. The ethanol levels, soluble protein and the pH of ikigage are 2.2% (v/v), 9.2 g·l-1 and 3.9 respectively (Lyumugabe et al., 2010).
5.2. Merissa of Sudan
24Merissa is a traditional alcoholic beverage manufactured in Sudan using malted red sorghum or millet. Dirar (1978) describes a complex procedure (Figure 3) for making merissa beer. The red sorghum grains are malted, dried and reduced into flour. Ungerminated sorghum is milled into a fine flour and cooked in two equal lots: the first lot is lightly cooked to a greyish brown paste while, the second lot is well cooked to a brown paste. These two lots are then mixed and allowed to cool. The resulting product, “futtara”, is a gelatinized solid material. One part malt flour is mixed with a quantity of water necessary for good humidification and is incubated at room temperature for 36 h until lactic fermentation occurs. The acid paste obtained (called “ajeen”) is cooked in a container, and, mashing is then carried out until the substance takes on a chestnut color, with a high acidification and a caramel flavor. This product (called “soorij”) is then cooled. Malt (5%), water and an inoculum of a previous merissa product are then added to the soorij and the mixture is left to ferment for 4-5 h. The resulting product (called “deboba”) is a vigorously fermenting, thick, dark suspension that is too sour to drink. After cooking, futtara is mixed with about 5% malt flour and is successively added to the deboba. After 8-10 h of fermentation, the product, merissa, is filtered through a suitable fabric mesh to partially retain the solid particles, while the liquid undergoes full fermentation. The resulting drink has a pH of 4 and an alcohol level of around 5% (v/v).
5.3. Doro of Zimbabwe
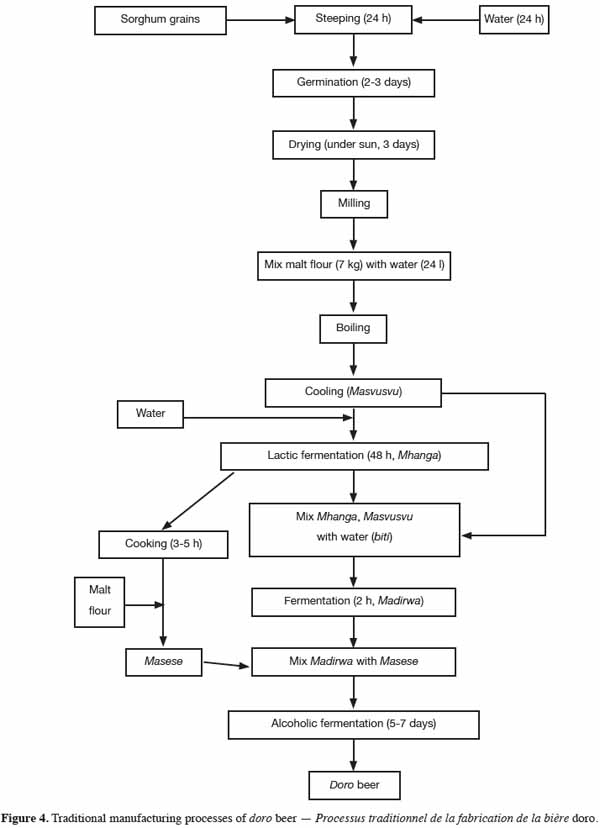
25The traditional sorghum beer of Zimbabwe is known as doro, chibuku, hwahwa, mhamba or uthwala in different regions of the country (Chamunorwa et al., 2002). The traditional doro brewing process (Figure 4) has been described by Benhura et al. (1989). The brewing process starts with the malting of red sorghum to produce a substance called masvusvu. Sorghum grains are soaked in water for 24 h at room temperature. They are then placed in a sack, washed and left to germinate for 2-3 days at room temperature. After this stage, the seedlings are sun-dried for 3 days and the semi-dry malt grains are ground or crushed. Approximately 24 l of water is mixed with 7 kg of sorghum malt flour. This mixture is heated while stirring until boiling. The masvusvu is cooled, diluted in clay pots and left to sour at ambient temperature for about 2 days. On the third day, the soured product (mhanga) is boiled for 3-5 h, reducing the original volume by a quarter in the process. The boiled mhanga is allowed to stand overnight after which time, more malt flour is added. Typically, the amount of malt added is about half the amount used at the beginning of the brewing process. On the sixth day, some masvusvu, two to three times more than the amount cooked on the first day, is prepared and allowed to cool. Meanwhile, a small portion of the mhanga is strained and kept separately. The strainings (masese), the rest of the unstrained mhanga and the fresh portion of cooled masvusvu are all mixed together with water to yield biti. The mixture is left to ferment for about 2 h and the resulting product, called madirwa, is then strained, mixed with the previously strained mhanga and left to ferment overnight. The fermentation process takes 5-7 days depending on ambient temperature. Ethanol is thought to be the main alcohol contained in the final doro beer (about 4% v/v).
5.4. Dolo of Burkina Faso
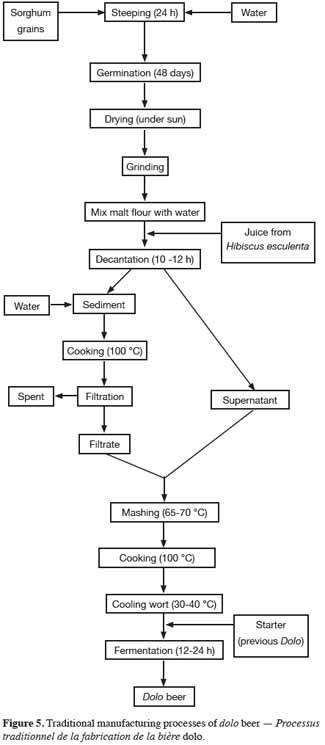
26Dolo is a popular traditional alcoholic beverage manufactured in Burkina Faso, and is most often made from red sorghum malt (Hilhorst, 1986). The traditional malting process for dolo (Figure 5) is similar to that described for the ikigage beer. The malt obtained is used by the traditional brewers (“dolotières”) to prepare the dolo beer. Sorghum malt flour is mixed with water (1:10, w/v) and the mixture is then decanted (for approximately 10-12 h) to separate off the enzymatic supernatant phase with precipitate containing starch. Water is added to the precipitate and the mixture is boiled to gelatinize starch, but the supernatant is not boiled (Dicko et al., 2006). After cooling, the precipitate is filtered to separate the soluble components (starch, sugars, proteins, etc.) and the residue (used as animal feed). The filtrate is mixed with previous supernatant and boiled at 65-70 ºC for 12-16 h in order to obtain the wort. This method seems to be a good traditional mashing process for producing sorghum wort with a high fermentable rate for sugars, because the process overcomes the problem of sorghum starch gelatinization and hydrolytic enzyme denaturation. After this stage, the wort is cooked and then cooled overnight to room temperature (30-40 ºC). The cooled wort is inoculated with a traditional leaven to start the fermentation process, leading to the dolo beer after 12-24 h (Griffon et al., 20011, cited by Maoura et al., 2009). The final dolo beer is opaque, with a red color, an alcohol content of 2-4% v/v and a pH of 4-5 (Dicko et al., 2006).
5.5. Pito and burukutu of Nigeria
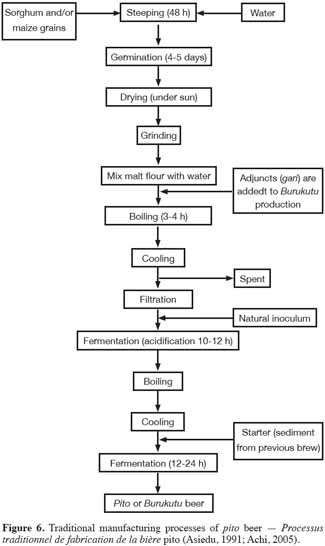
27Pito and burukutu are traditional Nigerian alcoholic beverages brewed with red or white sorghum malt and/or maize. The brewing process for pito (Figure 6) has been described by Ekundayo (1969). Briefly, sorghum grains are steeped in water (24-48 h) and then, drained. The grains are then allowed to germinate for four to five days and are sun-dried before grinding. The malt flour is mixed with water and the mixture is then boiled for 3-4 h to form a slurry. During the mashing stage of burukutu production, adjuncts are added in the form of gari (a farinaceous starchy powder produced from cassava, Manihot esculenta). However, adjuncts are not added during the production of pito (Faparusi et al., 1973; Ekundayo, 1969). After cooling, the paste is filtered and left in spontaneous lactic fermentation (acidification) at room temperature for approximately 12 h. More water is added and the mixture is then cooked for 3 h and cooled to around 20 to 29 °C. Cooled wort is subsequently left to ferment at room temperature for 12-24 h. The two resulting products are: a top clear supernatant, called “pito” and a thick brown suspension, called “burukutu”.
5.6. Amgba or bili bili of Cameroon
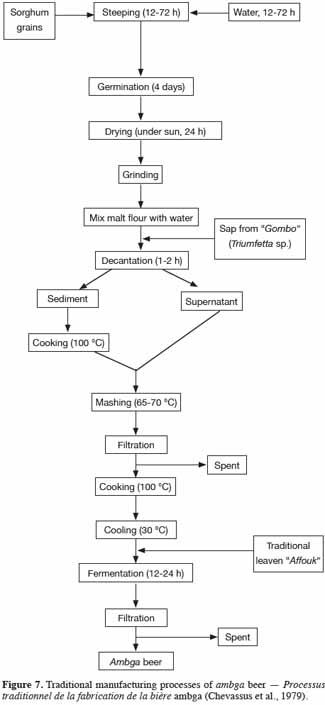
28Amgba, well known by the name bili bili, is a very popular traditional alcoholic beverage in Cameroon (among the Baya ethnic group). The drink is brewed primarily using sorghum malt (mouskouari or djigari variety), but millet malt (fonio variety) can also be associated with mouskouari. The traditional brewing process (Figure 7) for this beer has been described by Chevassus-Agnes et al. (1979). The sorghum grains are soaked in water for 12 to 72 h at room temperature in order to obtain a moisture level of 35 to 40%. The grains are then left in a heap in a container or spread out on a germination device (green plantain leaves, beaten soil, rocks) to form a layer (3 to 5 cm in thickness) and are kept covered until rootlets appear. If needed, initial moisture levels are maintained by spraying with water. The germination time is on average 4 days. After this step, the grains are dried under the sun for a maximum 1 day and ground into a fine flour. This malt flour is then mixed with water and sap (gombo) from trees. In particular gombo from Triumfetta sp. seems to improve the flocculation and filtration of the insoluble matter during decantation. This operation using the sap resembles that carried out during the clarification of barley beers in European breweries. However, the sorghum beer clarification process using gombo after fermentation has not been extensively investigated. After 1 to 2 h of decantation, the enzymatic supernatant phase is carefully collected, while the settled residue is cooked until boiling in order to gelatinize the starch. After cooking, the thick mash obtained is mixed with the previous supernatant at 65-70 °C. The mixture is then filtered by decantation or using a traditional device similar to the filter - tank used in industrial Western brewing. Very often, the traditional brewer leaves the filtrate in spontaneous lactic fermentation to acidify the wort. After boiling, the wort is cooled to approximately 30 °C and then inoculated with traditional leaven, “affouk” to start fermentation. After 12 to 24 h of fermentation, the resulting amgba can be consumed.
5.7. Tchoukoutou of Benin and Togo
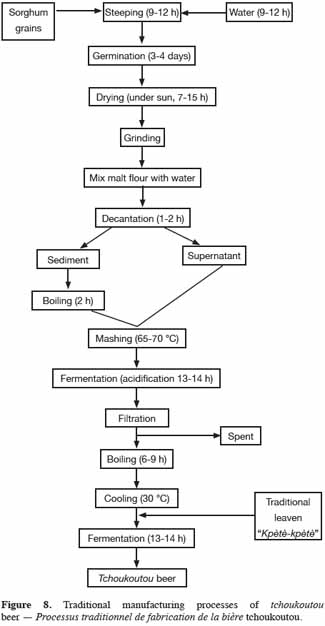
29Tchoukoutou, or chakpalo is a traditional alcoholic beverage produced in Benin and Togo principally using sorghum malt (red and brown varieties), but other starch sources, such as millet or maize can be used as adjuncts or as substitutes (Kayodé et al., 2005; Osseyi et al., 2011). Tchoukoutou and chakpalo are distinguishable by both their appearance and taste. Tchoukoutou is an opaque (turbid) and acidic beer while chakpalo is a clear and sweet fluid beer. The traditional brewing process (Figure 8) for tchoukoutou has been described by Kayodé et al. (2005, 2007b). Approximately 27 kg of grains are soaked in water for 9 to 12 h and then left to germinate during 72 to 85 h. After this step, the grains are dried under the sun (7-15 h) and ground into a fine flour. This malt flour is then mixed with water and left in decantation. After decantation (1-2 h), the enzymatic supernatant phase is collected and the residue containing starch is gelatinized by gradual heating until boiling for 2 h. The thick mash obtained is mixed with the previous supernatant phase and left in a state of acidifying (lactic) fermentation (13-14 h). After this stage, the mixture is filtered to obtain the wort. After cooking (6-9 h), cooled wort is inoculated with a traditional leaven (known as kpètè-kpètè in the Bariba, Denti and Yoruba languages) in order to start alcoholic fermentation. After 13 to 14 h of fermentation, tchoukoutou is ready for consumption. This beer is sour with a pH of 3.2: it contains a relatively high but variable level of solids and crude protein (Kayodé et al., 2007b) and has a 4% (v/v) alcohol content (Osseyi et al., 2011).
6. Socio-cultural aspects and nutritional status of sorghum beer
30African cereal beers (made from sorghum, millet, maize, etc.) have ancient origins. They may have originated in Egypt or Mesopotamia, where beers were being produced by at least 3,500 BC, and probably much earlier (Briggs et al., 1981). The first mentions of sorghum beer or millet beer come from the Arab travellers who, in the 6th and 7th centuries, praised the merits of beer manufactured in the Sahel region, in particular the merissa beer of Sudan (Huetz de Lemps, 2001). The manufacturing of sorghum beers is a tradition preserved by African women brewers and passed down to the next generation. In African tradition, sorghum beer symbolizes the woman, representing silence and a tacit acceptance of the “entente” between the peoples. In ancient times, royalties due to the local authorities were paid only in the form of sorghum or sorghum beer. Sorghum beer is called the “milk of the hoe” in Africa (amata y’isuka in the Rwandese language), affording the beer noble qualities (De Lame, 1995). Sorghum beer is an ancestral beverage widely used in various festivals and African ceremonies such as marriage, praying for rain, communication with ancestors, births, the handing-over of a dowry, circumcision, burial ceremonies, and the popular annual sorghum festival (Kayodé et al., 2007b; Lyumugabe et al., 2010). In Rwanda or Burundi, dowry handing-over ceremonies start initially with the consumption of traditional sorghum beer. The representatives of the two families greet each other around a clay jug (called an ikibindi) filled with sorghum beer because ikigage beer symbolizes the complementarity of the sexes (De Lame, 1996). Traditional sorghum beer is also consumed after community work or meetings of mutual associations, in order to provide energy (Van Liere, 19932, cited by Kayodé et al., 2007b).
31Traditional sorghum beer is mainly consumed by the poorest in society, and contributes significantly to the diet of millions of African people (Kayodé et al., 2007b). It is very rich in calories. It is also rich in the B-group vitamins including thiamine, folic acid, riboflavin, and nicotinic acid and is high in essential amino acids such as lysin (Table 2).
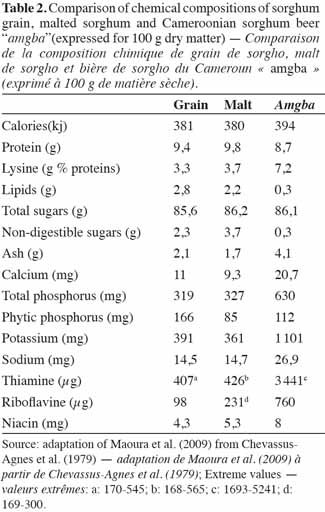
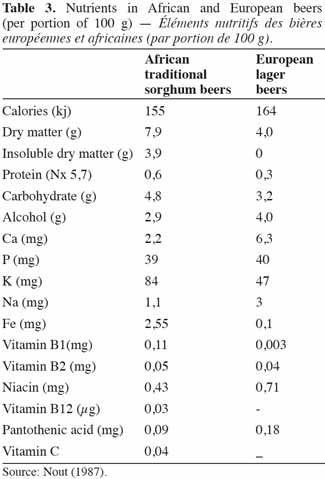
32According to Chevassus-Agnes et al. (1979), the significant dry matter losses during sorghum beer preparation seem to be balanced by the improvement in protein and amino-acid digestibility, mineral availability and vitamin content. Germination increases the digestive availability of essential amino acids, which is preserved in subsequent stages of production (Taylor, 1983). Fe solubility gradually increases during the beer making process (germination and fermentation) and is highly correlated with phytate and reactive phenolic compounds in the product. However, important losses of minerals occur during the beer making process, particularly during the mashing stage; thus, the quantity of Fe available to consumers is restricted (Kayodé et al., 2007a). Phenolic groups and tannins present in sorghum grain impair the grain’s nutritional value by sequestering exogenous and endogenous proteins in the form of indigestible complexes (Maoura et al., 2009). On the other hand the beer brewing process removes significant amounts of tannin (Dhanker et al., 1987; Osuntogun et al., 1989). Nevertheless, the nutritional value of sorghum beers is generally higher than that of European barley beers (Table 3) due to the presence of yeast, lactic acid bacteria and other suspended material. Due to their low alcohol content and the large quantity of suspended solids, many consumers consider these indigenous fermented sorghum beers to be more of a food than a beverage.
7. Shelf life of traditional sorghum beer
33Traditionally-made sorghum beers have a poor keeping quality. The limited shelf life (stability) of sorghum beers has been reported as the major problem confronting commercial brewers in Sudan (Dirar, 1978), in Tanzania (Tisekwa, 1989), in Nigeria (Sanni et al., 1999) and in Rwanda (Lyumugabe et al., 2010).
34Sorghum beer is consumed while it is still fermenting. The wort from which the beer is made is not heated – or otherwise treated prior to the addition of yeast, and the drink therefore always carries a residual microflora originating mainly from its ingredients. The resulting beer is thus microbiologically unstable i.e., infected at varying levels with yeasts and bacteria. Sanni et al. (1999) isolated the following bacteria from deteriorating sorghum beer (pito and burukutu): Aspergillus aceti, Aspergillus hansenii, Aspergillus pasteurianus, Lactobacillus plantarum, Lactobacillus fermentum, Lactobacillus brevis, Alcaligenes, Saccharomyces cerevisiae, Micrococcus spp., Candida spp., Bacillus licheniformis, Flavobacterium spp., Candida mycoderma, Hansenula anomala, Saccharomyces diastaticus (questionable), Bacillus spp. and Rhodotorula spp.
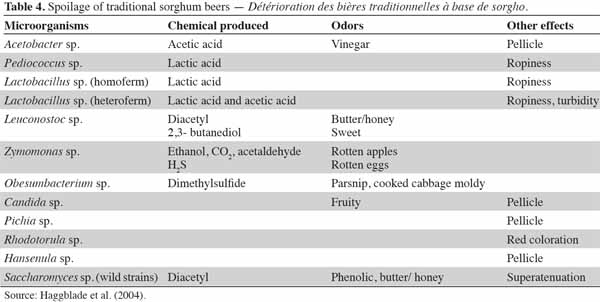
35Sorghum beers spoil rapidly because they are actively fermenting when solid, with organisms in addition to yeasts flourishing in the rich medium. During fermentation, yeasts initially increase in number. Then in the later stage of logarithmic growth the production of ethanol starts and proceeds during the stationary phase. It has been observed that during this time, very little or no increase in the number of contaminating organisms seems to occur. However, at the end of fermentation, the yeasts die, or else they undergo autolysis and their cell constituents are released into the beer. With little or no competition from yeasts for the readily available nutrients, contaminating microorganisms increase rapidly in number and their metabolites change the flavor of the beer. Because of the relatively high temperature of fermentation, these sequential events occur within a short time period. This period does not usually exceed more than 3 days in summer or 5 days in winter before this spoilage occurs. The metabolic activities of mesophilic lactic acid bacteria are primarily responsible for the spoilage. These bacteria, along with other undesirable bacteria (Acetobacter), produce acetic acid, volatile off-flavors, fruity odors, and pellicles which render the taste, odor and texture of the beer unacceptable to consumers (Van der Walt, 1956). Table 4 describes the types of spoilage during the conservation of sorghum beers.
36The flash-pasteurization method increases the shelf life of industrial European beers by destroying spoilage microbes. Unfortunately, this process is not applied in traditional sorghum beer-making. Early attempts at pasteurization failed because they led to an unacceptable increase in beer viscosity –through further gelatinization of starch and elimination of amylolytic enzymes – and also eliminated the beer’s characteristic effervescence by killing the active yeasts (Novellie et al., 1986). On the other hand, pasteurization of beer results in the killing of a large proportion of yeast cells, thereby making the B-group vitamins they contain available to human consumers of beer (Van Heerden, 1987). Post-fermentation pasteurization has enabled the shelf life of “tugela gold” sorghum beer to be extended to an extent comparable to that of European barley beers (Haggblade et al., 2004). Recently, Osseyi et al. (2011) were able to obtain stability in the tchoukoutou beer for at least 6 months after 3 h of bottle fermentation stopped by pasteurization in a water bath at 75-80 ºC for 15 min.
8. Use of starter cultures to improve sorghum beer
37A starter culture may be defined as a preparation or material containing large numbers of variable microorganisms selected for their properties and their harmlessness, which may be added to accelerate a fermentation process (Holzapfel, 2002).
38In Africa, starters are used in the form of traditional leaven, resulting from spontaneous fermentation of the wort. As a result, both the desirable and non-desirable strains contained in the leaven are reintroduced with fermentation, inducing the fermentation of the sorghum wort. For example, the fermentation of the ikigage beer is initiated by a traditional leaven (umusemburo), which contains Saccharomyces cerevisae, Candida inconspicua, Issatchenkia orientalis, Candida magnolia and Candida humilis, Lactobacillus fermentum, Lactobacillus buchneri, Aspergillus sp., Staphyloccocus aureus and Escherichia coli (Lyumugabe et al., 2010). Selected use of a dominant species (e.g. S. cerevisiae, Lactobacillus sp. or I. orientalis) could stabilize the organoleptic quality of this beer, increase its ethanol content and improve its hygienic quality.
39The use of starter cultures has been applied successfully to many products, and studies have been undertaken in the development of starter cultures for many other fermented foods from Africa (kivunde, ogi and togwa) (Teniola et al., 2001; Kirmaryo et al., 2002; Mugula et al., 2003). Research on improvement in the quality of traditional sorghum beer has focused on the adaptation of the starter culture. Sefa-Dedeh et al. (1999) used a pure culture of S. cerevisiae and a mixture culture comprised of S. cerevisiae with Kloeckera apiculata or Candida tropicalis, to produce in the laboratory a pito beer containing a high ethanol content compared to traditional pito. By contrast, they also found that a mixture of three cultures (S. cerevisiae, K. apiculata and C. tropicalis) as the starter produced a pito beer with a low ethanol content compared to the traditional pito beer. Orji et al. (2003) found that S. cerevisiae in combination with Lactobacillus plantarum, as a starter culture, also led to the satisfactory production of a pito beer with a taste and aroma similar to local pito beer, but with a low ethanol content. N’Guessan et al. (2010) successfully used S. cerevisiae in combination with C. tropicalis as starter cultures for the alcoholic fermentation of the tchapalo beer, but further investigations are required before a definitive conclusion. Glover et al. (2009) showed that dolo beer produced from starter combinations of one strain of L. fermentum and both S. cerevisiae strains had a taste and aroma that did not differ significantly from the local dolo beer. This kind of research needs to be widened to other types of sorghum beer because the microorganisms involved in spontaneous fermentation are very diverse.
40When the starter is adapted to the substrate, its use improves control of the fermentation process and the predictability of its products (Holzapfel, 1997). In addition, it facilitates control over the initial phase of fermentation (Holzapfel, 2002). In the same way, the hygienic quality and acceptability of African traditional foods could be improved with the use of a suitable starter (Gran et al., 2003). The use of starter cultures also reduces the organoleptic variations and the microbiological instability of African fermented foods (Kirmaryo et al., 2002).
41However, the use of starter cultures does not provide an absolute guarantee against failure of fermentation process, nor does it eliminate the health hazards associated with pathogens, toxinogens, or toxic components or residues (Holzapfel, 2002). The metabolic activities of desirable fermentation microorganisms must be supported by observing the basic principles of Good Manufacturing Practice (GMP).
9. Conclusion
42Traditional sorghum beers have a socio-cultural and nutritional value in Africa. Compared to the brewing of European beer with barley, the brewing of traditional sorghum beer is characterized by the complexity of the malting process, the speed and short time of alcoholic fermentation, and the existence of lactic fermentation.
43In Africa, the association of sorghum with other cereals (e.g. Eleusine coracana, Pennisetum glaucum, sweet potato) available in Africa could solve the problem of the lack of β-amylase in sorghum malt and provide a means of avoiding the use of the commercial enzymes and barley malt.
44The pasteurization of sorghum beer appears most promising for resolving the brewer’s perennial principal problem of a shorter shelf life. However, in order for this to happen, research will be needed to ensure the necessary refinements in pasteurization, and factory brewers would need to adopt the pasteurization process as their production standard (Haggblade et al., 2004).
45The presence of unspecified microorganisms from traditional leaven complicates the control of the fermentation process and yields products of variable quality. The use of starter cultures seems to be a good method to reduce organoleptic variations and to reduce the risk of contamination with pathogenic organisms. This approach would also increase the chances of preserving of traditional sorghum beer, giving it a longer shelf life. The existing variations in the production processes of African traditional sorghum beer could be incorporated into the development of a large variety of sorghum beers in Africa.
Bibliographie
Achi O.K., 2005. The potential for upgrading traditional fermented foods through biotechnology. Afr. J. Biotechnol., 5, 375-380.
Adenuga W., Olaleye O.N. & Adepoju P.A., 2010. Utilization of bitter vegetable leaves (Gongronema latifolium, Vernonia amygdalina) and Garcinia kola extracts as substitutes for hops in sorghum beer production. Afr. J. Biotechnol., 9, 8819-8823.
Agu R.C. & Palmer G.H., 1997a. The effect of temperature on the modification of sorghum and barley during malting. Process Biochem., 32, 501-507.
Agu R.C. & Palmer G.H., 1997b. Effect of mashing procedures on some sorghum varieties germinated at different temperatures. Process Biochem., 32, 147-158.
Agu R.C. & Palmer G.H., 1997c. Alpha-glucosidase activity of sorghum and barley malts. J. Inst. Brew., 103, 25-29.
Agu R.C. & Palmer G.H., 1998. A reassessment of sorghum for lager-beer brewing. Bioresour. Technol., 66, 253-261.
Agu R.C. & Palmer G.H., 1999. Development of micro-organisms during the malting of sorghum. J. Inst. Brew., 105, 101-106.
Aisien A.O., 1982. Enzyme modification of sorghum endosperm during seedling growth and malting. J. Sci. Food Agric., 33, 754-759.
Aisien A.O. & Palmer G.H., 1983. The sorghum embryo in relation to the hydrolysis of the endosperm during germination and seedling growth. J. Sci. Food Agric., 34, 113-121.
Aisien A.O. & Muts G.C.J., 1987. Micro-scale malting and brewing studies of some sorghum varieties. J. Inst. Brew., 93, 328-331.
Ajebesone P.E. & Aina J.O., 2004. Potential African substitutes for hops in tropical beer brewing. J. Food Technol. Afr., 9, 13-16.
Akingbala J.O., Rooney L.W., Palacios L.G. & Sweat V.E., 1982. Thermal properties of sorghum starches. In: Martin J.V., ed. International symposium on sorghum grain quality. Patancheru, India: ICRISAT, 251-261.
Asiedu J.J., 1991. La transformation des produits agricoles en zone tropicale : approche technologique. Paris : Karthala.
Bajomo M.F. & Young T.W., 1994. Fermentation of wort from 100% of raw sorghum and enzyme. J. Inst. Brew., 100, 79-84.
Benhura M.A. & Chingombe A., 1989. Traditional brewing methods of Zimbabwe. Zimbabwe Sci. News, 23, 69-70.
Beta T., Rooney L.W. & Waniska R.D., 1995. Malting characteristics of sorghum cultivars. Cereal Chem., 72, 533-538.
Beta T. & Corke H., 2001. Genetic and environmental variation in sorghum starch properties. J. Cereal Sci., 34, 261-268.
Blandino A. et al., 2003. Cereal-based fermented foods and beverages. Food Res. Int., 36, 527-547.
Boboye B.E. & Adetuyi F.C., 1994. Fungal population associated with raw materials and intermediate products of lager beer produced from Nigerian sorghum grains. J. Food Sci. Technol., 31, 148-150.
Briggs D.E., Hough J.S., Stevens R. & Young T.W., 1981. Malting and brewing science. Vol. 1. London: Chapman & Hall.
Chamunorwa A.T., Feresu S.B. & Mutukumira A.N., 2002. Identification of lactic acid bacteria isolated from opaque beer (Chibuku) for potential use as a starter culture. J. Food Technol. Afr., 7, 93-97.
Chevassus-Agnes S., Favier J.C. & Joseph A., 1979. Traditional technology and nutritive value of Cameroon sorghum beers. Cah. Onarest, 2, 83-112.
Dale C.J., Young T.W. & Makinde A., 1989. Extruded sorghum as a brewing raw material. J. Inst. Brew., 95, 157-164.
De Lame D., 1995. La bière en bouteille et le lait de la houe, parabole d'une colline rwandaise. In : Devisch R., De Boeck F. & Jonckers D., eds. Alimentations, traditions et développements en Afrique intertropicale. Paris : L'Harmattan, 116-153.
De Lame D., 1996. Une colline entre mille ou le calme avant la tempête : transformations et blocages du Rwanda rural. Annales sciences humaines, vol. 154. Tervuren, Belgique : Musée Royal de l’Afrique Centrale.
Demuyakor B. & Ohta Y., 1992. Malt characteristics of sorghum vulgare varieties from Ghana. J. Sci. Food Agric., 59, 457-462.
Dewar J., Taylor J.R.N. & Berjak P., 1997. Determination of improved steeping conditions for sorghum malting. J. Cereal Sci., 26, 129-131.
Dhanker N. & Chauhan B.M., 1987. Effect of temperature and fermentation time on phytic acid and polyphenol content rabaadi - a fermented pearl millet food. J. Food Sci., 52, 828-829.
Dicko M.H. et al., 2006. Sorghum grain as human food in Africa: relevance of content of starch and amylase activities. Afr. J. Biotechnol., 5(5), 384-395.
Dirar H.A., 1978. A microbiological study of Sudanese merissa brewing. J. Food Sci., 43, 163-168.
Dufour J.P., Melotte L. & Srebrnik S., 1992. Sorghum malts for the production of a lager beer. J. Am. Soc. Brew. Chem., 50, 110-119.
Ekundayo J.A., 1969. The production of pito, a Nigerian fermented beverage. J. Food Technol., 4, 217-225.
Etim M.U. & Etokakpan O.U., 1992. Sorghum brewing using sweet potato enzymic flour to increase saccharification. World J. Microbiol. Biotechnol., 8, 509-511.
Ezeogu L.I. & Okolo B.N., 1995. Effects of air rest periods on malting sorghum response to final warm water steep. J. Inst. Brew., 101, 39-45.
Faparusi S.I., Olofinboba M.O. & Ekwundayo J.A., 1973. The microbiology of burukutu beer. Z. Allg. Mikrobiol., 13, 563-568.
French B.J. & McRuer G.R., 1990. Malt quality as affected by various steep aeration regimes. Techn. Q. Master Brew. Assoc. Am., 27, 10-14.
Glennie C.W., 1984. Endosperm cell wall modification in sorghum grain during germination. Cereal Chem., 61, 285-289.
Glennie C.W. & Wight A.W., 1986. Dextrins in sorghum beer. J. Inst. Brew., 92, 384-386.
Glover R.L.K. et al., 2009. Utilization of Lactobacillus fermentum and Saccharomyces cerevisiae as starter cultures in the production of “dolo”. J. Appl. Biosci., 22, 1312-1319.
Goode D.L., Elke K. & Arendt E.K., 2003. Pilot scale production of a lager beer from a grist containing 50% unmalted sorghum. J. Inst. Brew., 109, 208-217.
Gran H.M., Gadaga H.T. & Narbhus J.A., 2003. Utilization of various starter cultures in the product of Amasi, a Zimbabwean naturally fermented raw milk product. Int. J. Food Microbiol., 88, 19-28.
Guerra N.P. et al., 2009. Use of amylolytic enzymes in brewing. In: Preedy V.R., ed. Beer in health disease prevention. Burlington, MA, USA: Elsevier Academic Press, 114-126.
Haggblade S. & Holzapfel H., 2004. Industrialization of Africa’s indigenous beer brewing. In: Streinrous K.H. Industrialization of indigenous fermented foods. 2nd ed. New York, USA: CRC Press.
Hilhorst R., 1986. Bierbereiding in Burkina Faso. PT/Procestechniek, 41, 93-95.
Holzapfel W., 1997. Use of starter cultures in fermentation on a household scale. Food Control, 8, 241-258.
Holzapfel W., 2002. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol., 75, 197-212.
Huetz de Lemps A., 2001. Boissons et civilisations en Afrique. Pessac, France : Presses universitaires de Bordeaux.
Igyor M.A., Ogbonna A.C. & Palmer G.H., 2001. Effect of malting temperature and mashing methods on sorghum wort composition and beer flavour. Process Biochem., 36, 1039-1044.
Jespersen L., 2003. Occurrence and taxonomic characteristics of strains of Saccharomyces cerevisiae predominant in African indigenous fermented foods and beverages. FEMS Yeast Res., 3, 191-200.
Kayodé A.P.P. et al., 2005. Quality of farmer’s varieties of sorghum and derived foods as perceived by consumers in Benin. Ecol. Food Nutr., 44, 271-294.
Kayodé A.P.P., Hounhouigana J.D. & Nout M.J.R., 2007a. Impact of brewing process operations on phyta, phenolic compounds and in vitro solubility of iron and zinc in opaque sorghum beer. LWT, 40, 834-841.
Kayodé A.P.P., Hounhouigana J.D., Nout M.J.R. & Niehof A., 2007b. Household production of sorghum beer in Benin: technological and socio-economic aspects. Int. J. Consum. Stud., 31, 258-264.
Khady B.A. et al., 2010. Étude comparative des composés phénolitiques, du pouvoir antioxydant de différentes variétés de sorgho sénégalais et des enzymes amylolytiques de leur malt. Biotechnol. Agron. Soc. Environ., 14, 131-139.
Kirmaryo V.M., Massawe G.A., Olasupo N.A. & Holzapfel W.H., 2002. The use of a starter culture in the fermentation of cassava for the production of “Kivunde’’, a traditional Tanzanian food product. Int. J. Food Microbiol., 56, 179-190.
Kumar L.S., Daudu M.A., Shetty H.S. & Malleshi N.G., 1992. Seed mycoflora and malting characteristics of some sorghum cultivars. J. Cereal Sci., 15, 203-209.
Lasekan O.O., Idowu M.A. & Lasekan W., 1995. Effect of germination and degree of grind (coarse/fine) on the extract and sugar content of sorghum malts. Food Chem., 58, 125-128.
Lasekan O.O., Lasekan W.O. & Babalola J.O., 1999. Effect of Vernonia amygdalina (bitter leaf) extract on brewing qualities and amino acid profiles of stout drinks from sorghum and barley malts. Food Chem., 64, 507-510.
Lineback D.R., 1984. The starch granule: organization and properties. Bakers Digest, 58, 16-21.
Lyumugabe L., Kamaliza G., Bajyana E. & Thonart Ph., 2010. Microbiological and physico-chemical characteristics of Rwandese traditional beer “Ikigage”. Afr. J. Biotechnol., 9, 4241-4246.
Maoura N. et al., 2005. Identification and typing of the yeast strains isolated from bili bili, a traditional sorghum beer of Chad. Afr. J. Biotechnol., 4, 646-656.
Maoura N. & Pourquie J., 2009. Sorghum beer: production, nutritional value and impact upon human health. In: Preedy V.R., ed. Beer in health disease prevention. Burlington, MA, USA: Elsevier Academic Press, 53-60.
Matumba L., Monjerezi M., Khonga E.B. & Lakudzala D.D., 2011. Aflatoxins in sorghum, sorghum malt and traditional opaque beer in southern Malawi. Food Control, 22, 266-268.
Mboto C.I. et al., 2009. Phytochemical properties and antimicrobial activities of combined effect of extracts of the leaves of Garcinia kola, Vernonia amygdalina and honey on some medically important microorganisms. Afr. J. Microbiol. Res., 3, 557-559.
Moll M., 1991. Bières. Paris : Lavoisier Tec & Doc.
Morrall P., Boyd H.K., Taylor J.R.N. & Van Der Walt W.H., 1986. Effect of germination time, temperature and moisture on malting of sorghum (Sorghum bicolor). J. Inst. Brew., 92, 439-445.
Mugula J.K., Nnkoa S.A.M., Narvhusb J.A. & Sorhaug T., 2003. Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. Int. J. Food Microbiol., 80, 187-199.
N’guessan F.K., N’diri D.Y., Camara F. & Djè M.K., 2010. Saccharomyces cerevisiae and Candida tropicalis as starter cultures for the alcoholic fermentation of tchapalo, a traditional sorghum beer. World J. Microbiol. Biotechnol., 26, 693-699.
Nikander P. et al., 1991. Ingredients and contaminants of traditional alcoholic beverages in Tanzania. Trans. R. Soc. Trop. Med. Hyg., 85, 133-135.
Nout M.J.R., 1987. Composition of foods: African traditional beers. Food Lab. Newsl., 8, 18-20.
Novellie L., 1962. Kaffircorn malting and brewing studies XI. Effect of malting conditions on the diastatic power of kaffircorn malts. J. Sci. Food Agric., 13, 115-120.
Novellie L., 1976. Beverages from sorghum and millets. In: Dendy D.A.V., ed. Proceedings of the International symposium on sorghum and millets for human food, 11-12th May 1976, 9th Congress of the International Association for Cereal Chemistry, Vienna. London: Tropical Products Institute, 73-77.
Novellie L., 1982. Fermented porridge. In: Proceedings of the International symposium on sorghum grain quality, 28-31 October 1981, Patancheru, India. Patancheru, India: ICRISAT, 121-128.
Novellie L. & De Schaepdrijver P., 1986. Modern developments in traditional African beers. Prog. Ind. Microbiol., 23, 73-157.
Nzigamasabo A. & Nimpagaritse A., 2009. Traditional fermented foods and beverages in Burundi. Food Res. Int., 42, 588-594.
Oboh F.O.J. & Masodje H.I., 2009. Nutritional and antimicrobial properties of Vernonia amygdalina leaves. Int. J. Biomed. Health Sci., 5, 51-56.
Ogundiwin J.O. & Ilori M.O., 1991. Development of stout from sorghum malt. Lebensm. Wiss. Technol., 24, 182-185.
Okafor N. & Aniche G.N., 1980. Brewing a lager beer from Nigerian sorghum. Brew. Distilling Int., 10, 32-35.
Okoh I.A., Babalola G.O. & Ilori M.O., 1995. Effect of methanol extract of Vernonia amygdalina on malting and brewing properties of sorghum. Q. Master Brew. Assoc. Am., 32, 11-14.
Okolo B.N. & Ezeogu L.I., 1996. Duration of final warm water steep as a crucial factor in protein modification in sorghum malts. J. Inst. Brew., 102, 167-177.
Okoro C.C. & Aina J.O., 2007. Effect of storage on the brewing properties of tropical hop substitutes. Afr. J. Biotechnol., 6(12), 1479-1483.
Okungbowa J., Obeta J.A.N. & Ezeogu L.I., 2002. Sorghum b-amylase production: relationship with grain cultivar, steep regime, steep liquor composition and kilning temperature. J. Inst. Brew., 108, 362-370.
Orji M.U., Mbata T.I., Anich G.N. & Ahonkhai I., 2003. The use of starter cultures to produce “pito”, a Nigerian fermented alcoholic beverage. World J. Microbiol. Biotechnol, 19, 733-736.
Osseyi E.G. et al., 2011. Stabilization of the traditional sorghum beer, “tchoukoutou” using rustic wine-making method. Adv. J. Food Sci. Technol., 3, 254-258.
Osuntogun B.A., Adewusi S.R.A., Ogundiwin J.O. & Nwasike C.C., 1989. Effect of cultivar steeping and malting on tannin, total polyphenol and cyanide content on Nigeria sorghum. Cereal Chem., 66, 87-89.
Owuama C.I. & Asheno I., 1994. Studies on malting conditions for sorghum. Food Chem., 49, 257-260.
Palmer G.H., 1989. Cereals in malting and brewing. In: Palmer G.H., ed. Cereal science and technology. Aberdeen: Aberdeen University Press, 61-242.
Palmer G.H., 1991. Enzymic degradation of the endosperm cell walls of germinated sorghum. World J. Microbiol. Biotechnol., 7, 17-21.
Palmer G.H., Etokakpan O.U. & Igyor M.A., 1989. Review: sorghum as brewing material. MIRCEN J. Appl. Microbiol. Biotechnol., 5, 265-275.
Pathirana R.A., Shivayogasundaram K. & Jayatissa P.M., 1983. Optimization of conditions for malting of sorghum. J. Food Sci. Technol., 20, 108-112.
Pelembe L.A.M., Dewar J. & Taylor J.R.N., 2004. Effect of germination moisture and time on pearl millet malt quality - with respect to its opaque and lager beer brewing potential. J. Inst. Brew., 110, 320-325.
Rooney L.W. & Serna-Saldivar S.O., 1991. Sorghum. In: Lorenz K.J. & Kulp K., eds. Handbook of cereal science and technology. New York, USA: Marcel Dekker Inc., 233-270.
Sanni A.I., Onilude A.A., Fadahusi I.F. & Afolabi R.O., 1999. Microbial deterioration of traditional alcoholic beverages in Nigeria. Food Res. Int., 32, 163-167.
Sawadogo-Lingani H. et al., 2007. The biodiversity of predominant lactic acid bacteria in dolo and pito wort, for production of sorghum beer. J. Appl. Microbiol., 103, 765-777.
Sefa-Dedeh S., Sanni AI., Tetteh G. & Sakyi-Dawson E., 1999. Yeasts in the traditional brewing of pito in Ghana. World J. Microbiol. Biotechnol., 15, 593-597.
Taylor J.R.N., 1983. Effect of malting on the protein and free amino nitrogen composition of sorghum. J. Sci. Food Agric., 34, 885-892.
Taylor J.R.N., 1992. Mashing with malted grain sorghum. J. Am. Soc. Brew. Chem., 50, 13-18.
Taylor J.R.N., 2009. Food security in Africa: the role of sorghum and millet. Brew. Distiller, 5, 22-25.
Taylor J.R.N. & Robbins D.J., 1993. Factors influencing beta-amylase activity in sorghum malt. J. Inst. Brew., 99, 413-416.
Taylor J.R.N. & Dewar J., 1994. Role of alpha-glucosidase in the fermentable sugar composition of sorghum malt mashes. J. Inst. Brew., 100, 417-419.
Teniola O.D. & Odunfa S.A., 2001. The effects of processing methods on the level of lysine and methionine and the general acceptability of ogi processed using starter cultures. Int. J. Food Microbiol., 63, 1-9.
Tisekwa B., 1989. Improvement of traditional manufacturing of sorghum beer (mtama) in Tanzania. PhD thesis: Ghent University (Belgium).
Uriyo M. & Eigel W.E., 1999. Duration of kilning treatment on α-amylase, β-amylase and endo-(1, 3) (1, 4)-β-D-glucanase activity of malted sorghum (Sorghum bicolor). Process Biochem., 35, 433-436.
Van der Aa Kühle A. et al., 2001. Identification and characterization of Saccharomyces cerevisiae strains isolated from West African sorghum beer. Yeast, 18, 1069-1079.
Van Der Walt J.P., 1956. Kaffircorn malting and brewing studies: studies on the microbiology of kaffir beer. J. Sci. Food Agric., 7, 105-113.
Van Heerden I.V. & Glennie G.W., 1987. Availability of B-vitamin in sorghum beer. Nutr. Rep. Int., 35, 147-155.
Verbruggen M.A., 1996. Glucuronoarabinoxylans from sorghum grain. PhD thesis: Wageningen Agricultural University (The Netherlands).
Waites M.J., Morgan N.L., Rockey J.S. & Higton G., 2001. Industrial microbiology: an introduction. London: Blackwell Science.
Zeigler P., 1999. Cereal beta-amylases. J. Cereal Sci., 29, 195-204.
Voetnoten
Om dit artikel te citeren:
Over : François Lyumugabe
Univ. Liege - Gembloux Agro-Bio Tech. Walloon Centre of Industrial Biology (CWBI). Unit of Bio-Industry. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: flyumugabe@gmail.com – National University of Rwanda. Faculty of Sciences. Unit of Biotechnologies. BP 117. RWA-Butare (Rwanda).
Over : Jacques Gros
Université Catholique de Louvain. Earth and Life Institute (ELIM). Unit of Brewery and Food Industries. Croix du Sud, 2 Bte 7. B-1348 Louvain-la-Neuve (Belgium).
Over : John Nzungize
Institut des Sciences Agronomiques du Rwanda (ISAR). Rue Député Kamuzinzi Kiyovu, 47. BP 5016. RWA-Kigali (Rwanda).
Over : Emmanuel Bajyana
National University of Rwanda. Faculty of Sciences. Unit of Biotechnologies. BP 117. RWA-Butare (Rwanda).
Over : Philippe Thonart
Univ. Liege - Gembloux Agro-Bio Tech. Walloon Centre of Industrial Biology (CWBI). Unit of Bio-Industry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






