- Accueil
- Volume 17 (2013)
- numéro 1
- Adventitious shoot regeneration from in vitro juvenile explants of black alder (Alnus glutinosa [L.] Gaertn.)
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Adventitious shoot regeneration from in vitro juvenile explants of black alder (Alnus glutinosa [L.] Gaertn.)

Notes de la rédaction
Received on April 24, 2012; accepted on October 12, 2012
Résumé
Régénération de pousses adventives à partir d'explants juvéniles in vitro de l'aulne glutineux (Alnus glutinosa [L. ] Gaertn.). Nous avons étudié la capacité de régénération in vitro de l'aulne glutineux (Alnus glutinosa L.) en utilisant des explants juvéniles et un milieu WPM modifié (mWPM). Dans notre première expérience (Exp. 1), des graines matures ont été cultivées en présence de différentes concentrations de thidiazuron (TDZ: 0, 1, 5 et 10 µM) pendant 2 ou 4 semaines à la lumière (photopériode de 16 h). Dans la seconde expérience (Exp. 2), des segments d'hypocotyle (Hyp), des nœuds cotylédonaires (CN) et des cotylédons (Cot) ont été excisés à partir de plantules in vitro âgées de 2 semaines et cultivées sur mWPM complété de différentes concentrations de 6-(γ, γ-dimethylallylamino)-purine (2iP: 0, 5, 10, 25 et 50 µM) et de 6-benzylaminopurine (BA: 1, 2, 3, 4 et 5 µM) pendant 2, 4 ou 6 semaines à l'obscurité. Après les périodes d'induction, les explants ont été transférés dans le milieu d'expression constitué de mWPM contenant 1 µM de BA à la lumière. Les fréquences de régénération et le nombre de bourgeons/pousses par explant régénéré ont été déterminés après 10 semaines pour l’Exp. 1 et 8 semaines pour l’Exp. 2. Pour l’Exp. 1, utilisant des graines matures, 1 µM de TDZ pendant 2 semaines à la lumière s’est avéré être le meilleur traitement d'induction, avec 91 % des explants produisant une moyenne de 11 bourgeons/pousses par explant, dont plus de 4 longues pousses (> 1 cm). Dans l’Exp. 2, il y avait de faibles fréquences de régénération de bourgeons/pousses, 3 % pour Hyp et 10 % pour Cot. En revanche, le CN a été l'explant le plus réactif, avec 100 % des explants produisant 12-14 bourgeons/pousses après le traitement d'induction le plus efficace, à base de 1-3 µM de BA pendant 2 semaines à l'obscurité.
Abstract
We studied the in vitro regeneration ability of black alder (Alnus glutinosa L.) using juvenile explants and a modified woody plant medium (mWPM). In our first experiment (Exp. 1), mature seeds were cultured in the presence of different concentrations of thidiazuron (TDZ: 0, 1, 5 and 10 µM) for 2 or 4 weeks under light conditions (16 h photoperiod). In the second experiment (Exp. 2), hypocotyl segments (Hyp), cotyledonary nodes (CN) and cotyledons (Cot) were excised from 2-week-old in vitro seedlings and cultured on mWPM supplemented with different concentrations of 6-(γ,γ-dimethylallylamino)-purine (2iP: 0, 5, 10, 25 and 50 µM) and 6-benzylaminopurine (BA: 1, 2, 3, 4 and 5 µM) for 2, 4 or 6 weeks in the dark. After the induction periods, the explants were transferred to the expression medium consisting of mWPM containing 1 µM BA under light conditions. The regeneration frequencies and the number of buds/shoots per regenerated explant were determined after 10 weeks for Exp. 1 and 8 weeks for Exp. 2. For Exp. 1, using mature seeds, 1 μM TDZ for 2 weeks under light conditions proved to be the best induction treatment, with 91% of explants producing an average of 11 buds/shoots per explant, including more than four long shoots (> 1 cm). In Exp. 2, there were low shoot regeneration frequencies, 3% for the Hyp and 10% for the Cot. In contrast, the CN was the most responsive explant, with 100% explants producing 12-14 buds/shoots after the most effective induction treatment, based on 1-3 µM BA for 2 weeks in the dark.
Table des matières
1. Introduction
1Black alder (Alnus glutinosa [L.] Gaertn., Betulaceae) is a widespread and popular tree species across Europe, well adapted to moist and wet soils (e.g., flood plains and along riverbanks). It is an interesting species because of its ability to form symbiotic relationships with mycorrhizal fungi and the nitrogen-fixing actinomycete Frankia sp., and also because of its multiple benefits, such as timber production, flood control, riverbank stabilization, polluted soil phytoremediation, and as a refuge for biodiversity (Roy et al., 2007; Claessens et al., 2010 and references therein). This important species is, however, threatened by a disease caused by Phytophthora alni that is now widespread in Europe, including Belgium (Webber et al., 2004). This disease represents a major risk for riverside alder trees, especially when flooding occurs. In Wallonia (Southern part of Belgium), where black alder accounts for 35% of the riparian species (Debruxelles et al., 2009), P. alni has affected more than 25% of the alder trees along riverbanks (Abras et al., 2005).
2In natural conditions, suitable conditions for the natural propagation of black alder in its distribution range are rarely met (Claessens et al., 2010). Most of the new stands have been established by planting 2-year-old seedlings. This species can, however, be readily vegetatively propagated either by stem cuttings (Martin et al., 1982; Ayan et al., 2006) or by in vitro tissue culture (Tremblay et al., 1984; Lall et al., 2005; Bajji et al., 2012).
3ECOLIRIMED is a cross-border (Wallonia-Lorrain-Luxembourg) project co-funded by the European Regional Development Fund under the Interreg IV-A “Greater Region” programme. Its main objective is to develop a woody ecotype production sector for sustainable fixing and phytoremediation of watercourse banks. One of its main actions was to set up an in vitro selection strategy for exploiting somaclonal variation and/or chemical mutagenesis in order to improve the potential use of woody plants (especially black alder) for accumulating and extracting heavy metals in polluted watercourse banks. The success of such a selection strategy, however, requires the regeneration of whole plants from cultured somatic tissues under selection conditions (Brown et al., 1995; Rai et al., 2011). An efficient protocol for adventitious shoot regeneration is therefore needed for this purpose. This regeneration method has been attempted for several woody plants (e.g., Barrueto Cid et al., 1999; Liu et al., 2002; Du et al., 2008; Nas et al., 2010) but, so far as we know, not yet for black alder. The objective of our study was therefore to develop a simple and efficient adventitious shoot regeneration system for black alder, using seeds and seedling parts as explants. Cytokinins, alone or in combination with auxins, are very effective in promoting adventitious shoot induction (Bates et al., 1992; Murthy et al., 1998; Barrueto Cid et al., 1999; Liu et al., 2002; Giri et al., 2004; Van Staden et al., 2008; Nas et al., 2010). In our preliminary experiments, thidiazuron (TDZ) for seed explants and 6-(γ,γ-dimethylallylamino)-purine (2iP) or 6-benzylaminopurine (BA) for seedling-derived explants were found to promote shoot regeneration when applied without any auxin addition.
2. Materials and methods
2.1. Plant material and culture conditions
4Mature seeds of black alder (Alnus glutinosa [L.] Gaertn.) were provided by the Forest Tree Seed Center of the Walloon Region (Belgium) and stored at 4°C until use. The basal medium used here was a modified woody plant medium (mWPM) (Lloyd et al., 1980) consisting of WPM mineral salts and vitamins supplemented with 300 µM Fe-EDDHA, 30 g·l-1 glucose and 5 g·l-1 Pastagar B (BIO-RAD, France) and with pH adjusted to 5.6 (Bajji et al., 2012). All cultures were maintained at 23 ± 1 °C, either in the dark or under a 16-h photoperiod (Sylvania Gro-lux fluorescent tubes, 35 µE·m-2·s-1).
2.2. Explant preparation
5Prior to disinfection, the seeds were soaked in water for about 24 h. They were then surface-disinfected under sterile conditions by soaking for 30 s in 90% ethanol, followed by 30 min in 30% H2O2 containing 0.1% Tween 20 and at least three rinses with sterile deionized water.
6The disinfected seeds were either directly used as explants (Exp. 1) or allowed to germinate in test tubes (150 x 25 mm) containing 10 ml of mWPM (half-strength macronutrients) for about 2 weeks (Exp. 2). From each seedling, five hypocotyl segments (Hyp, ~1.0 to 1.5 mm-long cut from the distal end), one cotyledonary node (CN) and two cotyledons (Cot) were excised and used as explants (Figure 1a).
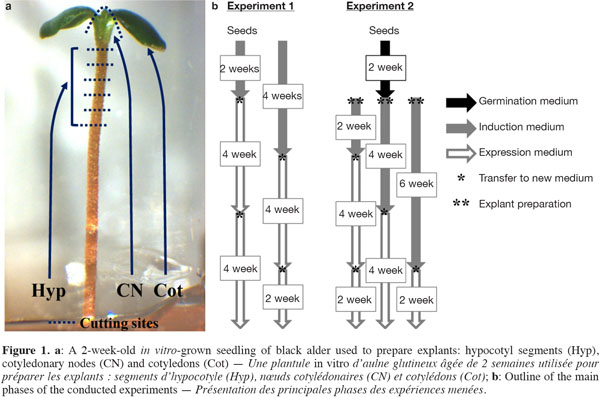
2.3. Experiment 1: adventitious shoot induction from mature seeds
7The disinfected seeds were placed on a solidified induction medium in 90 mm triple vent Petri dishes (25 ml per dish) consisting of mWPM supplemented with TDZ at different concentrations (0, 1, 5 and 10 µM) for 2 or 4 weeks under light conditions. In total, 30 seeds (5 per dish) were used per TDZ concentration and per induction period. After the induction period, two successive transfers to an expression medium consisting of mWPM, supplemented with 1 µM BA, were performed at 4-week intervals (Figure 1b).
2.4. Experiment 2: adventitious shoot induction from seedling-derived explants
8Hyp (25 per dish), CN (5 per dish) and Cot (10 per dish) were put on mWPM containing different concentrations of 2iP (0, 5, 25 and 50 µM) or BA (1, 2, 3, 4 and 5 µM) for 2, 4 or 6 weeks in the dark (Figure 1b). Explants were then transferred once (4 and 6 weeks) or twice (2 weeks) to the expression medium (mWPM + 1 µM BA) under a 16-h photoperiod. In all, 15 seedlings per 2iP or BA concentration and per induction period were used as explant sources.
2.5. Data collection and analysis
9Cultures were observed regularly at the different medium transfers. The regeneration response of the tested explants was evaluated at the end of the experimental periods (Figure 1b) by determining the percentages of callusing and regenerating explants, as well as the mean number of buds/shoots per regenerating explant. Reported data were pooled from two independent experiments. Percentage and bud/shoot number data were, respectively, arcsine square root and square root transformed, and then submitted to variance analysis (ANOVA) and the Tukey test at the 5% significance level using the Minitab program (Release 14, Minitab Inc., 2003).
3. Results
10The germination percentage of black alder seeds used in this study ranged from 45 to 50%, regardless of induction treatment (detailed data not shown). The percentage of seed infection after surface sterilization was about 5%. Only healthy germinated seeds were therefore considered in the quantified parameters.
3.1. Experiment 1: adventitious shoot induction from mature seeds
11In this experiment, we investigated the in vitro regeneration ability of black alder seeds in response to 2 or 4 weeks of incubation with different TDZ concentrations (0, 1, 5 and 10 µM). Seeds on the TDZ-free medium germinated and grew normally (Figure 2a). In contrast, seeds grown on media containing TDZ exhibited varying phenotypes after 2 to 4 weeks of culture. There was no or less epicotyl and root development, and the hypocotyl was shorter and more thickened (Figure 2b) than the control seedlings (Figure 2a). These changes increased over time with the appearance of calluses on the swollen parts. The roots that had not formed calluses became necrotic. Adventitious buds started to emerge after about 4 weeks of culture in the expression medium (Figure 2c). During the culture of explants arising from TDZ-containing media, calluses gradually invaded various parts of seedlings, reaching percentages ranging from 83-97% (2-week induction period) to 94-100% (4-week induction period) (detailed data not presented).

12Figure 3a shows the percentages of regenerated explants per TDZ treatment and per induction period after 10 weeks of culture. In the absence of TDZ, 100% of the germinated seeds produced at least one shoot per seed. With TDZ treatments, a 2-week induction period did not lead to a significant change in shoot production rate, regardless of TDZ levels; 4 weeks of induction, however, resulted in a significant decrease in shoot production rate when high TDZ concentrations (at 5 and 10 μM) were used.
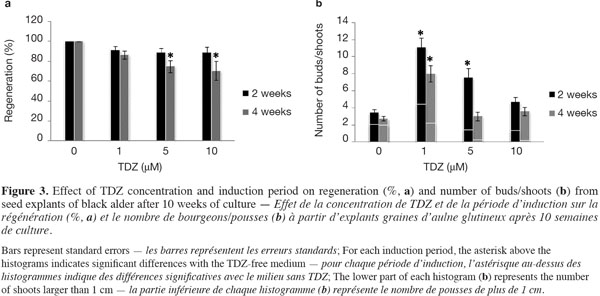
13The influence of TDZ concentration and induction period on the total number of buds/shoots per regenerated explant after 10 weeks of culture is shown in figure 3b. The lower part of each histogram represents the number of shoots longer than 1 cm (Figure 2d). In general, the total number of buds/shoots tended to be lower when seedlings had spent 4 weeks rather than 2 weeks on the TDZ induction medium. The formation of buds/shoots occurred in 92-100% of the seed explants (detailed data no shown) around the nodal region (distal part of the hypocotyl, between and behind the insertion points of the cotyledons) (Figures 2c, e). Significantly higher numbers of buds/shoots were recorded in response to 1 µM TDZ, regardless of the induction period, and to 5 µM TDZ with a 2-week induction period. TDZ at 1 µM for 2 weeks induced the highest shoot production rate (Figure 3a), more buds/shoots and longer shoots (Figure 3b) than the other treatments (TDZ concentration x induction period).
3.2. Experiment 2: adventitious shoot induction from seedling-derived explants
14In our preliminary experiments, shoot regeneration could not be induced from the root explants of in vitro-grown black alder seedlings. In Exp. 2, three types of explants (Hyp, CN, and Cot) were collected from 2-week-old seedlings and submitted to different 2iP (0, 5, 25 and 50 µM) or BA (1, 2, 3, 4 and 5 µM) concentrations for three induction periods (2, 4 or 6 weeks) in the dark (Figure 1). The time required to observe changes in the explants depended on the induction period. Explants treated for 2 weeks in the dark started to change (i.e., necrosis, callus formation at the cut end of Cot, bud/shoot neoformation at the CN level, Hyp swelling, etc.) after at least 2 weeks of culture. In contrast, such changes started to appear only after about 8 weeks of culture in explants treated for 6 weeks.
15The mean regeneration percentages for all treatments after 8 weeks of explant culture were 3.22 ± 0.29 for Hyp, 95.75 ± 1.12 for CN and 10.11 ± 2.56 for Cot (detailed data not shown). The evolution of CN over time was reminiscent of the seeds with an elongation of the hypocotyl side and the normal development of the epicotyl into a shoot in the cytokinin-free medium (Figure 2f), compared with media supplemented with 2iP or BA, where multiple buds/shoots appeared on swollen CN covered with calluses (Figure 2g). The regeneration frequency for the three explants was not significantly influenced by the induction period (Figure 4a). Callus formation was observed on almost 100% of the Hyp and Cot explants and on 78-93% of the CN explants. The calluses generally appeared at the cut surface of the Hyp and Cot and from the nodal region of the CN.
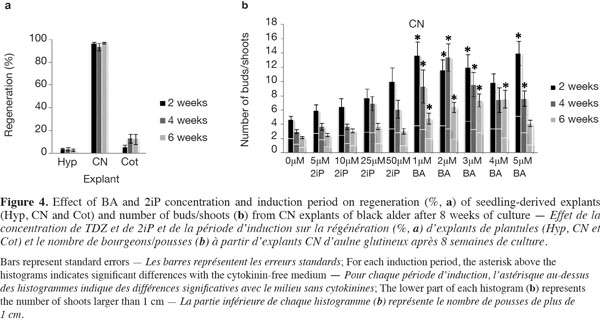
16For Hyp explants, the newly induced buds occurred mainly on the distal segment (near the CN) (Figure 2h). Those of Cot explants were often surrounded by small cotyledon-like structures (Figure 2i). Bud/shoot production was generally very low for Cot and, especially, for Hyp explants (detailed data not presented). In addition, no clear trends with cytokinin concentrations or induction periods could be found. The highest mean shoot number per Hyp explant (1.23) was observed with 25 µM 2iP for 2 weeks (3.3% germination). For Cot explants, the best shoot production (1.13) was obtained with 4 µM BA over 6 weeks (30% germination). When cytokinin concentrations were considered together, the mean shoot number tended to decrease (Hyp) or increase (Cot) with an increase in the induction period.
17CN explants yielded the best shoot production (Figure 4b). The ability of CN explants to produce buds/shoots depended on the treatment (cytokinin concentration x induction period), where the number of buds/shoots and the number of shoots longer than 1 cm decreased with an increase in the induction period. Buds/shoots were observed in all media and induction periods with a significant effect of BA compared to 2iP. For 2iP treatments, bud/shoot production increased as its concentration increased in response to the shortest (2 weeks) induction period; this effect tended, however, to decrease with the extension of the induction period (Figure 4b). In addition, values obtained with 2iP treatments were not statistically different from those obtained on the cytokinin-free medium. For BA treatments, 12 out of 15 cases (treatments) yielded significantly higher numbers of buds/shoots than the control (0 µM) (Figure 4b). The greatest number of shoots (> 1 cm), 5.15 ± 0.96/explant, was obtained with 5 µM BA and a 2-week induction period.
4. Discussion
18The lack of tissue culture information on in vitro adventitious shoot regeneration from the seed, seedling or clonal material of black alder prompted the present study. The ultimate goal of this work was to develop a simple and rapid protocol for shoot regeneration in black alder resulting in a large number of shoots that could be excised for rooting.
19For all four explant types tested, the best responses in terms of regeneration frequency, and especially the number of buds/shoots per regenerated explant, were obtained when the explants were pre-cultured for 2 weeks. Using mature seeds as explants, TDZ at 1 µM induced the formation of more buds/shoots, including long shoots (> 1 cm), compared with the other concentrations (0, 5 and 10 µM), along with a high percentage (> 90%) of regeneration (Figure 3). The first protocol to be retained from Exp. 1, where mature seeds were used as explants, was the use of mWPM supplemented with 1 µM TDZ for 2 weeks under lighting conditions before transfer to the expression medium. In Exp. 2, CN explants exhibited higher regeneration frequencies than Hyp and Cot explants (Figure 4a). BA was found to be more effective than 2iP in terms of bud/shoot formation (Figure 4b). Although the highest number of shoots that were at least 1 cm long was obtained with BA at 5 μM, these shoots showed some hyperhydricity symptoms, probably because of the high concentration of BA in the induction medium. The optimal shoot production conditions for CN explants were 15-day-old explants on mWPM containing 1-3 µM BA for 2 weeks in the dark for pre-culture, followed by transfer to the expression medium.
20In our preliminary studies, we could not induce shoot regeneration from root explants (data not shown). This does not seem to be specific to black alder; similar results have been reported for other species, such as Acacia mangium (Douglas et al., 2000) and Prunus microcarpa (Nas et al., 2010).
21In general, Cot explants have been reported to be more suitable than Hyp explants (Quoirin et al., 1998; Barrueto Cid et al., 1999; Douglas et al., 2000; Sanatombi et al., 2008; Nas et al., 2010). In this study, the highest frequency of adventitious bud/shoot formation was 10% for Hyp and it did not exceed 37% for Cot. Despite these low regeneration frequencies, Cot displayed higher regeneration percentages than Hyp, which accords with the findings reported by regeneration studies on other species (Barrueto Cid et al., 1999; Douglas et al., 2000; Nas et al., 2010). Nevertheless, buds produced by Cot were unable to elongate.
22Of all the explants used, seeds and CN were found to be the best in terms of shoot production. Multiple buds/shoots arose mainly from CN explants (Exp. 2) and from seed explants around the CN of the obtained seedlings (Exp. 1). The use of the CN as explants for shoot production has been successful in many species, including Acacia tortilis (Nandwani, 1995), Cercis canadensis (Distabanjong et al., 1997), Eucalyptus grandis x E. urophylla (Barrueto Cid et al., 1999) and Acacia mangium (Douglas et al., 2000). This ability of the nodal tissues to form new shoots could be explained by the origin of the calluses induced on these explants. The pre-existent meristems of these zones probably favored callus formation with high rates of adventitious bud initiation and shoot development, as already discussed (Barrueto Cid et al., 1999).
23As in other studies using CN as explants (Bates et al., 1992; Nandwani, 1995; Barrueto Cid et al., 1999; Douglas et al., 2000), it was not always easy to determine the exact number and, sometimes, the origin of the buds/shoots produced because they formed tight clusters around the CN. Buds/shoots that arose from seeds and CN explants of cytokinin-free treatments could have axillary or adventitious origins. In TDZ and BA-treated explants, however, bud/shoot formation occurred mainly at the callus level associated with CN explants (intermediate callus phase), suggesting that these buds/shoots could be considered as adventitious (Barrueto Cid et al., 1999; Gahan et al., 2008). We cannot, however, exclude the possibility that these organs might arise through the simple proliferation of the apical or nodal meristems of the seedling axis. Nevertheless, studies in which histological analysis was performed have indicated that the shoots were formed from actively dividing cells located in the axillary bud region (Distabanjong et al., 1997).
24As mentioned earlier, a simple and rapid protocol with high bud/shoot regeneration was selected from each experiment. Larger shoots (> 1 cm long) represented 37% (CN, 3 µM BA, 2 weeks) to 40% (seeds, 1 µM TDZ, 2 weeks) of the total number of buds/shoots and could be easily established as proliferating or rooting cultures. To promote bud elongation and thus enhance the number of large shoots, factors such as the effect of container size (Marques et al., 2011) or of adding activated charcoal (Humánez et al., 2011) during the expression phase could be tested. Black alder is a species that easily forms roots (in the presence of indole-3-butyric acid, and even spontaneously) and successfully acclimatizes to greenhouse conditions (Tremblay et al., 1984; Lall et al., 2005; Bajji et al., 2012). The rooting and acclimatization phases have never been a concern during the micropropagation of black alder and were therefore not considered in the present work.
25In conclusion, we developed a new, efficient and rapid adventitious shoot production protocol from either mature seeds or CN of 2-week-old in vitro-grown seedlings of black alder. As regeneration from clonal material has the advantage of retaining all the traits of the starting material (e.g., elite clone), our next step will be to develop an adventitious shoot regeneration protocol from mature explants (e.g., leaves or internodes) based on the procedures described in this study. The expected results should be very useful for black alder genetic improvement (in vitro selection or genetic transformation).
26List of abbreviations
27BA: 6-benzylaminopurine
28CN: cotyledonary nodes
29Cot: cotyledons
30Exp.: experiment
31Hyp: hypocotyl segments
32mWPM: modified Woody Plant Medium
33TDZ: thidiazuron
342iP: 6-(γ,γ-dimethylallylamino)-purine
35Acknowledgements
36This work was supported by the European Regional Development Fund under the Interreg IV-A “Greater Region” (Wallonia-Lorrain-Luxembourg) programme and by the Public Service of Wallonia (SPW) of Belgium.
Bibliographie
Abras S. et al., 2005. Phytosanitary monitoring of woody species from the banks of watercourses in Wallonia. Parasitica, 61, 69-80.
Ayan S. et al., 2006. The vegetative propagation possibilities of black alder (Alnus glutinosa subsp. barbata [C. A. Mey.] Yalt.) by softwood cuttings. Pak. J. Biol. Sci., 9, 238-242.
Bajji M. & Druart P., 2012. Protocol development for in vitro assessment of cadmium tolerance in black alder and basket willow at the callus and whole plant levels. Acta Hortic., 961, 123-131.
Barrueto Cid L.P., Machado A.C.M.G., Carvalheira S.B.R.C. & Brasileiro A.C.M., 1999. Plant regeneration from seedling explants of Eucalyptus grandis x E. urophylla. Plant Cell Tissue Organ Cult., 56, 17-23.
Bates S. et al., 1992. Thidiazuron stimulates shoot organogenesis and somatic embryogenesis in white ash (Fraxinus americana L.). Plant Cell Tissue Organ Cult., 31, 21-29.
Brown D.C.W. & Thorpe T.A., 1995. Crop improvement through tissue culture. World J. Microbiol. Biotechnol., 11, 409-415.
Claessens H., Oosterbaan A., Savill P. & Rondeux J., 2010. A review of the characteristics of black alder (Alnus glutinosa (L.) Gaertn.) and their implications for silvicultural practices. Forestry, 83, 163-175.
Debruxelles N., Claessens H., Lejeune P. & Rondeux J., 2009. Design of a watercourse and riparian strip monitoring system for environmental management. Environ. Monit. Assess., 156, 435-450.
Distabanjong K. & Geneve R.L., 1997. Multiple shoot formation from cotyledonary node segments of Eastern redbud. Plant Cell Tissue Organ Cult., 47, 247-254.
Douglas G. & McNamara J., 2000. Shoot regeneration from seedling explants of Acacia mangium Willd. In Vitro Cell. Dev. Biol. Plant, 36, 412-415.
Du N. & Pijut P.M., 2008. Regeneration of plants from Fraxinus pennsylvanica hypocotyls and cotyledons. Sci. Hortic., 118, 74-79.
Gahan P.B. & George E.F., 2008. Adventitious regeneration. In: George E.F., Hall M.A. & de Klerk G.-J., eds. Plant propagation by tissue culture. 1: the background. 3rd ed. Dordrecht, The Netherlands: Springer, 355-401.
Giri C.C., Shyamkumar B. & Anjaneyulu C., 2004. Progress in tissue culture, genetic transformation and applications of biotechnology to trees: an overview. Trees, 18, 115-135.
Humánez A. et al., 2011. Thidiazuron enhances axillary and adventitious shoot proliferation in juvenile explants of Mediterranean provenances of maritime pine Pinus pinaster. In Vitro Cell. Dev. Biol. Plant, 47, 569-577.
Lall S., Mandegaran Z. & Roberts A.V., 2005. Shoot multiplication in cultures of mature Alnus glutinosa. Plant Cell Tissue Organ Cult., 83, 347-350.
Liu G., Huang J., Chen L. & Bao M., 2002. Plant regeneration from excised hypocotyl explants of Platanus acerifolia Willd. In Vitro Cell. Dev. Biol. Plant, 38, 558-563.
Lloyd G.B. & McCown B.H., 1980. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc. Int. Plant Propagators Soc., 30, 421-437.
Marques N.T., Nolasco G.B. & Leitão J.P., 2011. Factors affecting in vitro adventitious shoot formation on internode explants of Citrus aurantium L. cv. Brazilian. Sci. Hortic., 129, 176-182.
Martin B. & Guillot J., 1982. Quelques essais de bouturage de l’aulne. Rev. For. Fr., 34, 381-391.
Murthy B.N.S., Murch S.J. & Saxena P.K., 1998. Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell. Dev. Biol. Plant, 34, 267-275.
Nandwani D., 1995. In vitro micropropagation of a tree legume adapted to arid lands Acacia tortilis subsp. raddiana. Ann. Sci. For., 52, 183-189.
Nas M.N., Bolek Y. & Sevgin N., 2010. The effects of explant and cytokinin type on regeneration of Prunus microcarpa. Sci. Hortic., 126, 88-94.
Quoirin M., Bittencourt J.M., Zanette F. & de Oliveira D.E., 1998. Effect of growth regulators on indirect organogenesis of Acacia mearnsii tissues cultured in vitro. Rev. Bras. Fisiol. Veg., 10, 101-105.
Rai M.K. et al., 2011. Developing stress tolerant plants through in vitro selection – An overview of the recent progress. Environ. Exp. Bot., 71, 189-198.
Roy S., Khasa D.P. & Greer C.W., 2007. Combining alders, frankiae, and mycorrhizae for the revegetation and remediation of contaminated ecosystems. Can. J. Bot., 85, 237-251.
Sanatombi K. & Sharma G.J., 2008. In vitro plant regeneration in six cultivars of Capsicum spp. using different explants. Biol. Plant., 52, 141-145.
Tremblay F. & Lalonde M., 1984. Requirements for in vitro propagation of seven nitrogen-fixing Alnus species. Plant Cell Tissue Organ Cult., 3, 189-199.
Van Staden J., Zazimalova E. & George E.F., 2008. Plant growth regulators. II: cytokinins, their analogues and antagonists. In: George E.F., Hall M.A. & De Klerk G.-J., eds. Plant propagation by tissue culture. 1: the background. 3rd ed. Dordrecht, The Netherlands: Springer, 205-226.
Webber J., Gibbs J. & Hendry S., 2004. Phytophthora disease of alder. Edinburgh: Forestry Commission.
Pour citer cet article
A propos de : Mohammed Bajji
Wallon Agricultural Research Centre (CRA-W). Life Sciences Department. Bioengineering Unit. Building Jean-Baptiste de La Quintinie. Chaussée de Charleroi, 234. B-5030 Gembloux (Belgium). E-mail: m.bajji@cra.wallonie.be
A propos de : Caroline Thunissen
Wallon Agricultural Research Centre (CRA-W). Life Sciences Department. Bioengineering Unit. Building Jean-Baptiste de La Quintinie. Chaussée de Charleroi, 234. B-5030 Gembloux (Belgium).
A propos de : Philippe Druart
Wallon Agricultural Research Centre (CRA-W). Life Sciences Department. Bioengineering Unit. Building Jean-Baptiste de La Quintinie. Chaussée de Charleroi, 234. B-5030 Gembloux (Belgium).






