Influence of sugar beet pulp on feeding behavior, growth performance, carcass quality and gut health of fattening pigs
Received on June 24, 2014; accepted on December 4, 2014
Résumé
Impact des pulpes de betteraves sur le comportement alimentaire, les performances de croissance, la qualité de carcasse et la santé du tube digestif des porcs charcutiers
Description du sujet. Les fibres alimentaires sont largement utilisées en production porcine, mais des effets contradictoires sur les performances et la santé apparaissent dans la littérature.
Objectifs. Cet article vise à clarifier les effets d’un aliment riche en fibres sur le comportement, la croissance, la qualité de carcasse et la santé digestive de porcs charcutiers.
Méthode. Deux lots de 24 porcs ont été divisés en deux groupes recevant soit un aliment standard à base de céréales (STD, 19% glucides non-amylacés), soit un aliment riche en fibres avec 23 % de pulpes de betteraves (HFD, 31% glucides non-amylacés).
Résultats. Le taux d’activité et la durée d’occupation des mangeoires ont augmenté respectivement de 57 % et 165 % avec HFD (P < 0,05). Le comptage des bactéries fécales a montré avec HFD une augmentation des Lactobacillus (10,21 vs 9,84 log10 de cfu·g-1 de matière sèche, P < 0,05) et Bifidobacterium (9,49 vs 8,88, P < 0,01), mais une diminution des Enterobacteriaceae (4,85 vs 5,97, P < 0,001). Le gain quotidien moyen et le rendement à l’abattage ont été réduits avec HFD (788 vs 876 g par jour et 75,7 vs 78,9 %, P < 0,001). Le score de lésion gastrique a diminué avec HFD (0,82 vs 1,55, P < 0,05). La surface relative occupée par des cellules caliciformes a été plus élevée dans le jéjunum (10,06 vs 7,99 %, P < 0,01) et le nombre de lymphocytes CD3 (1,24 vs 0,90·mm-², P < 0,05) plus élevé dans le colon avec HFD.
Conclusions. L’aliment HFD contribue à renforcer la santé du tube digestif de porcs charcutiers, mais détériore les paramètres de croissance et de carcasse.
Abstract
Description of the subject. Dietary fiber is largely used in pig production but some contradictions appear in the literature regarding the effects on performance and health.
Objectives. This paper aims to contribute to the clarification of the effects of a diet rich in sugar beet pulp on animal behavior, growth performance, carcass quality and gut health of fattening pigs.
Method. Two successive batches of 24 fattening pigs were each divided into two groups fed ad libitum either a standard diet based on cereals (STD, 19% NSP [non-starch-polysaccharides]) or a fibrous diet based on 23% sugar beet pulp (HFD, 31% NSP).
Results. Pigs activity rate and feeder occupancy duration were increased by 57% and 165% for group fed HFD, respectively (P < 0.05). The fecal bacteria counts showed increases with HFD for Lactobacillus (10.21 vs 9.84 log10 of cfu·g-1 of feces dry matter, P < 0.05) and Bifidobacterium (9.49 vs 8.88, P < 0.01) but decreases for Enterobacteriaceae (4.85 vs 5.97, P < 0.001). Reductions of the average daily gain (788 vs 876 g per day, P < 0.001) and the dressing percentage (75.7 vs 78.9%, P < 0.001) were observed with HFD. Gastric lesion score was decreased with HFD (0.82 vs 1.55, P < 0.05). For pigs fed HFD, the proportion of surface area occupied by goblet cells was increased in the jejunum (10.06 vs 7.99%, P < 0.01) and the number of CD3 lymphocytes was increased in the colon (1.24 vs 0.90·mm-², P < 0.05).
Conclusions. HFD contributes to strengthen the gut health of fattening pigs, but it impairs growth performance and carcass traits.
1. Introduction
1Dietary fiber in pig production has several advantages. It is a relatively cheap raw material, especially since the availability of numerous co-products from the agro-alimentary and biofuel industries. Fiber is also known to reduce stereotyped and aggressive behaviors, and to lower ammonia emissions from slurry (Philippe et al., 2008). Fiber impacts on pig growth and health are more contradictory. Intestinal fermentation of dietary fibers like sugar beet pulp are known to promote multiplication of bacterial populations such as Lactobacillus and Bifidobacterium that are usually considered beneficial to gut health, partly by preventing colonization of opportunist pathogens (Gibson et al., 1995; Bilic et al., 2003; Konstantinov et al., 2004). A lower risk of gastric ulcers has also been observed, as well as improved colonic mucosal integrity (Dirkzwager et al., 1998; Nofrarias et al., 2007; Millet et al., 2010). All these things help to strengthen the digestive health and protect against diarrhea (Bilic et al., 2003). Promoting gastrointestinal tract health is therefore expected to result in performance improvements. Conversely, more instances of diarrhea and colitis are sometimes reported with weaned piglets, for example in cases of soluble fiber supplementation which increases the viscosity of the digesta (Pluske et al., 2002). Some authors have noted deterioration in performance as a result of energy dilution of fibrous diets, faster intestinal transit and reduced ingestion due to early satiety (Anguita et al., 2007). Like for gut health, effects on performance can be variable according to the origin and type of fibre (Anguita et al., 2007).
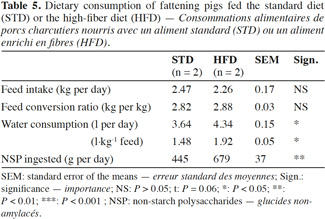
2Numerous experiments dealt with the effects of fibrous diet on performance and health, but many controversial findings were reported in the literature. Moreover, few of the former studies combined several types of impacts related to dietary fibers for pigs. Thus, this research aims to contribute to the understanding of the effects of sugar beet pulp on growth performance, behavior, carcass quality, stomach health, intestinal microbiota and the histomorphology of the gastrointestinal tract in fattening pigs.
2. Materials and methods
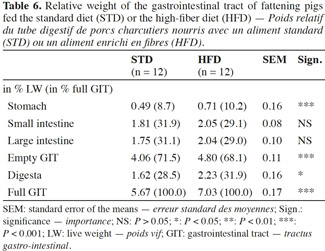
3The protocol was approved by the ethics committee for animal use and care of the University of Liege, Belgium, in compliance with Directive 2010/63/EU of the European Parliament and of the Council of the European Union.
2.1. Animals, diet and housing conditions
4Two successive batches of 24 Piétrain x Landrace fattening pigs were randomly divided into two groups of 12 animals according to age, body weight and sex (sex ratio 1:1), and housed separately in two identical pens (volume of 103 m3 and surface of 30 m2). The males were castrated before the age of 7 days. The age at the beginning of the fattening period was 95 and 88 days for the two successive batches, respectively. The pigs were fed ad libitum with the same diet per group during the whole fattening period and received either a standard diet (STD) based on cereals with 19.0% non-starch polysaccharides (NSP), or a high-fiber diet (HFD) based on sugar beet pulp with 31.4% NSP (Table 1). The diets were formulated by a commercial company, with concerns to balance the energy and the protein content despite dilution due to the dietary fiber. According to the InraPorc program® (INRA, 2006), the crude protein content was 16.0 and 15.6% for STD and HFD, respectively. The net energy content was 8.75 and 8.07 MJ·kg-1, respectively. Assignment of the groups (STD or HFD) to the pens was interchanged for replication. The floors of the pens were fully slatted. The available area was 9 m2, or 0.75 m2 per pig. A controlled ventilation system was used in order to maintain similar bioclimatic conditions in both pens (air temperature, ventilation rate and relative humidity). Feeding equipment was composed of two single-spaced feeders per pen with an integrated watering nipple. Feed intakes were weighted for each pen. Meters (Wateau®, EEC approval n°B02 314.29) were used to determine the water consumption per pen. The pigs were fattened to a body weight of about 110 kg. The date of slaughter was determined taking into account regular weighings of the pigs and estimation of the growth curve. The pigs were fasted 18 h before slaughter.
2.2. Activity rate and feeding behavior
5A video camera was set up in each room to film the whole pen. Recordings were made in the fourth, eighth and thirteenth week of fattening and during two days (2 × 24 h) each week. Every five minutes a frame was frozen and the posture of the pigs (sitting/standing or lying) and occupancy of the two feeders were noted. The data were processed to determine the animals’ activity rates and daily feeding durations. The activity rate for a given period was determined per pen and was defined as the ratio of the number of animals standing or sitting to the total number of animals. The average daily feeding duration was determined per pen and was estimated by multiplying the daily occupancy rate by the duration of a day, assuming occupancy of the feeder is devoted to feeding behavior. The average ingestion rate was determined by pen and was calculated by dividing the feed intake by the feeding duration. The data were expressed per pig by dividing the values by the number of animals in the pen.
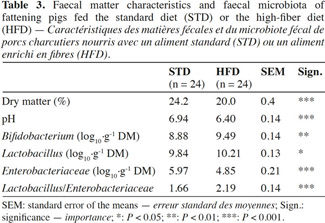
2.3. Fecal matter characteristics and fecal microbiota
6At the end of fattening, the day before slaughter, fecal matter (FM) was taken from each pig’s rectal outlet. Measurements of pH, dry matter (by desiccation at 105 °C) and microbiota count (Enterobacteriaceae, Lactobacillus and Bifidobacterium) were made on each sample. The culture media and incubation conditions were performed in accordance with protocols of Owusu-Asiedu et al. (2006) for Enterobacteriaceae, and Dave et al. (1996) for Lactobacillus and Bifidobacterium.
7The results are expressed as log10 cfu·g-1 DM (dry matter).
2.4. Zootechnical parameters and carcass traits
8The pigs were weighed individually at the start and at the end of the fattening (no fasting time before weighing). The second batch underwent intermediate weighing after eight weeks of fattening. Ingestion parameters (feed intake, water intake and feed conversion ratio) were determined for each group. The carcass traits were measured individually at the slaughterhouse and included hot carcass weight, dressing percentage (calculated as ratio between live weight and hot carcass weight), and muscle depth (M2 site), backfat depth (G2 site) and lean meat percentage measured between the 3rd and 4th last ribs, 6 cm off, parallel to the midline by Capteur Gras Maigre (CGM, Sydel, France).
2.5. Weight and histomorphology of the gastrointestinal tract
9The gastrointestinal tracts were collected and identified at the slaughterhouse. The weight of the gastrointestinal tract was determined before and after emptying. After emptying, the different portions of the gastrointestinal tract (stomach, small intestine and caecum/colon) were weighed separately. To assess the incidence of gastric ulcers the pars oesophagea was scored for lesions on a scale of 0 (intact) to 3 (ulcerated), adapted from the Pig Health Monitoring Service (1989) and described in table 2. From each gastrointestinal tract four samples of intestine (1 cm x 5 cm) were taken, two from the jejunum (200 cm from the ileocaecal junction) and two from the proximal colon (30 cm from the ileocaecal junction). Tissue samples were fixed in formol 10%, dehydrated and embedded in paraffin wax, sectioned at 5 μm, and stained with Alcian blue for mucous detection according to routine methods (Kiernan, 1990). A total of 5 intact, well-oriented, crypt-villus units were selected for each intestinal sample (10 crypt-villus units per pig and per intestinal section). The sections were scanned, digitalized using an imaging system for virtual microscopy (Dotslide, Olympus, Belgium), and analyzed with a Java image morphometric processing program (Image J software, National Institute of Health, USA). Morphological measurements, as the thickness of the different layers of the small and large intestinal wall, villus height, and crypt depth, were performed. The proportion of surface area occupied by epithelial goblet cells was evaluated and the number of T lymphocytes localized in the epithelium and in the lamina propria were determined. Briefly, sections were deparaffinised, rehydrated and treated in a Tris-EDTA buffer for 3 × 5 min in the microwave oven, a procedure for the retrieval of antigens. Sections were rehydrated and incubated for 1 h at room temperature with a rabbit polyclonal anti-human CD3 antibody. A secondary conjugated species-specific immunoglobulin peroxidase-labelled polymer (Amplification EnVision System-horseradish peroxidase [HRP]; Dako, Glostrup, Denmark) was applied for 30 min at room temperature. Peroxidase activity was revealed with 9-ethyl-3-aminocarbazole (Zymed, San Francisco, California, USA) combined with H2O2 as substrate. For each parameter, five measurements were made per sample except for the lymphocyte count (one measurement per sample). The histomorphological study was performed under blind conditions, and only on the pigs of the second fattening batch.
2.6. Statistical analyses
10For the behavioral parameters (with group as the experimental unit), the data were tested in the form of a mixed model for repeated measurements, including the fixed effects of the diet (1 df), the week of measurement (2 df), the interaction between the diet and the week of measurement (2 df) and the batch as random effect, with 48 (24 h x 2 d) successive measurements per week. The correlation between measurements was modelled using a type 1-autoregressive structure.
11For zootechnical performance and fecal matter characteristics (with pig as the experimental unit), the data were tested in the form of a mixed model, including the fixed effects of the diet (1 df), the sex (1 df), the interaction between the diet and the sex (1 df), and the random effects of the batch and the pig nested within interaction of the diet, the sex and the batch.
12For consumption parameters (feed intake, water intake and feed conversion ratio, with group as the experimental unit), the data were tested in the form of a mixed model, including the fixed effect of the diet (1 df) and the random effect of the batch.
13For the histomorphological study (with pig as the experimental unit), the data were tested in the form of a mixed model, including the fixed effects of the diet (1 df), the sex (1 df), the interaction between the diet and the sex (1 df), and the random effect of the pig nested within interaction of the diet and the sex.
14All statistical analyses were performed using SAS software (SAS Institute Inc., Cary, North Carolina, USA). The presented values are the least square means.
3. Results
3.1. Activity rate and feeding behavior
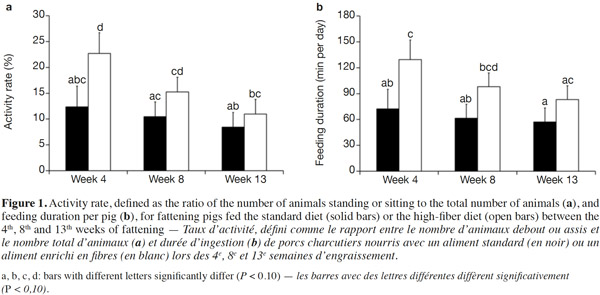
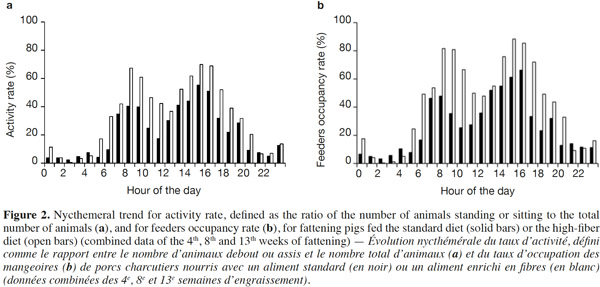
15Because of a technical glitch the data from the first batch are missing for week 4. Both activity rate and feeder occupancy duration were significantly higher for group fed HFD compared with STD with 16.3 vs 10.4% (P < 0.05) and 103.4 vs 63.6 min per day per pig (P < 0.01), respectively. The ingestion rate was 44% lower with HFD (21.8 vs 38.8 g·min-1, P = 0.09). The evolution of the activity rate and feeding duration throughout the fattening period (Figure 1) shows that the differences between the two types of diet were more marked at the start of fattening, then lessened over time. For pigs fed HFD, activity rate and feeding duration decreased in the course of time while for pigs fed STD, these were more stable. The nycthemeral trend showed a higher activity level in the diurnal period (7 h-19 h) compared with the nocturnal period (19 h-7 h) for both diets (Figure 2a): 18.0% vs 4.1% on the standard diet and 26.7 vs 6.4% on the fiber diet. Activity peaked twice during the day, once around 8 h and again around 15 h. The feeder occupancy rate (Figure 2b) followed the same nycthemeral trend as the activity rate.
3.2. Fecal matter characteristics and fecal microbiota
16In the case of HFD, a reduction in the fecal dry matter content was observed (20.0 vs 24.2%; P < 0.001) as well as a lower pH value (6.40 vs 6.94; P < 0.001) (Table 3). As regards the fecal microbiota, HFD resulted in an increase in Bifidobacterium and Lactobacillus counts (9.49 vs 8.88 log10·g-1 DM; P < 0.01 and 10.21 vs 9.84 log10·g-1 DM; P < 0.05) along with a reduction in the Enterobacteriaceae count (4.85 vs 5.97 log10·g-1 DM; P < 0.001).
3.3. Zootechnical parameters and carcass traits
17The pigs fed HFD had a significantly lower growth rate, with an average daily gain (ADG) reduced by more than 10% compared with STD (Table 4). This result was associated with a reduction of 9% in the quantity of feed ingested and an increase of 2% in the feed conversion ratio (FCR) (Table 5). However, these two ingestion parameters, measured per group, were statistically similar for both diets, contrarily to water consumption that was significantly increased by 19% with HFD (P < 0.05). The intermediate weighing in the second batch showed that pigs fed HFD grew more slowly in the first phase of fattening (from day 0 to day 57) compared with STD (ADG: 676 vs 757 g·d-1; P = 0.08), but no significant differences were observed in the second phase of fattening (up to day 90), with an ADG of 850 g·d-1 for both groups.
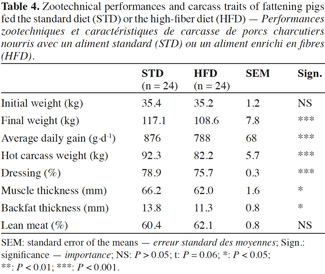
18With HFD, the slaughtering data showed a drop in carcass weight of 11% along with a lower dressing percentage. Since a reduction in muscle thickness of 6.5% was associated with a reduction in backfat thickness of 18% for HFD pigs, the lean meat percentage was similar for both diets, around 61.3%.
3.4. Weight and histomorphology of the gastrointestinal tract
19In the pigs fed HFD, all the digestive compartments and the contents were heavier compared with the pigs fed STD (Table 6). The relative weight (expressed as % of live weight) of the gastrointestinal tract (GIT) and the digesta were 18% and 38% higher with HFD compared with STD, respectively. The section of greatest difference was the stomach (+ 45%). The weight of the small and large intestines was not significantly affected by the dietary fibre content (P > 0.05). The empty GIT represented 4.80% of the total live weight with HFD against 4.06% with STD. The stomach accounted for 0.71% and 0.49% of the total live weight, respectively.
20The total jejunum wall tended to be thicker in the pigs fed HFD compared with STD (1,282 vs 1,422 µm; P < 0.10) (Table 7). This is mainly due to the greater thickness of the muscularis (+ 106 µm). The submucosa, the Muscularis mucosae and the mucosa showed no significant differences between the two diets, the average thickness being 167, 26 and 751 µm respectively. Likewise, the crypt depths and villus heights were identical in the two groups at about 230 and 414 µm, respectively. Within a defined zone, the goblet cells covered a larger area in the case of the fiber diet (10.06 vs 7.99%; P < 0.01). The number of CD3 lymphocytes did not differ significantly between diets, at about 1.19 lymphocytes per mm2.
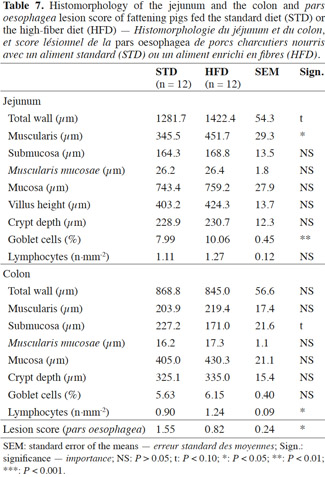
21In the colon, the submucosa tended to be less thick with HFD (171.0 vs 227.2 µm; P < 0.10). The total wall thickness and the thickness of the other colon layers did not differ significantly according to the dietary fiber content. Likewise, the crypt depths and the proportion of goblet cells were similar for the two diets at 330 µm and 5.9% of the area covered by goblet cells, respectively. The lymphocyte count was significantly higher in the pigs on the fiber diet (1.24 vs 0.90; P < 0.05).
22In regards to gastric ulcers, a significant reduction in the pars oesophagea lesion score was noted with the fiber diet (0.82 vs 1.55; P < 0.05).
4. Discussion
23The slower growth rate of the pigs fed HFD was associated to the higher FCR and lower feed consumption. The difference was more pronounced during the growing phase, whereas during the finishing phase the ADG remained the same for both treatments. Other studies have also reported a decline in performance, generally limited to the growing period (Anguita et al., 2007; Bruininx et al., 2009). For instance, Bruininx et al. (2009) showed that introducing 10% sugar beet pulp into the diet increased the FCR during the growing period (from 25 to 45 kg) but not during finishing (from 45 to 110 kg). However, a dose effect was noted since introducing 5% sugar beet pulp improved the FCR in both the growing and finishing phases. Other studies have also concluded that a high-fiber diet has no adverse effects on pig performance either at the growing or finishing stages although NE content was diluted due to introduction of fibrous feedstuff (Lizardo et al., 1997; Galassi et al., 2010). With equal NE contents, Millet et al. (2010) obtained no significant differences in feed consumption, ADG or FCR whereas up to 20% sugar beet pulp was introduced into the feed of pigs fattened from 20 to 105 kg. An optimum would therefore need to be found in regards to the ration of fiber content, which depends on the fiber source, the age of the pigs and their ability to adapt to a high-fiber diet.
24Former experiments about feeding behavior have shown that fattening pigs could compensate energy dilution of fibrous diets by spending more time eating with similar feed intake, whatever the fiber content (Schrama et al., 1998; Kallabis et al., 2012). Moreover, the increased eating time and lower energetic utilization of fibrous diet can be counterbalanced by the pigs by reducing their physical activity, as a potential consequence of better satiety (Schrama et al., 1998). These findings are in opposition to the results of the present study. Indeed, the increase in the feeding duration (+ 60%) was associated with the reduction in the quantity of feed consumed (- 9%), resulting in a lower feeding rate (defined as the ratio between feed intake and feeding duration). Moreover, the activity rate of HFD groups was also increased (16.3 vs 10.4%). This can be partly explained by the higher feeding duration that corresponds to 4.4 and 7.2% of the time for STD and HFD, respectively. Similar nycthemeral profiles of activity and feeders occupancy rates (Figure 2) illustrate the fact that standing activity is highly devoted to feeding behavior. Standing/sitting pigs that are waiting around the feeder for their meal also contribute to total activity. Besides, increased feeding duration with fibrous diets has to be considered to determine the number of feeders per pen. In this experiment, the daily feeders occupancy rate was around 43% in the HFD pen but higher than 75% during 6 h of the day. Thus, increasing the number of pigs per feeder should be avoided to prevent a greater waiting time before eating and potential aggressive behaviors between pigs.
25The lower feeding rate could be attributed to lower palatability of fiber diets (Philippe et al., 2008). Double choice tests have shown the animals to display a marked preference for cereal-based diet rather than high-fiber diet (Sola-Oriol et al., 2011). Another reason for the drop in feeding motivation may be that the animal is more quickly satiated on a fiber diet (Philippe et al., 2008). For the same energy supply, high-fiber diets fill greater volume and are generally coarser with a high water retention capacity. Thus, it increases the time spent on picking up and chewing, stimulates digestive secretions, promotes repletion by gastro-intestinal distension and delay feedback mechanisms that regulate feed intake (Anguita et al., 2007). However, the impact on feeding motivation could be transitory. Indeed, the effect on palatability can change over time, with the initial aversion to high-fiber diets possibly being merely temporary. Moreover, metabolic adaptation mechanisms and the increased capacity of the GIT are thought to progressively increase voluntary intake. In this study, the weight of the digestive tract and its contents was significantly higher with HFD. Previous studies showed that the effect is usually most pronounced in the stomach and colon, and appears to be little effect on the small intestine (Libao-Mercado et al., 2007; Hermes et al., 2009; Montagne et al., 2014). In the present experiment, whereas the weight of the rest of the GIT was not significantly affected by the fiber content, the relative stomach weight increased by 45% compared with the standard diet. Despite this increase, the global feed intake was not greater in the pigs fed HFD. Unfortunately, the experimental design did not allow to examine the intake trend over time. But similar ADG measured for both groups at the end of the fattening stage reflected the adaptation by the GIT to the high-fiber diet. The lower proportional weight of the GIT observed with STD could also reflect the decrease of the relative contribution of viscera to whole body weight with pig’s growth, as reported by Barea et al. (2006). Indeed, the body weight at slaughter of STD pigs was higher than HFD pigs as a result of faster growth rate. This contributes to explain the lower relative weight of the GIT.
26The drop in growth performance with HFD may also be attributed to the reduced net energy intake (- 16%). Moreover, decline in nutrient digestibility was frequently observed with rations rich in sugar beet pulp regarding dry matter, organic matter, crude proteins, fat and energy (Zhang et al., 2013). Increased mucus secretion accelerates the transit, reduces the accessibility of digestive enzymes and increases endogenous losses (Libao-Mercado et al., 2007). This may be partly offset by changes in the size and morphology of the gastrointestinal tract. Other factors such as the fiber lignification rate, fiber solubility, interaction with other nutrients, feeding level, live weight and the animals’ physiological state can also impact upon the digestibility of the various feed components (Le Goff et al., 2002).
27With regard to slaughtering data, literature seems to indicate that the dietary fiber content has little effect on most carcass traits (Magistrelli et al., 2009; Galassi et al., 2010; Smit et al., 2014). The only result reported in various studies is a drop in the dressing percentage (Lizardo et al., 1997; Bruininx et al., 2009; Magistrelli et al., 2009). The greater weight of the gastrointestinal tract and its contents is put forward as an explanation for this result (Lizardo et al., 1997; Magistrelli et al., 2009). The reduced body weight at slaughter, as this is the case for HFD pigs, could also explain the lower dressing percentage as reflecting the evolution of the metabolic activity in regards to the pigs’ age (Correa et al., 2006; Cerisuelo et al., 2010). The difference in carcass weight could also explain the difference in carcass composition. Indeed, authors have reported that higher carcass weight due to faster growth rate is associated with increased fat deposition and lower lean deposition (Correa et al., 2006; Cerisuelo et al., 2010; Trefan et al., 2013).
28Water content of the feces was higher with HFD, as a result of the presence of undigested fiber in the feces, the lower digestibility of other nutrients and the fiber’s water retention capacity. The lower pH of feces measured with HFD was explained by the higher volatile fatty acid production associated with fiber fermentation in the colon (Anguita et al., 2007; Lynch et al., 2008; Hermes et al., 2009; Ivarsson et al., 2014). With feed containing 8% sugar beet pulp, Anguita et al. (2007) showed a drop in rectal pH from 6.30 to 6.11 and an increase in colonic volatile fatty acid (VFA) from 107 to 119 μmol·g-1 digesta, compared with lower fiber content feed. Nevertheless, it should be noted that the pH value is not directly linked to VFA production. Other parameters such as ion absorption, buffering capacity of the digesta and the use of acids in the intestine also affect this value (Pluske et al., 2002). Anyway, a lower pH has proved advantageous in regards to digestive tract health, by creating unfavorable conditions for certain pathogens like spirochetes and specifically Brachyspira hyodysenteriae, which causes swine dysentery (Bilic et al., 2003).
29Regarding fecal microbiota, HFD was associated with increased numbers of Bifidobacterium and Lactobacillus, considered beneficial to GIT health, along with a reduction in the number of Enterobacteriaceae, comprising several potential GIT pathogens (Gibson et al., 1995). The differences were statistically significant but the absolute values differed only by about 1 log10. Thus, the results have to be taken cautiously. Nevertheless, it confirms former studies. Indeed, previous experiments showed that the addition of sugar beet pulp to the diet was found to increase the Lactobacillus population and reduce the coliform population (Konstantinov et al., 2004; Hermes et al., 2009). These effects help to stabilize the intestinal microbiota by increasing the resistance to colonization by pathogenic bacteria. Thus, high-fiber diets provide greater protection against diarrhea (Bilic et al., 2003). Conversely, the addition of highly fermentable, soluble, viscous fibers such as guar gum is considered to stimulate the proliferation of pathogenic strains of Escherichia coli, or the occurrence of swine dysentery (Thomsen et al., 2007; Wellock et al., 2008). The type of fiber therefore plays an important role in the intestinal microbiota response. Moreover, the feed protein content also intervenes with the fiber with respect to bacterial populations since protein fermentation in the digestive tract can increase the risk of intestinal dysbiosis and pathogen proliferation (Hermes et al., 2009; Chen et al., 2014).
30The histomorphology of the digestive tract was also modified by introducing fiber into the diet. In our experiment, an increase in muscularis thickness and a higher goblet cell count in the jejunum were observed, along with an increase in the colon wall lymphocyte count. Crypt depth and villus height appeared unaffected by the fiber content. The literature reports contradictory results for the effects of fiber on these parameters. For example, some authors noted an increase in villus height and crypt depth (Libao-Mercado et al., 2007; Serena et al., 2007), whereas others reported a reduction (Pluske et al., 2002; Martinez-Puig et al., 2007). According to Hedemann et al. (2006) the degree of solubility of the fiber was thought to affect crypt and villus response, with insoluble fiber promoting growth. Insoluble fiber is also thought to stimulate mucus production. However, these statements have not been verified in every case (Libao-Mercado et al., 2007; Serena et al., 2007). The fermentation products, chiefly VFA, also impact directly upon epithelium morphology. For instance, butyrate is the preferred energy source for colonocytes. It contributes to epithelium maturation, inhibits apoptosis of the colon crypt cells, stimulates mucus production and limits lymphocyte proliferation in the epithelium (Martinez-Puig et al., 2007). Studies have shown sugar beet pulp fermentation to reduce butyrate formation and benefit acetate (Anguita et al., 2007; Lynch et al., 2008). Comparing two diets containing 0 and 20% sugar beet pulp, respectively, Lynch et al. (2008) observed an alteration of VFA proportion with a reduction of butyric acid level (13 vs 17%) and an increase of the acetic acid level (61 vs 54%). That serves to account for the higher lymphocyte count observed in the colon wall. Nofrarias et al. (2007) suggested that this increase in lymphocyte number could be linked to greater exposure to bacterial antigens, a sign of more diversified intestinal microbiota.
31The pars oesophagea lesion scores were significantly lower when pigs fed HFD, which is in agreement with literature. By adding 5% whole sunflower hulls, Dirkzwager et al. (1998) reduced the number and severity of oesophagogastric lesions. Likewise, the addition of sugar beet pulp reduced the incidence of lesions by 43% in fattening pigs (Bruininx et al., 2009). Fiber, being generally coarser, is thought to act by stabilizing the feed layers in the stomach, thus limiting digesta fluidity, reflux and contact between irritants (H+ and bile acids) and the pars oesophagea (Dirkzwager et al., 1998). Several studies have shown the need for a high-fiber diet to be combined with a coarser texture, as coarse feed with a low fiber content and conversely fine texture with high-fiber feed do not reduce the incidence of gastric lesions (Dirkzwager et al., 1998; Millet et al., 2010). Fiber is also thought to stimulate the stomach wall and the intensity of the blood flow through it, consequently limiting the transient ischemia involved in the aetiology of ulcers (Dirkzwager et al., 1998). Usually, the reduction of gastric lesions is associated with better performance since inflammation, pain and stress due to ulcers could reduce the feed intake (Dirkzwager et al., 1998). However, only severe gastric lesions seem to impair performance (Dirkzwager et al., 1998) whereas some authors did not report any reduction in growth rate despite severe gastric lesions (Guise et al., 1997; Nielsen et al., 2000). In our study the lower lesion score was not associated with improved performance. A similar contradictory relationship between lesion score and performance had already been observed (Mavromichalis et al., 2000). The interactions with digestive, metabolic and behavioral effects of dietary fiber, as discussed above, may explain this result.
32In conclusion, adding 23% sugar beet pulp to the diet of fattening pigs improved the health of the gastrointestinal tract by reducing gastric lesions and strengthening the intestines’ protective barrier against bacterial invasions. The lower pH of the digesta, the higher goblet cell count in the jejunum, the higher lymphocyte count in the colon and the greater stability of the intestinal microbiota created unfavorable conditions for the development of potential pathogen. This is substantiated by the increase of beneficial bacterial population (Lactobacillus and Bifidobacterium) concurrent with the reduction of potential pathogen populations (Enterobacteriaceae). This way, high fiber diet can be used as a mean to mitigate some digestive disorders, like swine dysentery. However, these beneficial health effects are counterbalanced by a downturn in growth performance and carcass traits, which may be accounted for by reduced feed consumption, lack of feed palatability, greater repletion of the gastrointestinal tract and lower feed digestibility. Feeding duration and activity rate were increased with HFD. Nevertheless, the negative effect on growth was found to be transitory and the stomach size was also found to be significantly increased by the end of fattening, both signs of the gastrointestinal tract adapting to coarser feed. In order to prevent the decline in performance while still preserving the beneficial effects on gastrointestinal tract health, lower fiber content or gradual incorporation rate could be considered and tested in further experiments.
Acknowledgements
33This study was financially supported by the Operational Directorate-General for Agriculture, Natural Resources and the Environment of the Public Service of Wallonia, and the Veepeiler-varken. The authors thank Edwin Dawans, Valery Delleur, Jean-Noël Duprez, Joëlle Piret and Mélanie Rulens for their technical collaboration. The article revision provided by David Broderick is also acknowledged.
Bibliographie
Anguita M. et al., 2007. Effect of coarse ground corn, sugar beet pulp and wheat bran on the voluntary intake and physicochemical characteristics of digesta of growing pigs. Livest. Sci., 107, 182-191.
Barea R. et al., 2006. Effects of dietary protein content and feeding level on carcass characteristics and organ weights of Iberian pigs growing between 50 and 100 kg live weight. Anim. Sci., 82, 405-413.
Bilic B. & Bilkei G., 2003. Effect of highly fermentable dietary fiber on pig performance in a large unit, infected with endemic swine dysentery. Acta Vet., 53, 229-238.
Bruininx E.M.A.M. et al., 2009. The addition of pressed sugar beet pulp to a dry diet for growing-finishing pigs. Rosmalen, The Netherlands: Proefstation voor de Varkenshouderij; Sterksel, The Netherlands : Varkensproefbedrijf “Zuid- en West-Nederland”.
Cerisuelo A. et al., 2010. The inclusion of ensiled citrus pulp in diets for growing pigs: effects on voluntary intake, growth performance, gut microbiology and meat quality. Livest. Sci., 134, 180-182.
Chen H. et al., 2014. Impact of fiber types on gut microbiota, gut environment and gut function in fattening pigs. Anim. Feed Sci. Technol., 195, 101-111.
Correa J. et al., 2006. Effects of slaughter weight on carcass composition and meat quality in pigs of two different growth rates. Meat Sci., 72, 91-99.
Dave R.I. & Shah N.P., 1996. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus, and bifidobacteria. J. Dairy Sci., 79, 1529-1536.
Dirkzwager A., Elbers A.R.W., Van Der Aar P.J. & Vos J.H., 1998. Effect of particle size and addition of sunflower hulls to diets on the occurrence of oesophagogastric lesions and performance in growing-finishing pigs. Livest. Prod. Sci., 56, 53-60.
GalassiG. et al., 2010. Effects of high fibre and low protein diets on performance, digestibility, nitrogen excretion and ammonia emission in the heavy pig. Anim. Feed Sci. Technol., 161, 140-148.
Gibson G.R. & Roberfroid M.B., 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr., 125, 1401-1412.
Guise H.J. et al., 1997. Gastric ulcers in finishing pigs: their prevalence and failure to influence growth rate. Vet. Rec., 141, 563-566.
Hedemann M.S. et al., 2006. Intestinal morphology and enzymatic activity in newly weaned pigs fed contrasting fiber concentrations and fiber properties. J. Anim. Sci., 84, 1375-1386.
Hermes R.G. et al., 2009. Effect of dietary level of protein and fiber on the productive performance and health status of piglets. J. Anim. Sci., 87, 3569-3577.
INRA, 2006. InraPorc®, a decision support tool for pig nutrition, http://w3.rennes.inra.fr/inraporc/index_en.html, (13/01/2014).
Ivarsson E., Roos S., Liu H.Y. & Lindberg J.E., 2014. Fermentable non-starch polysaccharides increases the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs. Animal, 8, 1777-1787.
Kallabis K.E. & Kaufmann O., 2012. Effect of a high-fibre diet on the feeding behaviour of fattening pigs. Archiv Tierzucht., 55, 272-284.
Kiernan J.A., 1990. Histological and histochemical methods. Theory and practice. Oxford, UK: Pergamon Press.
Konstantinov S.R. et al., 2004. Specific response of a novel and abundant Lactobacillus amylovorus-like phylotype to dietary prebiotics in the guts of weaning piglets. Appl. Environ. Microbiol., 70, 3821-3830.
Le Goff G. et al., 2002. Digestibility and metabolic utilisation of dietary energy in adult sows: influence of addition and origin of dietary fibre. Br. J. Nutr., 87, 325-335.
Libao-Mercado A.J. et al., 2007. Effect of feeding fermentable fiber on synthesis of total and mucosal protein in the intestine of the growing pig. Livest. Sci., 109, 125-128.
Lizardo R., Peiniau J., Lebreton K. & Aumaitre A., 1997. Consequences of the inclusion of sugar beet pulp in diets for early weaned and growing pigs. Ann. Zootech., 46, 281-294.
Lynch M.B. et al., 2008. Effect of crude protein concentration and sugar-beet pulp on nutrient digestibility, nitrogen excretion, intestinal fermentation and manure ammonia and odour emissions from finisher pigs. Animal, 2, 425-434.
Magistrelli D., Galassi G., Crovetto G.M. & Rosi F., 2009. Influence of high levels of beet pulp in the diet on endocrine/metabolic traits, slaughter dressing percentage and ham quality in Italian heavy pigs. Ital. J. Anim. Sci., 8, 37-49.
Martínez-Puig D. et al., 2007. Long-term effects on the digestive tract of feeding large amounts of resistant starch: a study in pigs. J. Sci. Food Agric., 87, 1991-1999.
Mavromichalis I. et al., 2000. Enzyme supplementation and particle size of wheat in diets for nursery and finishing pigs. J. Anim. Sci., 78, 3086-3095.
Millet S. et al., 2010. Effect of grinding intensity and crude fibre content of the feed on growth performance and gastric mucosa integrity of growing-finishing pigs. Livest. Sci., 134, 152-154.
Montagne L. et al., 2014. Difference in short-term responses to a high-fiber diet in pigs divergently selected for residual feed intake. J. Anim. Sci., 92, 1512-1523.
Nielsen E.K. & Ingvartsen K.L., 2000. Effects of cereal disintegration method, feeding method and straw as bedding on stomach characteristics including ulcers and performance in growing pigs. Acta Agric. Scand. Sect. A, 50, 30-38.
Nofrarias M. et al., 2007. Long-term intake of resistant starch improves colonic mucosal integrity and reduces gut apoptosis and blood immune cells. Nutrition, 23, 861-870.
Owusu-Asiedu A. et al., 2006. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci., 84, 843-852.
Philippe F.-X. et al., 2008. Food fibers in gestating sows: effects on nutrition, behaviour, performances and waste in the environment. INRA Prod. Anim., 21, 277-290.
Pig Health Monitoring Service, 1989. A guide to abattoir procedures. Queensland, Australia: Department of Primary Industries.
Pluske J.R., Pethick D.W., Hopwood D.E. & Hampson D.J., 2002. Nutritional influences on some major enteric bacterial diseases of pigs. Nutr. Res. Rev., 15, 333-371.
Schrama J.W. et al., 1998. The energetic value of nonstarch polysaccharides in relation to physical activity in group-housed growing pigs. J. Anim. Sci., 76, 3016-3023.
Serena A., Hedemann M.S. & Bach-Knudsen K.E., 2007. Feeding high fibre diets changes luminal environment and morphology in the intestine of sows. Livest. Sci., 109, 115-117.
Smit M.N. et al., 2014. Feeding increasing inclusions of canola meal with distillers dried grains and solubles to growing-finishing barrows and gilts. Anim. Feed Sci. Technol., 189, 107-116.
Sola-Oriol D., Roura E. & Torrallardona D., 2011. Feed preference in pigs: effect of selected protein, fat, and fiber sources at different inclusion rates. J. Anim. Sci., 89, 3219-3227.
Thomsen L.E. et al., 2007. The effect of fermentable carbohydrates on experimental swine dysentery and whip worm infections in pigs. Vet. Microbiol., 119, 152-163.
Trefan L. et al., 2013. Meta-analysis of effects of gender in combination with carcass weight and breed on pork quality. J. Anim. Sci., 91, 1480-1492.
Wellock I.J. et al., 2008. The consequences of non-starch polysaccharide solubility and inclusion level on the health and performance of weaned pigs challenged with enterotoxigenic Escherichia coli. Br. J. Nutr., 99, 520-530.
Zhang W. et al., 2013. The effects of dietary fiber level on nutrient digestibility in growing pigs. J. Anim. Sci. Biotechnol., 4, 17.

