Changes in soil biological quality under legume- and maize-based farming systems in a humid savanna zone of Côte d'Ivoire
Université d’Abobo-Adjamé. Unité de Formation et de Recherche des Sciences de la Nature. 02 BP 801. Abidjan 02 (Côte d'Ivoire).
Université d’Abobo-Adjamé. Unité de Formation et de Recherche des Sciences de la Nature. 02 BP 801. Abidjan 02 (Côte d'Ivoire). E-mail: tondohj@yahoo.fr
Institut de Recherche pour le Développement (IRD). Laboratoire d’Ecologie des Sols Tropicaux. UMR 137 BIOSOL (Biodiversité et Fonctionnement du Sol). Avenue Henri Varagnat, 32. F-93143 Bondy Cedex (France).
Institut de Recherche pour le Développement (IRD). Laboratoire d’Ecologie des Sols Tropicaux. UMR 137 BIOSOL (Biodiversité et Fonctionnement du Sol). Avenue Henri Varagnat, 32. F-93143 Bondy Cedex (France) – Université des Antilles et de la Guyane. Faculté des Sciences naturelles. Laboratoire de Biologie et de Physiologie végétales. Unité de Recherche de la Dynamique des Ecosystèmes Caraïbes et Biologie des Espèces inféodées, Equipe d'Accueil 926 (DYNECAR EA 926). BP 592. F-97159 Pointe-à-Pitre Cedex (Guadeloupe, France).
Institut de Recherche pour le Développement (IRD). Unité de Recherche Séquestration du carbone et Bio-fonctionnement des sols (UR SeqBio). Montpellier SupAgro. 2 Place Viala. F-34060 Montpellier Cedex 1 (France).
Université de Cocody. UFR Biosciences. 22 BP 582 Abidjan 22 (Côte d'Ivoire).
Received on April 11, 2007, accepted on November 13, 2007
Résumé
Dynamique de la qualité biologique du sol sous différents systèmes de culture en zone de savane humide de Côte d'Ivoire. Il est important de disposer d’informations relatives à l’impact des systèmes de culture sur les caractéristiques du sol afin de proposer les plus appropriés pour une zone agroécologique donnée. Cette étude, menée dans une zone de savane humide en Afrique de l’Ouest, a pour but d’évaluer l’effet, à court terme, de différents systèmes de culture sur la teneur en carbone et certaines propriétés microbiologiques du sol (0-10 cm). Un dispositif en blocs aléatoires avec trois répétitions a été utilisé. Les traitements consistaient, d’une part, en des systèmes à base de légumineuses : Mucuna pruriens (Mucuna), Pueraria phaseoloides (Pueraria), Lablab purpureus (Lablab), l’association des trois espèces de légumineuses (Mixed-legumes) et d’autre part, en des systèmes de culture continue : maïs + urée (Maize-U), maïs + superphosphate triple (Maize-Sp), maïs + urée + superphosphate triple (Maize-USp) et maïs sans engrais (Maize-Tradi). Les résultats montrent que la teneur du sol en carbone augmente avec le temps sur les parcelles à légumineuses, de même que celles où les résidus de récolte (maïs) sont laissés sur place et associés à une fertilisation azotée. L’accroissement le plus important est observé sous le traitement à association de légumineuses (de 7,5 à 8,6 g.kg-1). Cependant, la teneur du sol en carbone ne montre pas de différence significative entre les traitements lorsqu’on tient compte des périodes d’échantillonnage. Quoique la biomasse microbienne du sol soit plus importante sous les systèmes à base de légumineuses, les différences avec les autres systèmes ne sont pas statistiquement significatives. En revanche, la minéralisation du carbone et la respiration spécifique du sol sont apparues comme des paramètres significativement influencés par les systèmes de culture, les valeurs les plus importantes étant observées avec ceux à base de légumineuses (42 ± 7,6 mg C-CO2.g-1 Corg et 0,4 mg C-CO2.g-1 biomasse C, respectivement), comparées à la culture continue de maïs (33,1 ± 1,6 mg C-CO2.g-1 Corg et 0,3 mg C-CO2.g-1 biomasse C, respectivement). Ceux-ci pourraient donc être utilisés comme indicateurs de qualité du sol permettant de discriminer des systèmes de culture dans le court terme. L’intégration des légumineuses herbacées dans les systèmes de culture en moyenne Côte d'Ivoire pourrait donc constituer une voie de gestion durable des sols.
Abstract
Studying the impact of farming systems on soil status is essential in determining the most relevant for a given agroecological zone. A trial was conducted in a West Africa humid savanna, aiming at assessing the short-term effects of farming systems on soil (0-10 cm) organic carbon (SOC) content and some soil microbiological properties. A randomized complete block experimental design with three replications, and the following treatments were used: Mucuna pruriens (Mucuna), Pueraria phaseoloides (Pueraria), Lablab purpureus (Lablab), a combination of these three legumes (Mixed-legumes), maize + urea (Maize-U), maize + triple super phosphate (Maize-Sp), maize + urea + triple super phosphate (Maize-USp), fertilizer-free maize continuous cropping (Maize-Tradi). Results indicated that SOC content was improved over time under legume-based systems. The relative increase was the highest with the legume association and Lablab, where SOC varied from 7.5 to 8.6 g.kg-1 (i.e. 14.7%) and from 7.2 to 8.3 g.kg-1 (i.e. 15.3%) respectively, between the start and the end of the trial. Besides, applying grass and maize residues as mulch on the ground, in association with inorganic fertilizers may be a way of improving SOC content in the short-term. Although legume-based systems exhibited highest values, microbial biomass carbon (MBC) did not show any statistical significant differences between treatments. However, soil C mineralization and soil specific respiration were influenced by the farming systems, with higher mean values under legume-based systems (42 ± 7.6 mg C-CO2.g-1 Corg and 0.4 mg C-CO2.g-1 biomass C, respectively), compared to maize continuous cropping systems (33.1 ± 1.6 mg C-CO2.g-1 Corg and 0.3 mg C-CO2.g-1 biomass C, respectively). Thus, these parameters can be used as sensitive indicators of the early changes in soil organic matter quality. The integration of legumes cover crops in farming systems may contribute to improve soil quality that would lead to sustainable agriculture in Côte d'Ivoire humid savannas.
1. INTRODUCTION
1Soil is an important natural resource, as it constitutes a medium for plant growth. However, most of the soils in West African moist savannas are characterized by low quality level, leading to major problems in crop production (Sanchez, 1976; Moorman et al., 1978; Vanlauwe et al., 2002; Oorts et al., 2003). According to Doran et al. (1994), soil quality is “ the capacity of a soil to function within an ecosystem and sustain biological productivity, maintain environmental quality and promote plant and animal health ”. Scientists identified and quantified chemical and biological soil-quality indicators for agroecosystems, which are useful in evaluating the soil quality status owing to their early reaction to soil condition change (Knoepp et al., 2000; Emmerling et al., 2002). Among these indicators, microbiological and biochemical properties have been widely reported (Dick, 1994; Knoepp et al., 2000; Palma et al., 2000). These are furthermore related to the accumulation and the cycling of soil organic matter (SOM), a key component of soil quality (Sparling, 1997; Koutika et al., 2001). In the absence of high exchange-capacity clay, SOM becomes the main nutrient storage site for soils of the tropics (Ayalanja et al., 1991). Therefore, the role of SOM in the retention of plant nutrients emphasizes the need for management strategies designed to maintain adequate levels of soil organic matter (Okpara et al., 2005).
2The low fertility level of moist savanna soils, common in middle Côte d'Ivoire, was reported by Riou (1974) and needs to be improved. It is known that one way of improving soil quality involves the addition of organic products because they regulate soil microbial biomass, affect carbon mineralization and turnover of organic matter and contribute to higher N-availability for crops (Albiach et al., 2000; Mayer et al., 2003). In order to improve the quality of soils in the region, legumes cover crop-based farming systems have been used. Indeed, leguminous cover crops may be an appropriate component of sustainable food-production systems in the savannas of West Africa (Carsky et al., 1998; Segda et al., 1998). However, information related to their impact on soil status is required in order to propose the most relevant for both soil resources conservation and optimization of production (Okpara et al., 2005). The potential of herbaceous legumes as green manure crops has been documented, but up to now, data that focus on the response of soil organic carbon (SOC) content or soil organic matter-related microbiological parameters in West Africa, particularly in Côte d'Ivoire, are scarce (Autfray et al., 2001).
3The aim of this study was to examine, in the short run, the efficiency of legumes cover crops to improve some soil quality parameters (SOC content, SOC mineralization, soil specific respiration and soil microbial biomass carbon, MBC), as compared to maize-based systems. Ultimately, this study intended to determine soil parameters that can be used as indicators of early changes in soil organic matter status under different farming systems.
2. MATERIAL AND METHODS
2.1. Study site
4This experiment was carried out in the surrounding zone of the Lamto reserve (6°13’ N and 5°20’ W) located in middle Côte d'Ivoire. Vegetation structure was a mosaic of secondary forests, shrubby and woody savannas, Chromolaena odorata fallows and agroecosystems. The study site was located in the shrubby savanna, well represented in middle Côte d'Ivoire. This facies was dominated by Hyparrhenia diplandra and Imperate cylindrica grasses and shrub species such as Bridelia ferruginea, Cussonia barteri, Crossopteryx febrifuga, Terminalia glaucescens, Nauclea latifolia, Annona senegalensis. Climate was characterized by constant temperatures (average 27°C) throughout the year. Four seasons can be distinguished: a long dry season from December to February, a long wet season from March to July, a short dry season in August and a short wet season from September to November. Mean annual rainfall during the study period (2003-2004) was 1 305 mm. Soils are Alfisols (American system), with a sandy loam upper layer (0-30 cm) overlying sandy clay (30-70 cm) and stony (> 70 cm) horizons. They are characterized by low contents in organic matter and phosphorus (Riou, 1974).
2.2. Experimental design
5A randomized complete-block design, with three replications, was set up in a 6 240 m2 area from 2003 to 2004. The blocks were separated by 4 m intervals, each of them included eight plots of 192 m2 (8 m x 24 m), separated by 2 m intervals. Eight treatments, involving four legumes cover crops and four maize-based treatments, were used:
6- Mucuna pruriens (Mucuna)
7- Pueraria phaseoloides (Pueraria)
8- Lablab purpureus (Lablab)
9- a combination of the legumes (Mixed Legumes)
10- maize fertilized with urea (50 kg.ha-1) (Maize-U)
11- maize fertilized with triple super phosphate (30 kg.ha-1) (Maize-Sp)
12- maize fertilized with the two fertilizers at the same rate (Maize-USp)
13- continuous maize cropping without fertilizer (Maize-Tradi).
14Savanna land used for experimentation was slashed, and then plots were hoe-weeded prior to the sowing. Legumes and maize were hand-sown during the rainy season. Legumes have grown for 12 months (May 2003 - April 2004) at 0.5 x 0.5 m plant spacing. Maize plots were cropped twice in the same period at 31 000 plants.ha-1: from May to August 2003, and from October 2003 to January 2004. Triple super phosphate was applied once at sowing, while urea was split into two applications, 1/3 at sowing and 2/3 at 40 days after sowing, according to the standard of the International Institute of Tropical Agriculture (IITA) (Kang, 1997). Maize plots were weeded twice during the cycle, 1 and 2 months after sowing. After maize harvest, maize residues were left on the ground. The above ground grass (Imperata cylindrica) was weeded and also left on soil surface as mulch.
2.3. Soil sampling
15Soil from each treatment was sampled before land clearance, 6 and 12 months after treatments establishment, in order to monitor the dynamic of measured parameters. Nine soil samples from the 0-10 cm layer were collected, from the inner one-third (8 m x 8 m) of each plot using an auger and then, mixed into a composite sample. Thus, there were three replicates per treatment. Then, the samples were air-dried, crushed and 2 mm sieved and stored in plastic bags for further chemical analyses.
2.4. Soil analyses
16Soil organic carbon (SOC) is known to be a key component of soil quality (Gregorich et al., 1997). Therefore, assessing how farming systems impacts it is of a great importance. This parameter was determined using the Near Infrared Reflectance Spectroscopy (NIRS) technique, based on the close relationship between spectral absorbance and the biochemical composition of a sample (Dalal et al., 1986; Morra et al., 1991; Ludwig et al., 2002; McCarthy et al., 2002). The samples were scanned in a spectrophotometer, FOSS 5 000 model (Foss NIRSystems, Silver Spring, MD, USA) in the reflectance mode from 1 100 to 2 500 nm to produce spectra with 700 data points. The spectral data obtained were recorded as the logarithm of the inverse of reflectance [log (1/R)]. They were analysed using WinISI III-version 1.50e software (Foss NIRSystems, Infrasoft International). A reference set was selected using the 119 first most representative samples identified with the internal algorithm used by Winisi software as detailed by Shenk et al. (1991). Then, these samples were analysed in an elemental micro-analyser CHN Carlo Erba (CE Instruments, Milan, Italy) to determine total C content. In the absence of carbonates, the total carbon is assumed as organic carbon. Finally, the calibration model stemmed from the reference set was applied to the spectral data of all soil samples to predict C content.
17Soil carbon mineralization (Cmin), which may control the amounts of available nutrients (Knoepp et al., 2000) or the organic matter degradability, was measured directly through CO2 emission by soil microorganisms. Seventy grams of dry soil were brought to 80% of field capacity with distilled water and put into a jar with open pipes to allow aeration. The jars were incubated in an oven at a constant temperature of 30°C before CO2 measurements after 7, 14, and 21 days. The easily degradable carbon was determined using an infrared CO2 meter (Dräger Polytron IR CO2) according to the dynamic closed chamber method (Bekku et al., 1997).
18Soil microbial biomass carbon (MBC) has been proposed as an indicator of the state of total SOM (Knoepp et al., 2000). It was measured using the chloroform fumigation-extraction method (Vance et al., 1987; Horwarth et al., 1994). Twenty grams of soil samples of each treatment was fumigated with an ethanol-free chloroform solution. Simultaneously, 10 g of unfumigated samples were kept in a desiccator in a dark room. After a 24 hours incubation period, C from fumigated and control samples was extracted with 0.5M K2SO4 solution after shaking, centrifugation and filtration of the soil suspension. The next step consisted in determining Dissolved Organic Carbon (DOC) of the soil solutions by measuring chemical oxygen demand (COD) with a spectro-colorimeter DR/700 (HachTM method). The microbial biomass C was determined as the difference between DOC from fumigated and unfumigated soil samples.
19Statistical analyzes were performed using STATISTICA 6.0 Software. Because of the low number of replications (3), mean comparisons were done using the Mann-Whitney and the Kruskal-Wallis ANOVA tests at α = 0.05 level. Correlation analyses (Pearson) were used to test relationships between variables.
3. RESULTS AND DISCUSSION
3.1. Initial soil characteristics
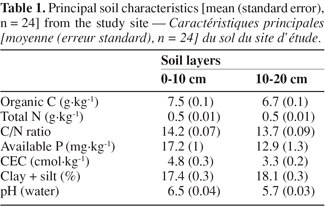
20The soil was slightly acidic, with pH values around 6. Surface soil fine fraction amount was low (ca 17%). The fertility level is low, as indicated by the following chemical characteristics, measured on the 0-10 and 10-20 cm layers respectively: soil organic C: 7.5-6.5 g.kg-1, total N: 0.52-0.49 g.kg-1, available P: 17-13 g.kg-1, cation exchange capacity (CEC): 4.8-3.3 cmol.kg-1 (Table 1). Riou (1974) attributed the low fertility level of soils encountered in the study region to their coarse texture and the low exchange capacity-clay (kaolinite) they contain. In addition, organic matter was reported to be rapidly mineralized in these soils (Abbadie, 1990).
3.2. Soil organic carbon (SOC)
21With respect to the type of treatment, soil organic carbon showed different dynamics. It increased significantly over time under all the legume-based treatments. Concerning the maize-based treatments however, this was not the case, except with Maize-USp (Table 2). Six months after the start of the trial, the increase in SOC content was significant under Mucuna and Lablab. The significance was reached for the other treatments after 12 months, regardless of Mucuna and Lablab. Finally, the extent of increase was the highest with the legume association and Lablab, where SOC varied from 7.5 to 8.6 g.kg-1 (i.e. 14.7%) and from 7.2 to 8.3 g.kg-1 (i.e. 15.3%) respectively, between the start and the end of the trial.
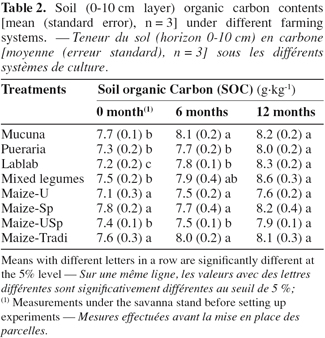
22As reported in other studies (Azontonde et al., 1998; Tian et al., 2001; Dinesh et al., 2004), legumes continuously provided great amounts of litter to the soil. This may explain the increase in SOC observed under legumes in the soil layer. The delay in SOC increase recorded under Pueraria, compared to the other legume-based treatments, could simply be attributed to the delay in litter accumulation due to the initial lower growth of the species. Indeed, litter accumulation began four to five months after Pueraria, and one or three months after Mucuna and Lablab establishments. Leaving crop residues as mulch on soil surface is known to contribute to organic matter building up in soil, particularly when combined with fertilizer N (Omay et al., 1997; Tonye et al., 1997; Shrestha et al., 2002; Whitbread et al., 2003). Our results with Maize-USp treatment were consistent with these findings. As a matter of fact, their C-to-N ratio decreases with addition of N, increasing their decomposability by microorganisms. The time span, necessary to observe an increase in soil organic matter, is a function of soil type (Six et al., 2002). It is shorter with sandy soil than with clayey soil. Thus, the increases in soil organic carbon observed in the short term also might be due to the high proportion of sand in the top (0-10 cm) soil layer.
23When compared at each time of sampling, the treatments were not significantly different. However, it could be expected that longer duration of experiment allows best discrimination between treatments.
3.3. Soil microbial biomass C (MBC)
24Although MBC was higher under cover crop-based systems compared to continuous cropping systems (Table 3), statistical significance was not established. Similar results have previously been reported by Haggar et al. (1993), in a study involving legumes and maize treatments. Since microbial biomass is likely to respond more quickly to change in soil management strategies (Knoepp et al., 2000), this result may be a consequence of either high field spatial variability of the parameter or the low number of replicates (Bross et al., 2007). As observed in this study, it has been reported that the soil microbial biomass displays pronounced spatial and temporal variability in tropical soils (Cleveland et al., 2004; Bross et al., 2007). In such conditions, other indicators more robust than the fumigation-extraction method derived-MBC should have been used as recommended by Bross et al. (2007). Nevertheless, after 12 months, highest values were recorded under the legume combination and Lablab, with 176.7 and 168.3 mg.kg-1 respectively. Furthermore, the highest relative increases in MBC, compared to the initial soil, occurred under the two treatments. MBC increased significantly by 50.7% and 86% under Lablab and legume combination respectively. The result obtained with legume-based treatments in general could be explained by the supply of great quantities of readily decomposable organic matter to the soil (Wick et al., 1998; Manjaiah et al., 2000). Although our data did not show any statistically significant correlation between soil organic C and MBC, the increase in SOC availability is likely to explain higher values of MBC, as C has been reported to be the principal energy source to soil microorganisms (Cleveland et al., 2004; Demoling et al., 2007).
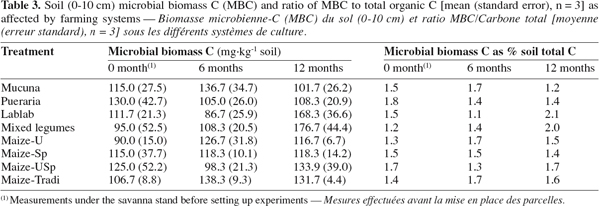
25The ratio of MBC to soil organic C is potentially a sensitive index of farming system effects on SOM (Omay et al., 1997). However, in this study, the ratio of MBC to total C did not vary significantly among treatments nor over time. Mean values varied in a narrow range: from 1.1 to 1.7 after 6 months and from 1.2 to 2.1 after 12 months (Table 3). Thus, these fell within the 1-5 % range of values reported by Jenkinson et al. (1981).
3.4. Soil carbon mineralization and specific soil respiration
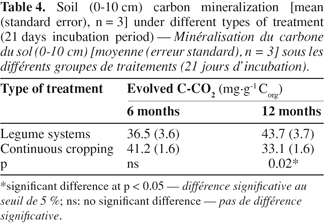
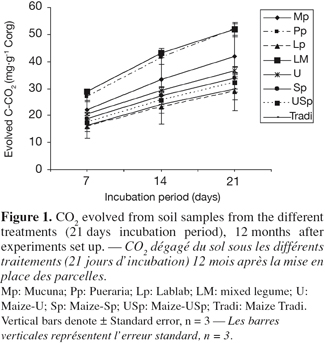
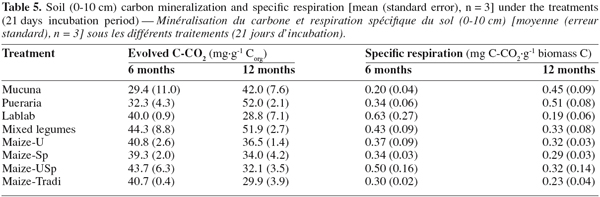
26When considering the farming systems into two types, i.e. legume systems versus continuous cropping, carbon mineralization exhibited significant difference after 12 months, and it was greater under legumes system than under the continuous cropping one (Table 4). However, soil carbon mineralization was not significantly influenced by the treatments, considered separately (Figure 1), although CO2 production seemed higher under Pueraria and Mixed-legumes (52 mg C-CO2.g-1 Corg), and lower under under Maize-Tradi (29.9 mg C-CO2.g-1 Corg) at the 12 month sampling time (Table 5).
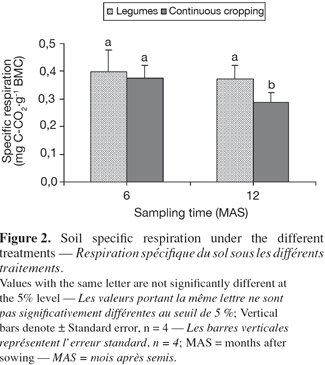
27When considering the two groups as described above, specific soil respiration, an indicator of soil microbial activity, showed significant difference after 12 months. The legumes group exhibited higher values (0.37 ± 0.05 mg C-CO2.g-1 biomass C), compared to continuous cropping system (0.29 ± 0.03 mg C-CO2.g-1 biomass C) (Figure 2). However, specific soil respiration was not significantly influenced by treatments, considered separately (Table 5). Values varied from 0.63 under Lablab to 0.2 under Mucuna after six months, and from 0.51 under Pueraria to 0.19 under Lablab, after 12 months. The specific soil respiration was more important at 12 than that at 6 months under Mucuna and Pueraria, while the opposite trend was observed under the other treatments.
28Plant material analysis (data not shown) showed that legume materials are of a higher quality with lower C-to-N ratio, compared to graminea-derived materials as reported by Bernhard-Reversat et al. (2000). Thus, legume provided more readily decomposable materials that stimulated soil microorganisms. This may explain why soil respiration was higher under legume systems, compared to maize-based ones. Such conditions under legume might allow an increase in microbial biomass C instead of a decrease as observed under Mucuna and Pueraria. Nonetheless, soil microorganism's activity increased under both Mucuna and Pueraria as indicated by soil specific respiration measured at 12 months, and this may be a consequence of an increase in active microbial fraction.
29Rhizodeposition (root-derived compounds and plant material released) into the soil can make up a high proportion of below-ground carbon and nitrogen which serve as substrate for soil microorganisms (Paterson, 2003; Böhme et al., 2006; Wichern et al., 2007). In the present study, Lablab and Mucuna completed their cycles two and three months before the final soil sampling, respectively, whereas legumes stands were still alive on both the Pueraria and the legume combination plots up to 12 months after sowing (as P. phaseoloides is a perennial and drought resistant legume). Thus, the continuous turnover of Pueraria roots and release of exudates through rhizodeposition may explain the higher soil respiration recorded for these plots. Moreover, soil temperature was lower and constant relative to Mucuna and Lablab plots. At that moment (12 months after sowing), the senescence of roots increased under Mucuna and Lablab, and structural root components (with higher C-to-N ratio) probably contributed more to rhizodeposition, leading to a rhizodeposition having a lower availability to microorganisms (Wichern et al., 2007). The decomposition of Lablab leaf litter was so rapid (data not shown) that at the final sampling (12 months), there was almost no litter on the ground. Since plant-derived carbon is prominently used (compared to that of the SOM) as energy source by microorganisms (Kramer et al., 2006), the breaking in organic supply may have caused the decline in soil respiration.
30Soil microorganisms are likely to be affected or dormant under continuous cropping, explaining the observed low specific soil respiration as compared to the legume-based systems. However, for sandy soils with low organic C content, other microbiological parameters such as enzyme activities might have better discriminated the farming systems as suggested by Böhme et al. (2006).
4. Conclusion
31This study showed that soil organic carbon content was significantly improved over time under legume-based systems, contrarily to most of the continuous maize-based systems. However, it did not show any significant influence of farming systems on SOC content. Likewise, microbial biomass C did not appear to be influenced by farming systems. One reason could be that 12 month duration is short for farming system discrimination. Besides, the variability of carbon mineralization and microbial biomass measurements are generally not negligible, and lower the precision of comparisons. However increase in soil organic matter during the experiment showed that crop and weed residues, in combination with fertilizers are likely to be a way of improving SOC storage in the short run. It is apparent that soil mineralizable C, and to some extent, soil specific respiration were influenced by farming systems. However, their use as indicators of early changes in SOM quality is likely to be limited by the small-scale spatial heterogeneity of the soil. Nevertheless, integration of legumes cover crops, as fallow in farming systems, may contribute to improve soil quality and crop production in humid savannas of Côte d'Ivoire.
32Acknowledgements
33Special thanks to the International Foundation for Sciences (IFS) for having funded this study. The authors are grateful to Dr Martine Tahoux, and Dr Souleymane Konate for facilitating field access. They would like also to thank N’Goran Yao, Martin Kouakou Loucou and Jean N’Guessan Konan (Lamto Ecological Research Station) for their assistance in fieldwork.
Bibliographie
Abbadie L., 1990. Aspects fonctionnels du cycle de l’azote dans la strate herbacée de la savane de Lamto. Thèse de doctorat : Université Pierre et Marie Curie, Paris.
Albiach H., Canet R., Pomares F. & Ingelmo F., 2000. Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Biores. Technol., 25, 43-48.
Autfray P. & Oliver R., 2001. N available for maize (Zea mays) on living cover crops of Chromolaena odorata and Pueraria phaseloides in forest areas of Côte d'Ivoire. In: Garcia-Torres L., Benitez J. & Martinez-Vilela A., eds. Conservation agriculture, a worldwide challenge. Vol. 2. Proc. First world congress on conservation agriculture, 1-5 October 2001, Madrid, Spain. Roma: FAO; Brussels: European Conservation Agriculture Federation (ECAF), 395-399.
Ayalanja S.A., Sanwo J.O. & Ago-Iwoye, 1991. Management of soil organic matter in the farming systems of the low land humid tropics of West Africa: a review. Soil Technol., 4, 265-279.
Azontonde A.H., Feller C., Ganry F. & Remy J.-C., 1998. Le mucuna et la restauration des propriétés d’un sol ferrallitique au sud du Bénin. Agric. Dév., 18, 55-62.
Bekku Y., Koizumi H., Oikawa T. & Iwaki H., 1997. Examination of four methods for measuring soil respiration. Appl. Soil Ecol., 5, 247-254.
Bernhard-Reversat F., Masse D. & Harmand J.M., 2000. Qualité des litières et décomposition dans les jachères naturelles. In : Floret Ch. & Pontanier R., eds. La jachère en Afrique tropicale. Vol. 1. Paris : John Libbey Eurotext, 194-203.
Böhme L. & Böhme F., 2006. Soil microbiological and biochemical properties affected by plant growth and different long-term fertilisation. Eur. J. Soil Biol., 42, 1-12.
Bross K. et al., 2007. Limitations of soil microbial biomass carbon as an indicator of soil pollution in the field. Soil Biol. Biochem., 39, 2693-2695.
Carsky J.R. & Ndikawa R., 1998. Identification of cover crops for the semi-arid savanna zone of West Africa. In: Buckles D. et al., eds. Cover Crops in West Africa. Ottawa: IDRC publications.
Cleveland C.C., Townsend A.R. & Contance B.C., 2004. Soil microbial dynamics in Costa Rica: searsonal and biogeochemical constraints. Biotropica, 36, 184-195.
Dalal R.C. & Henry R.J., 1986. Simultaneous determination of moisture, organic carbon, and total nitrogen by near-infrared reflectance spectrophotometry. Soil Sci. Soc. Am. J., 50, 120-123.
Demoling F., Figueroa D. & Baath E., 2007. Comparison of factors limiting bacterial growth in different soils. Soil Biol. Biochem., 39, 2485-2495.
Dick R.P., 1994. Soil enzyme activities as indicators of soil quality. In: Doran J.W., Coleman D.C., Bezdicek D.F. & Stewart B.A., eds. Defining soil quality for a sustainable environment. Proc. Symposium 4-5 November 1992, Minneapolis, MN, USA. Madison, WI, USA: Soil Science Society of America, Special Publication n° 35; American Society of Agronomy, 107-124.
Dinesh R., Suryanarayana M.A., Chaudhuri Ghoshal S. & Sheeja T.E., 2004. Long-term influence of leguminous cover crops on the biochemical properties of a sandy clay loam fluvientic sulfaquent in a humid tropical region of India. Soil Tillage Res., 77, 69-77.
Doran J.W. & Parkin T.B., 1994. Defining and assessing soil quality. In: Doran J.W., Coleman D.C., Bezdicek D.F. & Stewart B.A., eds. Defining soil quality for a sustainable environment. Proc. Symposium 4-5 November 1992, Minneapolis, MN, USA. Madison, WI, USA: Soil Science Society of America, Special Publication n° 35; American Society of Agronomy, 3-21
Emmerling C., Schloter M., Hartman A. & Kandeler E., 2002. Functional diversity of soils organisms – a review of recent research activities in Germany. J. Plant. Nutr. Soil Sci., 165, 408-420.
Gregorich E.G. et al., 1997. Biological attributes of soil quality. In: Gregorich E.G. & Carter M.R., eds. Soil quality for crop production and ecosystem health. New York, USA: Elsevier, 81-114.
Haggar J.P., Tanner E.V.J., Beer J.W. & Kass D.C.L., 1993. Nitrogen dynamic of tropical agroforestry and annual cropping system. Soil Biol. Biochem., 25, 1363-1378.
Horwarth W.R. & Paul E.A., 1994. Methods of soil analysis. Part 2: Microbiological and biochemical properties. Madison, WI, USA: Soil Science Society of America, Book Series no5; American Society of Agronomy.
Jenkinson D.S. & Ladd J.N., 1981. Microbial biomass in soil: measurement and turnover. In: Paul E.A. & Ladd J.N., eds. Soil Biochemistry, vol. 5. New York, USA: Dekker, 415-471.
Kang B.T., 1997. Les engrais : définition et calculs. Guide de phytotechnie de l'IITA, n°1. Ibadan, Nigeria : International Institute of Tropical Agriculture (IITA).
Knoepp J.D., Coleman D.C., Crossley Jr D.A. & Clark J.C., 2000. Biological indices of soil quality: an ecosystem case study of their use. Forest Ecol. Manage., 138, 357-368.
Koutika L.-S., Hauser S. & Henrot J., 2001. Soil organic matter assessment in natural regrowth, Pueraria phaseoloides and Mucuna pruriens fallow. Soil Biol. Biochem., 33, 1095-1101.
Kramer C. & Gleixner G., 2006. Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol. Biochem., 38, 3267-3278.
Ludwig B., Khanna P.K., Bauhus J. & Hopmans P., 2002. Near infrared spectroscopy of forest soils to determine chemical and biological properties related to soil sustainability. Forest Ecol. Manage., 171, 121-132.
Manjaiah K.M., Voroney R.P. & Sen U., 2000. Soil organic carbon stocks, storage profile and microbial biomass under different crop management systems in tropical agricultural ecosystem. Biol. Fertil. Soils, 31, 273-278.
Mayer J. et al., 2003. Estimating N Rhizodeposition of grain legumes using 15N in situ stem labelling method. Soil Biol. Biochem., 35, 21-28.
McCarthy G.W., Reeves V.B., Follet R.F. & Kimble J.M., 2002. Mid-infrared and near infrared diffuse reflectance spectroscopy for soil carbon measurement. Soil Sci. Soc. Am. J., 66, 640-646.
Moorman F.R. & Wambeke A.V., 1978. Soils of lowland rainy tropical climate, vol. 2. Proc. 11th International Society of Soil Science (ISSS) congress, 19-27 June 1978. Edmonton, Canada: University of Alberta, 272-279.
Morra M.J., Hall M.H. & Freeborn L.L., 1991. Carbon and nitrogen analysis of soil fractions using near-infrared reflectance spectroscopy. Soil Sci. Soc. Am. J., 55, 288-291.
Okpara D.A., Ikeorgu J.E.G. & Njoku J.C., 2005. Potential of cover crops for short fallow replacement in low-input systems of maize production in the humid tropics. Trop. Subtrop. Agroecosyst., 5, 109-116.
Omay A.B., Rice C.W., Maddux L.D. & Gordon W.D., 1997. Changes in soil microbial and chemical properties under long-term crop rotation and fertilization. Soil Sci. Soc. Am. J., 61, 1672-1678.
Oorts K., Vanluwe B. & Merckx R., 2003. Cation exchange capacity of organic matter fractions in a Ferric Lixisol with different organic matter inputs. Agric. Ecosyst. Environ., 100, 61-171.
Palma R.M., Arrigo N.M., Saubidet M.I. & Conti M.E., 2000. Chemical and biochemical properties as potential indicators of disturbances. Biol. Fertil. Soils, 32, 381-384.
Paterson E., 2003. Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur. J. Soil Sci., 54, 741-750.
Riou G., 1974. Les sols de la savane de Lamto. In : Analyse d’un écosystème tropical humide : la savane de Lamto (Côte d'Ivoire). Les facteurs physiques du milieu. Paris : Laboratoire de Zoologie de l’ENS, 3-38.
Sanchez P.A., 1976. Properties and management of soils in the tropics. New York, USA: Wiley-Interscience Publications.
Segda Z., Hien V., Lompo L. & Becker M., 1998. Gestion améliorée de la jachère par l'utilisation de légumineuses de couverture. In: Buckles D. et al., eds. Cover Crops in West Africa. Ottawa: IDRC publications.
Shenk J.S. & Westerhaus M.O., 1991. Population definition, sample selection, and calibration procedure for near infrared reflectance spectroscopy. Crop Sci., 31, 469-474.
Shrestha R.K., Ladha J.K. & Lefroy R.D.B., 2002. Carbon management for sustainability for an intensively managed rice-based cropping system. Biol. Fertil. Soils, 36, 215-223.
Six J., Conant R.T., Paul E.A. & Paustian K., 2002. Stabilization mechanisms of soil organic matter: implications for C saturation of soils. Plant Soil, 241, 155-176.
Sparling G.P., 1997. Soil microbial biomass, activity and nutrient cycling as indicators. In: Pankhurst C., Doube B.M. & Gupta V.V.S.R., eds. Biological indicators of soil health. New York, USA: CAB International, 97-120.
Tian G., Sakalo F.K. & Ishida F., 2001. Replenishment of C, N, and P in a degraded alfisol under humid tropical conditions: effect of fallow species and litter polyphenols. Soil Sci., 16609, 614-621.
Tonye J., Ibewiro D. & Duguma B., 1997. Residue management of a planted fallow on an acid soil in Cameroon: crop yields and soil organic matter fractions. Agroforestry Syst., 37, 199-207.
Vance E.D., Brookes P.C. & Jenkinson D.S., 1987. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem., 19, 703-707.
Vanlauwe B. et al., 2002. Fertility status of the derived savanna and northern Guinea savanna and response to major plant nutrients, as influenced by soil type and land use management. Nutr. Cycl. Agroecosyst., 62, 139-150.
Whitbread A. et al., 2003. Managing crop residues, fertilizers and leaf litters to improve soil C, nutrient balances, and the grain yield of rice and wheat cropping systems in Thailand and Australia. Agric. Ecosyst. Environ., 100, 251-263.
Wichern F., Mayer J., Joergensen R.G. & Müller T., 2007. Rhizodeposition of C and N in peas and oats after 13C-15N double labelling under field conditions. Soil Biol. Biochem., 39, 2527-2537.
Wick B., Kühne R.F. & Vlek P.L.G., 1998. Soil microbial parameters as indicators of soil quality under improved fallow management systems in south-western Nigeria. Plant Soil, 202, 97-107.

