Growth and nutrition of Tetraclinis articulata (Vahl) Mast. cultivated in different rhizosphere soils collected from Tetraclinis stand
Forest Research Centre. Sylviculture Department. Root Symbiosis Laboratory. BP. 763. MA-Agdal-Rabat (Morocco). E-mail: abbayouns@gmail.com – Mohamed V University. Faculty of Science. Plant Biology Laboratory. MA-Rabat (Morocco).
Forest Research Centre. Sylviculture Department. Root Symbiosis Laboratory. BP. 763. MA-Agdal-Rabat (Morocco).
University of Montpellier II. Supagro. Tropical and Mediterranean Symbioses Laboratory (LSTM). UMR 113 CIRAD, IRD. TA10J. F-34398 Montpellier Cedex 5 (France).
Mohamed V University. Faculty of Science. Plant Biology Laboratory. MA-Rabat (Morocco).
Forest Research Centre. Sylviculture Department. Root Symbiosis Laboratory. BP. 763. MA-Agdal-Rabat (Morocco).
University of Montpellier II. Supagro. Tropical and Mediterranean Symbioses Laboratory (LSTM). UMR 113 CIRAD, IRD. TA10J. F-34398 Montpellier Cedex 5 (France).
Received on March 7, 2011; accepted on November 8, 2012
Résumé
Croissance et nutrition du thuya de Berbérie (Tetraclinis articulata [Vahl] Mast.) mis en culture dans différents sols rhizosphériques de peuplement de thuya. Cinq espèces végétales (Withania frutescens Pauquy, Lavandula multifida L., Pistacia atlantica Desf., Olea europaea L. subsp. oleaster (Hoffmanns. & Link) Negodi et Tetraclinis articulata [Vahl] Mast.), rencontrées au niveau de l’écosystème tétraclinaie au Maroc ont été sélectionnées afin d'évaluer leur potentiel mycorhizien et de comparer l’impact de leur sol rhizosphérique sur la croissance et la nutrition des jeunes plants de thuya (Tetraclinis articulata). Les racines des plantes sélectionnées ont été abondamment colonisées par les champignons mycorhiziens arbusculaires (AM). Les racines de L. multifida ont montré le pourcentage le plus élevé de mycorhization (100 %). Nous avons ensuite isolé les spores de champignons AM à partir du sol de ces cinq espèces de plantes et nous avons constaté que le nombre total de spores isolées de la rhizosphère de L. multifida et T. articulata a été sensiblement différent de celui des autres plantes. Le potentiel mycorhizien des sols indigènes a été évalué en utilisant l’approche du nombre le plus probable (MPN). Nous avons constaté que ce nombre était significativement plus élevé dans les sols récoltés près des cinq espèces de plantes, que celui dans le sol sans couvert végétal, soit une moyenne du MPN par 100 g de sol sec de 11,8 (compris entre 5,6 et 25,0) dans le sol nu et 228,5 (compris entre 108,0 et 476,0) dans la rhizosphère de L. multifida. Ce résultat indique que le sol entourant les plantes cibles peut être utilisé comme inoculum mycorhizien. En pépinière forestière, les plants de thuya mis en culture dans le sol sous la lavande ont montré les meilleurs pourcentages de mycorhization et ont pu améliorer leur croissance. Toutefois, la production en arbuscules du thuya se développant dans la rhizosphère de la lavande n'a pas significativement affecté la croissance ou la nutrition des plants par rapport à ceux se développant dans un sol sous le thuya, sauf pour la concentration du potassium. Nos recherches ont montré clairement que le sol sous L. multifida peut être utilisé comme inoculum mycorhizien efficace dans la production de T. articulata en pépinières forestières, et peut participer ainsi au maintien de l’écosystème tétraclinaie.
Abstract
Five representative plant species (Withania frutescens Pauquy, Lavandula multifida L., Pistacia atlantica Desf., Olea europaea L. subsp. oleaster (Hoffmanns. & Link) Negodi and Tetraclinis articulata [Vahl] Mast.) were selected from Moroccan Tetraclinis woodland in order to evaluate their mycorrhizal potential and to compare the impact of their rhizosphere soil on growth and nutrition of Tetraclinis articulata seedlings. We observed that roots of selected plants were highly colonized by Arbuscular Mycorrhizal (AM) fungi. Lavandula multifida roots showed the highest colonization percentage (100%). We recovered AM fungal spores from the rhizosphere soils of the five plant species and we found that the spores number from L. multifida and T. articulata rhizosphere was significantly different from that of other plants. We assessed the mycorrhizal potential of the indigenous soils by using the Most Probable Number (MPN) approach. We found that MPN in soils collected near the five plant species was significantly higher than the one in the bare soil. The average of MPN per 100 g of dry soil was 11.8 (from 5.6 to 25.0) in the bare soil and 228.5 (from 108.0 to 476.0) in L. multifida rhizosphere. This result indicates that the soil surrounding the target plants can be used as inoculum for mycorrhization. We conducted a nursery experiment in which L. multifida soil yielded a high mycorrhizal percentage in T. articulata plants, thus contributing to improve the plant growth. However, the highest formation of arbuscules in T. articulata grown in Lavandula soil did not significantly affect the growth or the nutrition of plants compared to Tetraclinis soil, except for potassium concentration. Our investigation clearly showed that L. multifida soil can be used as biofertilizer to inoculate nurseries for T. articulata production. This will greatly contribute to sustain Tetraclinis woodland.
1. Introduction
1Moroccan forest ecosystems are predisposed to degradation because of demographic expansion of the local populations, which is coupled with increased requirements for cultivable lands, commercial exploitation of certain forest trees and climatic constraints (i.e. drought, erosion, fire, parasitic attacks, etc.). This degradation results in the reduction of biological diversity, leading over time to the alteration of physico-chemical and biological soil properties (Kennedy et al., 1995; Warren et al., 1996; Carrillo-García et al., 1999).
2Arbuscular mycorrhizal symbiotic fungi are regarded as a “key” microbial group in ecosystems due to their capacity to promote the development of plants in degraded areas. The mycorrhizal propagules are involved in the functioning of the resource islands that are developed around plant roots (Azcon-Aguilar et al., 2003; Koide et al., 2004; Ouahmane et al., 2006a; Ouahmane et al., 2006b). In the forest land, the mycorrhizal effect can be obtained either by introducing a fungal symbiont previously selected for its ability to stimulate the growth of the host plant under given environmental conditions (Duponnois et al., 2005), or by the management of the native soil mycorrhizal potential (Duponnois et al., 2001). The success of the second approach is in keeping with the use of highly mycotrophic plant species (grasses and shrubs) that promotes dissemination of mycorrhizal propagules in the soil and enhances the ability of target plants to become inoculated. Barea et al. (1990) reported that in degraded zones, the mycorrhizal component may disappear or, at least, be severely depleted and so it may be necessary to reinforce or replace it by appropriate inoculation.
3In Mediterranean areas, the regeneration of a great number of tree and shrub species shows a spatial pattern associated with established plants, suggesting a net positive balance in plant-plant interactions (Zamora et al., 2004). A good shrub cover is the best insurance to avoid soil erosion, to achieve natural regeneration of woodlands, and to boost the success of reforestation (Zamora et al., 2004).
4Several studies support the potential for exploiting the natural diversity of mycorrhizal fungi as inoculum sources in revegetation programs. Azcon et al. (1997) showed that Lavandula plants must be mycorrhized in order to thrive in degraded soils from desertification-threatened areas in typical Mediterranean ecosystems, and the consequences of the mycorrhization of these plants with regards to a revegetation strategy are obvious.
5Recently, Ouahmane et al. (2006b) observed that growth and mycorrhizal colonization of Cupressus arizonica and L. multifida were both improved when the two species were grown together. These results emphasize the role of “resource islands” and “nurse plants” of Lavandula species in the regeneration processes of forest trees such as Cupressus spp. Thus, the conservation of ecosystems is highly dependent on the composition of the soil microbial communities, especially, mycorrhizae.
6In Tetraclinis woodlands, T. articulata is an endomycorrhizal-dependant species (Diaz et al., 1993; Abbas et al., 2006) and many shrub species are observed in the natural distribution area of this forest plant, including Withania frutescens Pauquy, Lavandula multifida L., Pistacia atlantica Desf., Pistacia lentiscus L., Olea europaea L. subsp. oleaster (Hoffmanns. & Link) Negodi. However, there are no studies on the potential of those species to produce arbuscular mycorrhizal (AM) inoculum for selected plant species to be used in revegetation strategies. In addition, plants growing under natural conditions differ in their ability to enrich the soil by the mycorrhizal propagules and the effectiveness of AM depends on the native host species (Azcón-Aguilar et al., 2003; Caravaca et al., 2005). Therefore, the aim of this study was to assess whether the most distributed shrub species in Tetraclinis woodlands could form a reservoir of mycorrhizal propagules at the root system layers of T. articulata and could be used as an efficient mycorrhizal inoculum for T. articulata production in forest nurseries.
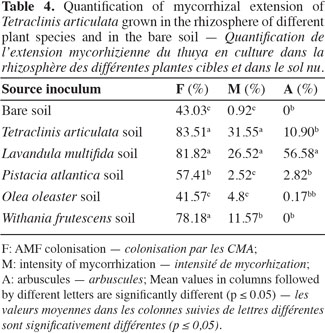
2. Materials and methods
2.1. Study site
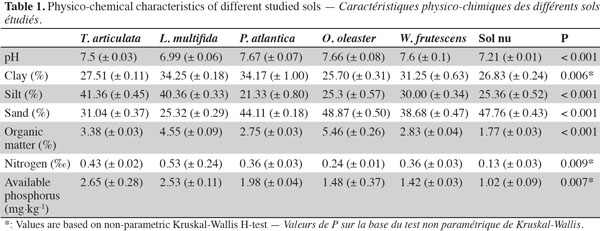
7The experimental area is located in Khémisset, Morocco (33’ 52 557 N, 005’ 54 907 W, 279 m above sea level). The climate is similar to that expected in semi-arid Mediterranean environments, with an average annual rainfall of 577 mm and a mean annual temperature of 17.8 °C. The substratum is schistose or siliceous with a neutral to neutral-alcaline pH and low amounts of nitrogen and phosphorus, respectively 0.50‰ and 3.8 mg·kg-1 of soil. Tetraclinis articulata, P. atlantica, L. multifida, W. frutescens and O. oleaster were the perennial shrubs the most widely distributed in this site. They were always recorded in the vicinity of Tetraclinis articulata adult and then selected as target species in the sampling area. The physico-chemical analysis of soil from those plants is summarized in table 1.
2.2. Field sampling
8Soil samples were collected from the rhizospheres of the five target species (W. frutescens, L. multifida, P. atlantica, O. oleaster and T. articulata) in the experimental area. For each target species, five individual plants were randomly chosen and rhizosphere soil samples were taken. Each sample consisted of five sub-samples (100 cm3) collected at a depth of 10-20 cm. Control samples were randomly collected from bare soil site, away from plant influence. Roots of target species (about 100 g fresh root per species) were randomly collected within the experimental area at 20 cm depth and conserved in alcohol (50%).
2.3. Mycorrhizal quantification
9Roots were washed with tap water, clarified and stained according to the method of Phillips and Hayman (1970). They were then placed on a slide in a drop of polyvinyl alcohol-lactic acid-glycerol (PVLG) (Koske et al., 1983) for microscopic observation (Trouvelot et al., 1986). About 90 root pieces (1 cm) were observed per plant.
10Mycorrhizal development was evaluated by the method of Trouvelot et al. (1986) and expressed as frequency of AM colonization (F%, percentage of root fragments showing fungal colonization), intensity of AM colonization (M%, which gives an estimation of the amount of root cortex that became mycorrhized and is referred to the whole root system) and arbuscule abundance (A%, which gives an estimation of the arbuscule richness in the whole root system).
11AM fungal spores were extracted from the rhizosphere of each plant and from bare soils by wet sieving and decanting method, followed by sucrose centrifugation (Sieverding, 1991). Then the supernatant was poured through a 50 µm sieve and rinsed with tap water. Spores were counted using a stereomicroscope and grouped according to their morphological characteristics. The uniformity of the morphological groups was confirmed under an optical microscope and the different morphotypes were identified according to genus. Spore identification was assessed mainly using spore size and color, wall structure and hyphal attachment (Walker, 1983; INVAM, 1997).
2.4. Indigenous soil inoculum potentials
12The mycorrhizal potential of the rhizosphere soil samples collected from the five target species and bare soil was measured by the well-known “Most Probable Number” method (MPN), using the dilution technique (Sieverding, 1991). Six dilutions of each soil were made by thoroughly mixing the original soil in 1:4 proportions with an autoclaved sandy soil (121 °C, 40 min, two times). After autoclaving, its physical and chemical characteristics were as follows: pH (H2O) 6.7; clay 3.8%; coarse silt 0.8%; fine sand 34.3%; coarse sand 61.1%; carbon 1.89%; total nitrogen 0.08%; total phosphorus 134 mg·kg-1. Five replicates were prepared for each dilution. The seeds of Sorghum vulgare (surface sterilized with 10% sodium hypochlorite) were pre-germinated for 2-days on humid filter paper. One germinated seed was then transplanted into each of small plastic pots filled with 100 g of different soil dilutions, and pots were placed in the forest nursery. After one month, the entire root system of each seedling was collected, washed under tap water, cleared and stained by the method of Phillips and Haymann. Each entire root system was mounted on a microscope slide and observed at a 250 x magnification under a compound microscope to observe the presence of arbuscular mycorrhizal structures. Data were expressed as the number of AM fungal propagules in 100 g of dry soil and the confidence limits, superior or inferior at 95%, were assigned according to Fisher et al. (1970).
2.5. Growth and nutrition of Tetraclinis articulata seedlings
13Each soil sample from the rhizosphere of the five target plants and of the bare soil was packed in 500 ml pots. Seeds of T. articulata were sown directly on top of the soil. The pots were arranged in a randomized complete block design with five replicates per treatment. Plants were grown in a forest nursery under natural conditions. After nine months, the heights and diameters of the stems were measured. Tetraclinis articulata plants were uprooted and the root systems gently washed with tap water. The extent of AM fungal colonization was assessed as described above. After drying (65 °C, 72 h), the dry weight of shoots was measured and samples of shoot tissues were burnt to ash at 600 °C for 3 h and digested in a high acid mix (HCl and HNO3). The phosphorus was analyzed with a PerkinElmer spectrophotometer and the potassium by atomic spectrophotometry (GBC 906AA).
14The total nitrogen was analyzed by Kjeldahl method at the LAMA-US 191 (Laboratory analysis of IRD Centre in Dakar, Senegal).
2.6. Statistical analysis
15Statistical analyses were conducted using XLSTAT-2006 at 5 %. We used ANOVA parametric tests when the normality (Kolmogorov–Smirnov test) and homoscedasticity (Levene test) assumption were met and non-parametric tests (Kruskal-Wallis H-test) when the data lacked normality and homoscedasticity. Comparison among means was made using Student Newman-Keuls test calculated at P < 0.05. To assess the relationship between mycorrhizal parameters, the non-parametric Spearman's rank correlation coefficient was used. When significant correlations occurred a simple linear regression was developed.
3. Results
3.1. Mycorrhizal quantification in the rhizosphere of the associated shrub species
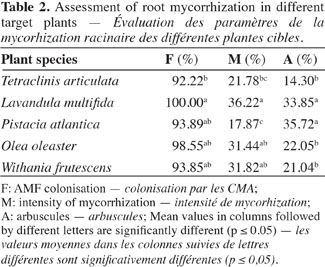
16Natural mycorrhizal root colonization. The examination of the roots from the main mature plants growing in the ecosystem (L. multifida, W. frutescens, P. atlantica and O. oleaster) showed that all of them were mycorrhizal and densely colonized. They all had typical mycorrhizal structures (coils, arbuscules, hyphae, and vesicles) but in terms of mycorrhizal intensity (M) and arbuscules, a few plants, like L. multifida and P. atlantica, showed some potential for mycorrhizal infectivity and could be more receptive to the settlement of mycorrhizal structures (Table 2). Furthermore, statistical analysis showed that no correlations were found between all mycorrhization parameters except for P. atlantica, where the root colonization was negatively correlated with the mycorrhizal intensity (Rs = -0.975, P = 0.005). Indeed, the linear regression showed that the two variables are sharply related (Estimate (±SE) = -0.786 ± 0.129; t = -6.100; P = 0.009; R2 adjusted = 0.901).
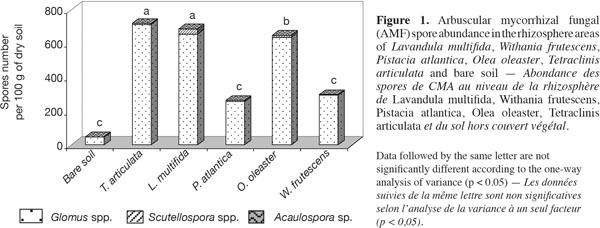
17Population of spores. The number of spores found in the root zones of T. articulata, L. multifida and O. oleaster was higher than in the other species with spores of three different genera of AM fungi (Glomus, Scutellospora and Acaulospora) observed in the different rhizospheres prospected. A significant difference was observed in the total number of AM fungal spores isolated from the rhizosphere soil of L. multifida and T. articulata with respect to the other soils. Also, the number of Scutellospora spores was slightly higher in L. multifida than in T. articulata soils (Figure 1). In our experiment, the number of Glomus spores was higher in all the rhizosphere areas. The main species collected were Glomus aggregatum, Glomus constrictum and Glomus etunicatum identified according to the classical phenotypic parameters (spore wall, coloration, etc..) (Palenzuela Jiminez Eulogio Javier, Estacion Experimental del Zaidin, Granada-Spain, pers. com.)
3.2. Indigenous soil inoculum potentials
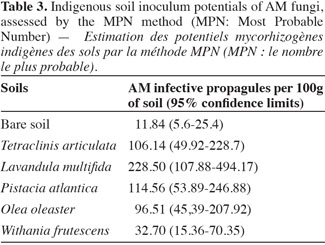
18The number of mycorrhizal propagules in soils collected near the five plant species was significantly higher than the one in the bare soil. The average of MPN per 100 g of dry soils tested was in the following order: L. multifida > P. atlantica > T. articulata > O. oleaster > W. frutescens > bare soil. This result indicates that L. multifida was the most important plant that was able to improve its rhizosphere soil in mycorrhizal propagules. Indeed, the average of MPN per 100 g of dry soil was 88.93% higher in L. multifida rhizosphere (228.5) than in the bare soil (11.8) (Table 3).
3.3. Growth and nutrition of Tetraclinis articulata seedlings on the rhizosphere soils collected from the different target plants and the bare soil
19Mycorrhizal quantification. Data summarized in table 4 showed that the mycotrophy intensity of the existing vegetation in the studied ecosystem differed from one plant to another. The intensity of AM fungal colonization was significantly higher in T. articulata growing in L. multifida and T. articulata rhizosphere soils than in the other soils. In terms of arbuscules the L. multifida soil showed however an enhancement in those structures considered, as a principal site of exchange between plant and fungus in the roots of T. articulata. Indeed, analysis showed that, only in L. multifida, a positive correlation was found between mycorrhizal intensity and number of arbuscules (Rs = 0.900, P = 0.037). The linear regression showed that the two variables are highly related (Estimate (±SE) = 0.311 ± 0.057; t = 5.454; P = 0.012; R2 adjusted = 0.878).
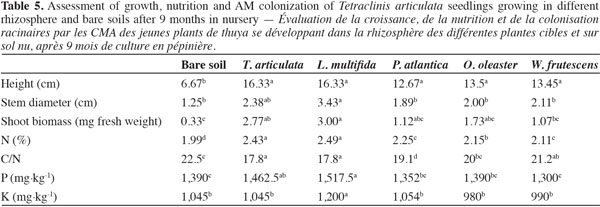
20Growth and nutrition of Tetraclinis articulata seedlings. After nine months of T. articulata growing in the rhizosphere soil of P. atlantica, O. oleaster and W. frutescens, only the plant heights and nitrogen leaf contents were significantly higher compared to plants in the bare soil (Table 5). However, it is noteworthy that T. articulata and L. multifida soils were the most effective source of inoculum to improve stem diameter and phosphorus contents by T. articulata species. Differences between Tetraclinis plants grown in bare soil and rhizosphere soil from Tetraclinis or Lavandula were 145 and 145% for height, 90.4 and 174% for stem diameter, 739 and 809% for shoot fresh biomass, 22 and 25% for N concentration, 5.2 and 9.5% for P contents and 0 and 14% for K concentration, respectively. Only the K concentration in soil from Lavandula was significantly different from that one in Tetraclinis soil.
4. Discussion
21Because of the key ecological functions played by AM symbiosis (Jeffries et al., 2003), management of the native soil mycorrhizal potential through native and highly mycotrophic plant species is one of the main reclamation strategies to increase revegetation success (Duponnois et al., 2001; Azcon-Aguilar et al., 2003). A previous work has shown that Tetraclinis woodlands contain a high relative abundance of AMF spores (Abbas et al., 2006), and this ecosystem is characterized by a patchy distribution of individual plant species. Thus, this study was carried out to assess the role of shrubby plants mainly encountered in Tetraclinis woodlands in improving, by mycorrhizal colonization, the quality of T. articulata production in forest nurseries. Indeed, many experiments in the Mediterranean area have shown the importance of native mycorrhizal potential as a source of AM inoculum for shrubs and trees. Caravaca et al. (2003b) noted that indigenous AM may be considered as a preferential inoculation strategy to guarantee the re-establishment success of native shrub species in a semi-arid degraded soil. Gasque et al. (2004) showed that Stipa tenacissima, for example, acts as a nurse plant providing a physical barrier to the water and sediment circulation down slope, which results in habitat improvement for the establishment of Pinus halepensis.
22In the present study, soil samples were collected from the rhizosphere of five target species (W. frutescens, L. multifida, P. atlantica, O. oleaster and T. articulata) existing in the experimental area. Mycorrhizal status and effectiveness on Tetraclinis seedlings were analyzed. Our results showed that all roots of the target plants examined from the field were highly infected by AM fungi. These results agree fairly well with other survey studies carried out in Mediterranean areas (Requena et al., 1996; Requena et al., 1997), but natural effectiveness of the different target plants was not the same. Lavandula multifida and P. atlantica showed the best percentage of mycorrhizal intensity (M) and arbuscules (A), and seemed to be more receptive to the establishment of mycorrhizal structures. This feature seems to be important to improve growth and nutrition of T. articulata seedlings, because different AM fungal species have different effects on plant performance and nutrient uptake (Allen et al., 1995; Jeffries et al., 2001). Quantification of spore abundance in the rhizospheres of the target plants and in the bare soil showed a predominance of Glomeraceae species which is in agreement with a previous report based on morphological characters of Moroccan Tetraclinis woodland spores (Abbas et al., 2006). The dominance of Glomus species in a disturbed ecosystem can be explained by the ability of the Glomeraceae to colonize via fragments of mycelium or mycorrhizal root pieces (Biermann et al., 1983). The relative abundance of spores of AM fungi varies with the nature of target species, as sporulation can depend on the AM fungus, the host plant, the soil characteristics and the climatic conditions (Sieverding, 1991). Morphological description showed that only three to five AM fungal spore morphotypes were detected in the rhizosphere of the target plant species and in the bare soil, which can be a signal for degradation of the ecosystem. Indeed, high diversity in natural ecosystems may principally be based on a great variety of plant species (Sieverding, 1991). Thus, vegetable cover has a great benefit for AM propagules density that can be affected by its degradation.
23In the current study, counting infective AM propagules from field samples showed that the five target species differ in their capabilities to enrich the soil with mycorrhizal propagules. Lavandula multifida was the most effective plant to provide a high number of infective propagules per unit of soil weight. Previous reports have already described that many plants from the Mediterranean area form arbuscular mycorrhizae association and have been classified as “obligatory mycorrhizal” or as “highly dependent on mycorrhiza” (Brundrett, 1991; Habte et al., 1991; Azcon et al., 1997; Caravaca et al., 2003a; Caravaca et al., 2003b; Ferrol et al., 2004). AM fungi can besides enhance the growth of native shrub species in the short term, which in turn creates a more favorable environment for the development of ecosystem processes (Caravaca et al., 2003b). For this reason, autochthonous plant species are widely used for reclaiming degraded lands in Mediterranean semi-arid areas (Caravaca et al., 2002).
24Growth of T. articulata in the different rhizosphere soils showed different response levels but, generally, it was significantly higher in the rhizosphere of the target species than in the bare soils. The same result was found in glasshouse experiments by Ouahmane et al. (2006a), where the growth of Cupressus atlantica seedlings was significantly higher in the C. atlantica soil and in the shrub species soils than in the bare soils. Other studies on L. multifida and Cupressus arizona corroborated the above mentioned results and emphasized the role of “resource islands” and “nurse plants” of Lavandula species in the regeneration processes of forest trees such as Cupressus spp. (Ouahmane et al., 2006b).
25The uptake of nitrogen was significantly stimulated when T. articulata was grown in different target soils. This result involves yet again the beneficial effect of AM fungi in this nutrient acquisition as found in previous studies (Tobar et al., 1994a; Tobar et al., 1994b; Azcon et al., 1997). Lavandula multifida and T. articulata soils showed the best percentage of this improvement. As those soils are poor in N content (Abbas et al., 2006), we can suppose that beneath the rhizosphere of the two types of species there are other microorganisms, different from the AM fungi, involved also in nitrogen uptake.
26With regard to P and K uptake, except for Lavandula and Tetraclinis soils, there was no significant difference detected between the other soils. Van der Heijden et al. (1998) demonstrated that the increase of AM fungal diversity led to an increased phosphorus content in plant material and, consequently, to a more efficient exploitation of soil phosphorus. In the soil, though, AM fungi are found as spores, hyphae or infected root pieces, and all these propagules can be considered as inoculum sources (Duponnois et al., 2001). It is possible that in our study the AM mycelia network was the main source of inoculum, as previously observed in semi-arid and arid ecosystems (Brundrett et al., 1991; Ouahmane et al., 2006a). However, the highest arbuscule production, in T. articulata growing in Lavandula soil, did not significantly affect plant growth or nutrition compared with Tetraclinis soil, except for K concentration. This nutrient is one of the most important inorganic solutes, and has an important role in processes such as water balance, cell extension and solute transport in the xylem (Porras-Soriano et al., 2009). So, under dry conditions, the K concentration improvement by AM inocula from Lavandula soil may be very important to maintain a high tissue water level, even under conditions of osmotic deficiency.
27Recently, the diversity of the AMF community composition in roots of two plant species (T. articulata and L. multifida) that exist alone or co-occur in Moroccan Tetraclinis woodlands was characterized using molecular techniques (Bakkali et al., 2011). The highest diversity was found in the roots of T. articulata alone, followed by the associated T. articulata-L. multifida and finally L. multifida alone. Many AMF were shared between the two species, although a few of them had a preference for one or the other (Bakkali et al., 2011). Maybe particular plant-fungus combinations are flavored according to the ecological conditions. Therefore, further research, using molecular tools (PCR/sequencing), is needed to identify and select the most efficiency spore species to be used in inoculation tests of T. articulata. Also, it is important to address in Tetraclinis woodlands the relationship between AM fungi sporulation, AM mycelia network and morphotype of AM fungi associated to T. articulata roots.
28Finally, more research input is also needed to test the effect in the field, with different ecological conditions, of AM propagules isolated from the Lavandula rhizosphere to enhance the development and nutrition of T. articulata.
29The present study supports the beneficial effects from a shrub species established in Tetraclinis woodland. Lavandula multifida rhizosphere seems to be of great interest and could be used as an efficient mycorrhizal inoculant for the production of T. articulata in forest nurseries.
30Acknowledgements
31We thank Pr. Rosario AZCON for the revision of this manuscript. This work was supported by the Moroccan-France cooperation (PRAD project number 06/05). The authors gratefully thank the laboratory analysis of IRD Centre in Dakar, Senegal for support providing in technical analysis.
Bibliographie
Abbas Y. et al., 2006. Biodiversity of arbuscular mycorrhizal fungi in Moroccan Tetraclinis articulata (Vahl.) Masters forests. Ann. For. Sci., 63, 285-291.
Allen E.B. et al., 1995. Patterns and regulation of mycorrhizal plant and fungal diversity. In: Collins H.P., Robertson G.P. & Klug M.J., eds. The significance and regulation of soil biodiversity. Amsterdam, The Netherlands: Kluwer Academic Publishers, 47-62.
Azcon R. & Barea J.M., 1997. Mycorrhizal dependency of a representative plant species in Mediterranean shrublands (Lavandula spica L.) as a key factor to its use for revegetation strategies in desertification-threatened areas. Appl. Soil Ecol., 7, 83-92.
Azcon-Aguilar C. et al., 2003. Analysis of the mycorrhizal potential in the rhizosphere of representative plant species from desertification-threatened Mediterranean shrublands. Appl. Soil Ecol., 22, 29-37.
Bakkali Yakhlef S. et al., 2011. Effective AMF population in roots of Tetraclinis articulata and Lavandula multifida in Moroccan Tetraclinis woodlands. Mycology, 2(2), 79-86.
Barea J.M., Salamanca C.P., Herrera M.A. & Roldán-Fajardo B.E., 1990. Microorganisms-plant symbioses in the establishment of a vegetal cover on degraded lands. In: Albaladejo J., Stocking M.A. & Díaz E., eds. Soil degradation and rehabilitation in Mediterranean environmental conditions. Murcia, Spain: CSIC, 139-158.
Biermann B. & Linderman R.G., 1983. Use of vesicular arbuscular mycorrhizal roots, intraradical vesicles and extraradical vesicles as inoculum. New Phytol., 95, 97-105.
Brundrett M.C., 1991. Mycorrhizas in natural ecosystems. In: Macfayden A., Begon M. & Fitter A.H., eds. Advances in ecological research. Vol. 21. London: Academic Press Ltd., 171-313.
Brundrett M.C. & Kendrick B., 1991. The mycorrhizal status, root anatomy, and phenology of plants in a sugar maple forest. Can. J. Bot., 66, 1153-1173.
Caravaca F., Barea J.M., Figueroa D. & Roldán A., 2002. Assessing the effectiveness of mycorrhizal inoculation and soil compost addition for enhancing reafforestation with Olea europaea subsp. sylvestris through changes in soil biological and physical parameters. Appl. Soil Ecol., 20, 107-118.
Caravaca F. et al., 2003a. Re-establishment of Retama sphaerocarpa as a target species for reclamation of soil physical and biological properties in a semi-arid Mediterranean land. For. Ecol. Manage., 182, 49-58.
Caravaca F. et al., 2003b. Establishment of shrub species in a degraded semi-arid site after inoculation with native or allochthonous arbuscular mycorrhizal fungi. Appl. Soil Ecol., 22, 103-111.
Caravaca F., Alguacil M.M., Barea J.M. & Roldán A., 2005. Survival of inocula and native AM fungi species associated with shrubs in a degraded Mediterranean ecosystem. Soil Biol. Biochem., 37, 227-233.
Carrillo-García A., Leon de la Luz J.L., Bashan Y. & Bethlenfalvay G.J., 1999. Nurse plants, mycorrhizae, and plant establishment in a disturbed area of the Sonoran Desert. Restor. Ecol., 7, 321-335.
Diaz G. & Honrubia M., 1993. Arbuscular mycorrhizae on Tetraclinis articulata (Cupressaceae): development of mycorrhizal colonisation and effect of fertilisation and inoculation. Agronomie, 13, 267-274.
Duponnois R., Plenchette C., Thioulouse J. & Cadet P., 2001. The mycorrhizal soil infectivity and arbuscular mycorrhizal fungal spore communities in soils of different aged fallows in Senegal. Appl. Soil Ecol., 17, 239-251.
Duponnois R., Founoune H., Masse D. & Pontanier R., 2005. Inoculation of Acacia holosericea with ectomycorrhizal fungi in a semi-arid site in Senegal: growth response and influences on the mycorrhizal soil infectivity after 2 years plantation. For. Ecol. Manage., 207, 351-362.
Ferrol N. et al., 2004. Analysing arbuscular mycorrhizal fungal diversity in shrub-associated resource islands from a desertification threatened semi-arid Mediterranean ecosystem. Appl. Soil Ecol., 25, 123-133.
Fisher R.A. & Yates F., 1970. Statistical tables for biological agriculture and medical research. 6th ed. Davien, CT, USA: Hafner Publishing Company.
Gasque M. & Garcıa-Fayos P., 2004. Interaction between Stipa tenacissima and Pinus halepensis: consequences for reforestation and the dynamics of grass steppes in semi-arid Mediterranean areas. For. Ecol. Manage., 189, 251-261.
Habte M. & Manjunath A., 1991. Categories of vesicular-arbuscular mycorrhizal dependency of host species. Mycorrhiza, 1, 3-12.
INVAM (International Culture Collection of [Vesicular] Arbuscular Mycorrhizae), 1997. http://www.invam.caf.wvu.edu/
Jeffries P. & Barea J.M., 2001. Arbuscular mycorrhiza: a key component of sustainable plant-soil ecosystems. In: Hock B., ed. The Mycota. IX. Fungal Associations. Berlin: Springer, 95-113.
Jeffries P. et al., 2003. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils, 37, 1-16.
Kennedy A.C. & Smith K.L., 1995. Soil microbial diversity and the sustainability of agriculture soils. Plant Soil, 170, 75-86.
Koide R.T. & Mosse B., 2004. A history of research on arbuscular mycorrhiza. Mycorrhiza, 14, 145-163.
Koske R.E. & Tessier B., 1983. A convenient permanent slide mounting medium. Mycol. Soc. Am. Newsl., 34(2), 59.
Ouahmane L. et al., 2006a. Some Mediterranean plant species (Lavandula spp. and Thymus satureioides) act as potential “plant nurses” for the early growth of Cupressus atlantica. Plant Ecol., 185, 123-134.
Ouahmane L. et al., 2006b. Lavandula species as accompanying plants in Cupressus replanting strategies: effect on plant growth, mycorrhizal soil infectivity and soil microbial catabolic diversity. Appl. Soil Ecol., 34, 190-199.
Phillips J.M. & Hayman D.S., 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc., 55, 158-161.
Porras-Soriano A., Soriano-Martin M.L., Porras-Piedra A. & Azcon R., 2009. AM fungi increased growth, nutrition uptake and tolerance to salinity in olive trees under nursery conditions. J. Plant Physiol., 166, 1350-1359.
Requena N., Jeffries P. & Barea J.M., 1996. Assessment of natural mycorrhizal potential in a desertified semi-arid ecosystem. Appl. Environ. Microbiol., 62, 842-847.
Requena N., Jimenez I., Toro M. & Barea J.M., 1997. Interactions between plant-growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in Mediterranean semi-arid ecosystems. New Phytol., 136, 667-677.
Sieverding E., 1991. Vesicular arbuscular mycorrhiza management in tropical agrosystems. Eschborn, Germany: GTZ.
Tobar R.M., Azcon R. & Barea J.M., 1994a. Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water stressed conditions. New Phytol., 126, 119-122.
Tobar R.M., Azcon R. & Barea J.M., 1994b. The improvement of plant N acquisition from an ammonium-treated, drought-stressed soil by the fungal symbiont in arbuscular mycorrhizae. Mycorrhiza, 4, 105-108.
Trouvelot A., Kouch J. & Gianinazzi-Pearson V., 1986. Mesure du taux de mycorhization VA d’un système radiculaire : recherche de méthodes d’estimation ayant une signification fonctionelle. In : Actes du 1er Séminaire sur les mycorhizes : physiologie et génétique, 1-5 juillet 1985, Dijon, France. Paris : Inra, 217-221.
Van der Heijden M.G.A. et al., 1998. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature, 396, 69-72.
Walker C., 1983. Taxonomic concepts in the Endogonaceae. I. Spore wall characteristics in species description. Mycotaxon, 18, 443-455.
Warren A., Sud Y.C. & Rozanov B., 1996. The future of deserts. J. Arid Environ., 32, 75-89.
Zamora R., García-Fayos P. & Gómez-Aparicio L., 2004. Las interacciones planta-planta y planta-animal en el contexto de la sucesión ecológica. In: Valladares F., ed. Ecología del bosque mediterráneo en un mundo cambiante. Madrid: Ministerio de Medio Ambiente, EGRAF, 371-393.

