- Startpagina tijdschrift
- Volume 17 (2013)
- numéro 3
- Bacteriological assessment of smoked game meat in Lubumbashi, D.R.C.
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Bacteriological assessment of smoked game meat in Lubumbashi, D.R.C.

Nota's van de redactie
Received on December 21, 2012; accepted on May 8, 2013
Résumé
Qualité bactériologique de la viande boucanée de gibier à Lubumbashi, R.D.C. La qualité bactériologique de la viande boucanée de gibier n’était pas bien connue à Lubumbashi jusqu’à présent. Pour cette étude, 182 échantillons de viande boucanée provenant de trois espèces de gibier, à savoir Syncerus caffer (n = 63), Phacochoerus aethiopicus (n = 60) et Sylvicapra grimmia (n = 59) ont été achetés au détail dans les marchés de Lubumbashi et analysés. Le dénombrement de Escherichia coli (en moyenne 4,87 ± 0,6 log10 CFU·g-1 d’échantillon) dans 81,3 % d’échantillons montre une contamination fécale significative de viandes boucanées de gibier. Pour l’ensemble de l’échantillonnage, nous avons déterminé par culture des prévalences de 0,0 %, 4,3 % [IC95 % 1,4-7,4], 3,8 % [IC95 % 1,1-6,6] et 14,2 % [IC95 % 9,2-19,4] respectivement pour Escherichia coli producteur de Shiga toxine (STEC), Salmonella spp., Campylobacter jejuni and Campylobacter coli. Par contre, à l’aide de la PCR (Polymerase Chain Reaction), ces prévalences ont été de 2,2 % [IC95 % 0,1-4,3], 6,0 % [IC95 % 2,6-9,5], 3,8 % [IC95 % 1,1-6,6] et 15,9 % [IC95 % 10,6-21,3] respectivement pour STEC, Salmonella spp., C. jejuni et C. coli. Syncerus caffer a été établi comme vecteur potentiel de STEC portant les gènes stx1 (3,2 %), stx2 (1,6 %) et la combinaison des gènes stx2 et eae (1,6 %). Sur base de ces données, nous avons suggéré la nécessité de développer des plans de surveillance pour la production, la préparation et la distribution des viandes boucanées de gibier à Lubumbashi.
Abstract
The bacteriological quality of smoked game meat in Lubumbashi has not been studied much to date. The present study focused on the analysis of 182 samples of smoked game meat from three species, Syncerus caffer (n = 63), Phacochoerus aethiopicus (n = 60) and Sylvicapra grimmia (n = 59), sold at retail outlets in Lubumbashi. The isolation of Escherichia coli from 81.3% of samples (mean 4.87 ± 0.6 log10 CFU·g-1 of sample) confirms significant faecal contamination of smoked game meat. The study has determined by culture prevalences of 0.0%, 4.3% [CI95% 1.4-7.4], 3.8% [CI95% 1.1-6.6] and 14.2% [CI95% 9.2-19.4] respectively for Shiga toxigenic Escherichia coli (STEC), Salmonella spp., Campylobacter jejuni and Campylobacter coli. Using Polymerase Chain Reaction, these prevalences were of 2.2% [IC95% 0.1-4.3], 6.0% [IC95% 2.6-9.5], 3.8% [IC95% 1.1-6.6] and 15.9% [IC95% 10.6-21.3] respectively for STEC, Salmonella spp., C. jejuni and C. coli. Syncerus caffer was established as a potential vehicle of STEC carrying stx1 gene (3.2%), stx2 gene (1.6%) and the combination of stx2 and eae genes (1.6%). On the basis of these data, we suggested the need for developing monitoring plans of the production, preparation, handling and distribution of smoked game meat in Lubumbashi.
Inhoudstafel
1. Introduction
1Wild mammals contribute widely to the diet of African populations (Bachand et al., 2012). Their meat is considered to be a good alternative to beef meat (Onyango et al., 1998). Chomel et al. (2007) have reported that in the Congo basin, trade and consumption of game meat could reach up to 4.5 million tons annually. In Katanga, the southern province of Democratic Republic of Congo (D.R.C.), smoked game meat is generally provided by rural populations living around woodlands. In Lubumbashi, the main city of Katanga, as well as in others countries of Central Africa, smoked game meat is highly appreciated by consumers because of its organoleptic characteristics, such as taste, smell and color. Smoked game meat is of great value for traditional ceremonies or when welcoming an important host. Traditional and fetish practitioners often include it in magical preparations, illustrating its major importance in superstitious beliefs. Smoking is a transformation process for meat aimed at prolonging their shelf life and improving their organoleptic properties. This procedure was developed because of the lack of an appropriate storage system for transporting meat, as hunting is usually done in villages far from urban centers. Thus, game meat could be transported to dealers in urban centers in the form of dried or smoked meat. The process of smoking game meat consists of boiling the game meat, draining it, then placing it on metal sieves above a low heat. Smoke is produced by burning wood or sawdust beneath the sieves.
2Numerous factors may be responsible for the spoilage of fresh game meat, such as conditions of evisceration, and exposure to ambient temperature and relative humidity. Macroscopic alterations like the presence of moisture, mould, maggots and a nauseating smell are often observed on the meat at the selling point to consumers. This situation can be explained by illegal practices by salesmen, which involve injection of water into smoked game meat or soaking of smoked game meat in order to add volume to the dried muscle tissue. Consumption of such meat can constitute a threat to public health. However, there is no study conducted to assess the bacteriological quality of this type of meat in Lubumbashi. Although zoonotic transmission of enteric pathogens to human through the consumption of contaminated food such as meat has been reported (Ojo et al., 2010). In developed world, studies focusing on the microbiological quality of game meat as a threat to public health have been conducted previously (Atanassova et al., 2008; Miko et al., 2009). Indeed, wildlife could act as a potential source of human Salmonellosis (Hilbert et al., 2012). In developing world, enteric diseases are a major threat to public health (Bachand et al., 2012) and more than 60% of human infectious diseases are shared with wild or domestic animals (Karesh et al., 2012). Pathogenic Escherichia coli, Campylobacter spp. and Salmonella spp. are the most incriminated in gastroenteritis from zoonotic origin (Mohammed, 2012; Goualié et al., 2012). Immunocompromised people and children under the age of 5 are the most affected and 4.9 deaths per 1,000 among children are reported every year (Fhogartaigh et al., 2009).
3The objective of this study is to assess, by means of culture and Polymerase Chain Reaction (PCR), the prevalence of major Enterobacteriaceae such as STEC and Salmonella, as well as other major foodborne pathogens such as Campylobacter jejuni and Campylobacter coli in smoked game meat sold at retail outlet in Lubumbashi.
2. Materials and methods
2.1. Sample collection
4In the city of Lubumbashi, game meat is mainly sold in the central market of Lubumbashi municipality and in the central market of Kenya municipality; both sites were used to acquire samples of game meat. A total of 182 samples of smoked game meat were collected between December 2009 and December 2010 in the city of Lubumbashi (D.R.C.). Ninety-six samples were collected from the municipality of Kenya and 86 from the municipality of Lubumbashi. Sampling was performed monthly, 3 to 6 samples of each type of game meat were collected on the day of harvest according to the availability of meats on the market. Stalls selling smoked game meat are located in semi-open sheds, among stalls of other foods, including dried and salted fish, fried or dried and smoked fish. Various game meats like Potamocherus porcus (Red River Hog), Cephalopus sp. (Duiker), Lepus saxatilis (Scrub Hare), Paraxerus boehmi (Boehm’s Bush Squirrel) and Cryptomys hottentotus (African Mole Rat) are consumed in Lubumbashi. But we have included in this study three species that are common and sold in retail cuts. These included Syncerus caffer (African buffalo called Mbo in Swahili, the local language of Lubumbashi; n = 63), Phacochoerus aethiopicus (warthog or Lupenge in Swahili; n = 60) and Sylvicapra grimmia (common duiker or Nkasha in Swahili; n = 59). Smoked meat is sold as specified cuts like thigh, shoulder and ribs, but also as unspecified retail cuts of 50 to 100 g, hand-separated or separated with a sharp instrument such as a knife, axe, machete or hacksaw. We have included in this study only unspecified retail cuts of smoked game meat prior to sale.
2.2. Sample preparation and enrichment procedure
5Analyses were performed at the Laboratory of Expertise, Hygiene and Technology of Food from Animal Origin, University of Lubumbashi. All samples were transported to the laboratory in an isothermal box at 4 °C and analyzed within 2 to 4 h after collection.
6Isolation, enumeration and presumptive identification of E. coli. The isolation of E. coli was performed on samples diluted in Buffered Peptone Water (BPW) (dilution 10-1) and further diluted in sterile water up to 10-4. Diluted samples were then spread onto Violet Red Bile Agar with Lactose (VRBL) (Merck, Darmstadt, Germany) and incubated for 24 h at 44 °C. Violet colonies of 5 mm in diameter were counted. For presumptive identification 1 to 4 violet colonies were streaked onto Eosin Methylene Blue (EMB) agar (Merck) and incubated at 37 °C for 24 h (protocol of enumeration of E. coli in the Laboratory of Expertise, Hygiene and Technology of Food from Animal Origin, University of Lubumbashi). Green colonies with a metallic sheen were characterized as E. coli using the API 20E (bioMérieux, France). Data on the enumeration of E. coli were entered into Excel and transformed into log10 Colony-Forming Units per gram (CFU·g-1) of food sample.
7According to Congolese criteria, an E. coli colony count between 2.75 to 3.70 log10 CFU·g-1 in a food sample is considered high and above 3.70 log10 CFU·g-1 the food is then declared unsuitable for human consumption. For the interpretation of our results, we considered positive only the samples that showed a count above 3.70 log10 CFU·g-1.
8Isolation and presumptive identification of STEC. A quantity of 25 g of each sample was mixed with 225 ml of BPW supplemented with vancomycin, cefsulodin and cefixime (BPW-VCC) according to the protocol of Chapman (1995), and then homogenized using a Stomacher 400 (Seward, London, UK). A 1 ml volume of the BPW-VCC mixture was then enriched in brain heart broth (Merck) in order to enhance the bacterial level necessary for PCR sensitivity. The enrichment mixture was incubated at 37 °C for 18 to 24 h and each enriched sample was stored at -20 °C for further characterization by PCR in the Laboratory of Medical Microbiology, University of Liege. The interpretation of PCR results was based on the “presence” of “absence” of the target gene in a 25 g sample. Isolation of STEC was carried out on sorbitol MacConkey Agar (SMAC) (Merck). The characterization of E. coli strain was carried out with the API 20E (bioMérieux). In addition, agglutination of O157 antigen using dry SpotTM for identification of E. coli O157 (Oxoid, Basingstoke, UK) was used for colonies suspected to be STEC of O157 serotype.
9Isolation and presumptive identification of Salmonella spp. A quantity of 25 g of each sample was mixed with 225 ml of BPW. Then the mixture was homogenized using a Stomacher 400 (Seward). Following the techniques described by Woldemariam et al. (2005), 1 ml of the BPW mixture was enriched in selenite broth (Merck) and incubated at 37 °C for 18 to 24 h. Enriched samples were stored at -20 °C for further characterization by PCR in the Laboratory of Medical Microbiology, University of Liege. Cultures of Salmonella were performed on Xylose-Lysine-Deoxycholate (XLD) agar (Merck) and incubated at 37 °C for 24 h. The characterization of Salmonella was achieved using the API 20E (bioMérieux).
10Isolation and presumptive identification of Campylobacter spp. A quantity of 25 g of each sample was mixed with 225 ml of BPW and homogenized using a Stomacher 400 (Seward). A volume of 1 ml of the mixture was further enriched in Bolton broth (Oxoid, Basingstoke, UK) and incubated at 42 °C for 18 to 24 h under microaerophilic conditions. Enriched samples were stored at -20 °C for further characterization by PCR in the Laboratory of Medical Microbiology, University of Liege. Campylobacter jejuni and C. coli were isolated on Blood Free Campylobacter selective agar base (Merck) supplemented with Charcoal Cefoperazone Deoxycholate Agar Supplement (CCDA selective supplement) (Merck) and incubated at 42 °C for 48 h in a microaerophilic atmosphere, using GENbox microaer, atmosphere generators (bioMérieux). The morphology of colonies, the motile characteristics and biochemical tests, such as production of oxidase (Merck) and catalase (Merck) were used to confirm Campylobacter spp. Then, the hippurate hydrolysis test (Rosco, Taasturp, Denmark) was used to differentiate between C. jejuni and C. coli, following the protocols described by Sallam (2007) and Enokimoto et al. (2007).
2.3. DNA extraction
11DNA was directly extracted from enriched samples. Thus, 200 µl of each enriched sample were centrifuged and the bacterial pellets were collected. For cell lysis, 100 µl of a buffer containing 50 mM KCl, 10 mM Tris-HCl pH 8.3, 2.5 mM MgCl2, 0.5% Nonidet P40 (Applied Biosystem, Branchburg, USA. Manufactured by Roche®) and 0.5% Tween 20 (Promega®, Madison, USA) supplemented with 3 µl of proteinase K (20 mg·ml-1) (Qiagen®, Hilden, Germany) were added to bacterial pellets and heated for 30 min at 60 °C and then 30 min at 100 °C. Cell debris were removed by centrifugation at 13,000 rpm for 5 min using a centrifuge 5415R (Eppendorf, Hamburg, Germany). The supernatant was used as the DNA template for PCR assays.
2.4. PCR assays
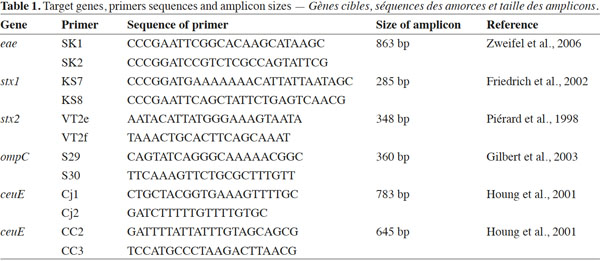
12Simplex PCRs for identification of stx1 and stx2 genes encoding type I and type II Shiga toxins and for identification of the eae gene encoding intimin responsible for lesions of attachment-effacement caused by STEC were developed. Another simplex PCR was developed to detect the ompC gene of Salmonella spp. Finally, 2 simplex PCR were developed to identify C. jejuni and C. coli based on ceuE genes present in both species. Target genes, primers, primers sequences and amplicon sizes are listed in table 1.
2.5. Reference strains
13The PCR experiments were performed on reference strains of E. coli O157:H7 ATCC 43890, EH 384 and EH 1340; Salmonella typhi LO8493, Salmonella spp. 04 and Salmonella spp. 4818; C. jejuni N204, T276 and L359 and C. coli Y58, T287 and A747 provided respectively, from the Reference Laboratory of Enterohemorrhagic Escherichia coli, VUB (Belgium); the Laboratory of Medical Microbiology, UHC of Liege (Belgium); and the Reference Laboratory of Campylobacter, UHC Saint Pierre (Belgium).
2.6. Statistical analysis
14Statistical analysis of results was conducted via IBM SPSS Statistics (Version 19.0 Copyright©, SPSS Inc., Chicago, USA), and the methods used were the chi-squared contingency table test and Fisher’s exact test to determine whether there were associations between prevalence of researched bacteria with species of game meat analysed. The level of agreement according to detection rate by culture and by PCR was expressed using Coehen’s kappa coefficient.
15To minimize error related to sampling, Confidence Interval (CI) with 95% confidence was calculated as follows:
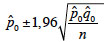
16where p0 is the observed percentage, q0 the complementary percentage, n the number of observations and 1.96 the coefficient for a risk error of 5%.
3. Results
17Faecal contamination was found in 81.3% of all sampling. The prevalence of E. coli in different groups was of 100%, 78.3% and 64.4% respectively from meat of Syncerus caffer, Phacochoerus aethiopicus and Sylvicapra grimmia. The mean count of E. coli was ranging from 4.8 ± 0.8 log10 CFU·g-1 in the meat of Syncerus caffer; 4.8 ± 0.8 log10 CFU·g-1 in the meat of Phacochoerus aethiopicus meat; and 4.9 ± 0.9 log10 CFU·g-1 in the meat of Sylvicapra grimmia. Other findings by culture determined for the all sampling prevalences of 0%, 4.3% and 18.1% respectively for STEC, Salmonella spp. and Campylobacter spp. Among Campylobacter spp., 3.8% were C. jejuni and 14.2% were C. coli. Nevertheless, it is important to mention that during the whole sampling process no culture could yield positive results for STEC, even though culture tests were specifically performed to identify STEC of serotype O157: absence of fermentation of sorbitol in < 24 h and colorless on Sorbitol MacConkey agar (SMAC). Biochemical and serological tests performed on suspected colonies were negative.
18By PCR, we determined for the all sampling prevalences of 2.2%, 6.0% and 19.7% respectively for STEC, Salmonella spp. and Campylobacter spp. Among Campylobacter spp. 3.8%, were characterized as C. jejuni and 15.9% as C. coli. STEC were especially recovered from the meat of Syncerus caffer (6.3%) while cultures were negatives. Among the STEC-positive samples, two were carrying the stx1 gene (3.2%), one was harboring the stx2 gene (1.6%) and the last one was carrying the combination of stx2 and eae genes (1.6%). Comparing both methods, agreement between the PCR and the cultural method was observed (κ = 0.834, κ = 1.000 and κ = 0.936 respectively for Salmonella, C. jejuni and C. coli), although the detection rate was slightly higher by PCR than by culture (Table 2).
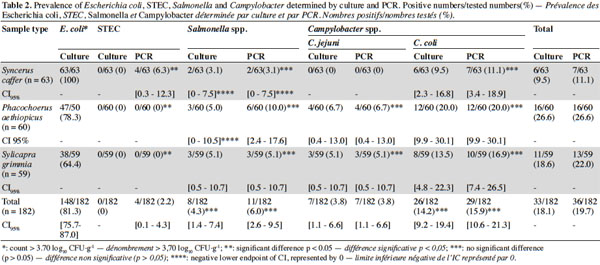
19The highest prevalence of Salmonella (10%), C. jejuni (6.7%) and C. coli (20.0%) were found by PCR in meat of Phacochoerus aethiopicus, there were statistically no difference of level of contamination compared to other types of smoked game meat (p = 0.44, p = 0.13 and p = 0.39 respectively). A significant difference was observed in prevalence of STEC in meat of Syncerus caffer compared to other types of smoked game meat (p = 0.02). In the other hand, the association between the prevalence of E. coli and the prevalence of pathogens was not established (p = 0.33; p = 0.10; p = 0.76 and p = 0.24 respectively for STEC, Salmonella, C. jejuni and C. coli).
4. Discussion
20Bacteriological analyses by culture and PCR were conducted to assess the contamination of smoked game meat by STEC, Salmonella, C. jejuni and C. coli in Lubumbashi. Our findings showed higher prevalences of these pathogens by PCR than by cultural method. We can explain these findings by the sensitivity of PCR as a diagnostic tool, which has been demonstrated in many studies (Debruyne et al., 2008; Shelton et al., 2008; Karmali et al., 2010).
21Faecal contamination of smoked game meat was assessed by the count of E. coli colonies. In the all sampling the mean count of E. coli was ranging from 4.8 ± 0.6 log10 CFU·g-1, and the prevalence of E. coli was of 81.3%. This illustrates an important faecal contamination of smoked game meat as the acceptable Congolese criteria is < 3.7 log10 CFU·g-1. Such faecal contamination could be explained by the lack of hygiene during the preparation of smoked meat, during storage or during the display of smoked meat for sale. In contrast to our case, lower values were found in a study performed in South Africa, on the microbiological quality of matured game salami produced from springbok (Antidorcas marsupialis), gemsbok (Oryx gazelle), kudu (Tragelaphus strepsiceros) and zebra (Equus burchelli). Indeed, the count of E. coli ranged from 1.5 ± 0.5 to 2.0 log10 CFU·g-1. These values were within acceptable limits, as the southern African retail norm for red meat is < 4.7 log10 CFU·g-1 for E. coli (van Schalkwyk et al., 2011). In Nigeria, the highest count for E. coli (7.5 log10 CFU·g-1) was found in beef meat when studying the safety of street-vended meat products (Ologhobo et al., 2010). This is much higher than in Lubumbashi. In the developed world, several studies have demonstrated faecal contamination of fresh game meat, such as red deer, roe deer and wild boar in France (Membré et al., 2011), kangaroo meat in Australia (Holds et al., 2008) and carcasses of bison in Canada (Gill, 2007).
22In Lubumbashi, STEC were only found in smoked game meat of Syncerus caffer. Buffaloes belong to the Bovidae family, of which domestic cattle is a member as well as the main reservoir of STEC. These animals are generally considered as healthy carriers of STEC and therefore they can constitute a source of contamination for other animals (Ateba et al., 2011). Meat is often contaminated at slaughtering and dressing of carcasses. This would explain the presence of STEC in smoked game meat of Syncerus caffer. In our case, it cannot be excluded that contamination of meats due to lack of hygiene occurs during evisceration or when smoking the game meat. Indeed, the tools used for slaughtering and evisceration are often used during smoking. There are few data on contamination of game meat by STEC in Africa. However, in an outbreak of haemorrhagic diarrhoea reported in Ngoïla in Cameroon, STEC was isolated in 50% of samples from contaminated people after consumption of smoked game meat (Germani et al., 1998). In the other hand, this pathogen has been reported in various livestock such as healthy cattle in Uganda (Kaddu-Mulindwa et al., 2001), cattle, sheep, goats and pigs in Nigeria (Ojo et al., 2010), meats like beef meat and meat products in South Africa (Abong’o et al., 2009; Ateba et al., 2011), smoked zebu meat in Central African Republic (Raji et al., 2006) and poultry in Senegal (Nzouankeu et al., 2010).
23In the developed world, studies performed by PCR-based detection of STEC in game meat are rare. However, STEC serotype O157:H7 was isolated by culture from stool of patients contaminated following consumption of sausages made from roe deer in Missouri (Ahn et al., 2009); and from the faeces of wild ruminants such as Cervus elapsus, Capreolus capreolus, Dama dama, Ovis musimon and wild boar in Spain (Sánchez et al., 2009; Sánchez et al., 2010). To avoid underestimation of the real STEC prevalence in Lubumbashi, great care should be taken in order to optimize the STEC isolation methods such as the combination of immunomagentic separation and culture on SMAC supplemented with Cefixim and Tellurite, which has showed an increased isolation rate of STEC O157 (de Boer, 1998). In addition, serological tests and PCR could allow the characterization of STEC serotypes potentially involved in food contamination in Lubumbashi. The presence of one STEC organism can be a threat to public health, as demonstrated by Paton et al. in 1996. Thus, much attention must be accorded to this pathogen.
24The presence of Salmonella in game meat has never been reported in D.R.C. In Zimbabwe, southern Africa, Madsen (1996) determined 20% of Salmonella respectively from refrigerated and frozen crocodile meat. In other regions of Africa, Salmonella was reported in a wide range of sources: various livestock like sheep and goats, poultry dishes, fresh sausages and chicken carcasses (Cardinale et al., 2005; van Nierop et al., 2005; Woldemariam et al., 2005; Mrema et al., 2006). But there is a lack of data focused on the contamination of smoked game meat by Salmonella.
25In developed countries, contamination of fresh game meat by Salmonella has been previously described. Among 385 kangaroo carcasses, 4 (1.0%) were identified as being contaminated by Salmonella in Australia (Holds et al., 2008). But this prevalence is much lower than the prevalence determined in game meat from Lubumbashi. According to Congolese standards, the presence of one Salmonella spp. per 25 g of analyzed food sample is not acceptable, and food must be considered unsuitable for human consumption. Thus, in addition to our qualitative study, quantitative studies must be conducted to determine the levels of bacteria that can constitute a threat for public health as usually the food is cooked at high temperature, which significantly reduces the bacterial load.
26Little is known about contamination of game meat by Campylobacter in Africa. However, Campylobacter contamination of food animals like sheep, goats, chickens, meats and meats products, such as chicken dishes, beef meat, goat meat, sheep meat, has been already reported (Cardinale et al., 2005; Kassa et al., 2007; Dadi et al., 2008; Woldemariam et al., 2009). In Lwiro, the eastern region of D.R.C., among patients who attended as outpatients because of diarrhoea and among children admitted in the same hospital because of malnutrition, C. jejuni was identified in 13.7% of outpatients and in 24.0% of inpatients. The same study was also conducted on animals handled by the local population and C. jejuni was found in goats (13.0%), pigs (38.4%) and chickens (40.0%) (De Mol et al., 1983).
27In our study, C. coli was more detected (15.9%) than C. jejuni (3.8%). Similar findings were reported in developed world, although the prevalence of Campylobacter in game meat was very low. Among 1.0% of Campylobacter detected in meat samples of wild boar and feral pigs, two were identified as being C. coli and one as C. jejuni (Atanassova et al., 2008). In contrast to us, Gill (2007) could not detect any Campylobacter from fresh game meat sold at retail. Consideration must be taken for C. jejuni and C. coli as major foodborne pathogens, though the Congolese standard does not stipulate a standard level of Campylobacter spp. in food. Further studies should be conducted on characterization of the Campylobacter species frequently isolated, as well as determining the antibiotic susceptibility of these pathogens. This, in order to prevent emergence or dissemination of antimicrobial-resistant Campylobacter, which has increased the concern that treatment of foodborne campylobacterisis can be compromised in cases of severe symptoms or in immunocompromised patients (Ge et al., 2003; Uaboi-Egbeni et al., 2011).
28This is the first assessment of bacteriological quality of smoked game meat conducted in Lubumbashi. However, the study encountered limitations particularly the accessibility to villages to meet hunters and/or producers of smoked game meat, and to convince these people to participate in our survey. The cost of smoked game meat was also a limitation for the sampling process, as all the samples were purchased.
29In conclusion, smoked game meat sold at retail outlets in Lubumbashi is contaminated by STEC, Salmonella, C. jejuni and C. coli, with respective detection rates of 2.2%, 6.0%, 3.8% and 15.9% determined by PCR. These results provide reliable epidemiological data on the bacteriological quality of smoked game meat in Lubumbashi. Further studies should be conducted on serotyping of STEC and Salmonella, and characterization of the Campylobacter species frequently isolated in meat Lubumbashi. Although trade in smoked game meat is allowed, it remains difficult to control the production chain of such meat, because sometimes game meat comes from hunting by poaching in national parks or from occasional hunting which are generally illegal and the markets are often supplied with game meat from unknown origin. With the establishment of supervised hunting within defined hunting periods and a quota of game per season, in addition to the identification of hunters and intermediate sellers of game between hunters, smoked game meat dealers and salesmen in local markets, it would be possible to develop monitoring plans of the production chain and commercialization of smoked game meat in Lubumbashi.
30Acknowledgements
31The authors would like to thank all individuals who participated to the study, as well as those who took part by identifying sites of smoked game meat preparation and interviewing game meat salesmen. Special thanks are also extended to the people who helped to carry out the sampling and later analyses to the laboratories in Lubumbashi (DRC) and Liege (Belgium). Thanks to the Reference Laboratory of Campylobacter/UHC Saint-Pierre (Belgium) and the Reference Laboratory for Enterohemorrhagic E. coli/VUB (Belgium) for providing reference strains. The Belgian Technical Cooperation is sincerely thanked for its financial support.
Bibliographie
Abong'o B.O. & Momba M.N., 2009. Prevalence and characterization of Escherichia coli O157:H7 isolates from meat and meat products sold in Amathole District, Eastern Cape Province of South Africa. Food Microbiol., 26(2), 173-176.
Ahn C.K. et al., 2009. Deer sausage: a newly identified vehicle of transmission of Escherichia coli O157:H7. J. Pediatr., 155(4), 587-589.
Atanassova V., Apelt J., Reich F. & Klein G., 2008. Microbiological quality of freshly shot game in Germany. Meat Sci., 78(4), 414-419.
Ateba C.N. & Mbewe M., 2011. Detection of Escherichia coli O157:H7 virulence genes in isolates from beef, pork, water, human and animal species in the northwest province, South Africa: public health implications. Res. Microbiol., 162, 240-248.
Bachand N. et al., 2012. Public health significance of zoonotic bacterial pathogens from bushmeat sold in urban markets of Gabon, Central Africa. J. Wildl. Dis., 48(3), 785-789.
Cardinale E. et al., 2005. Risk factors for contamination of ready-to-eat street-vended poultry dishes in Dakar, Senegal. Int. J. Food Microbiol., 103(2), 157-165.
Chapman P.A., 1995. Verocytotoxin-producing Escherichia coli: an overview with emphasis on the epidemiology and prospects for control of E. coli O157. Food Control, 6(4), 187-193.
Chomel B.B., Beletto A. & Meslin F.-X., 2007. Wildlife, exotic pets, and emerging zoonoses. Emerg. Infect. Dis., 13(1), 6-11.
Dadi L. & Asrat D., 2008. Prevalence and antimicrobial susceptibility profiles of thermotolerant Campylobacter strains in retail raw meat product in Ethiopia. Ethiop. J. Health Dev., 22(2), 195-200.
de Boer E., 1998. Update on media for isolation of Enterobacteriaceae from foods. Int. J. Food Microbiol., 45(1), 43-53.
Debruyne L. et al., 2008. Comparative performance of different PCR assays for the identification of Campylobacter jejuni and Campylobacter coli. Res. Microbiol., 159(2), 88-93.
de Mol P. et al., 1983. Enteropathogenic agents in children with diarrhoea in rural Zaire. Lancet, 1(8323), 516-518.
Enokimoto M. et al., 2007. Enumeration and identification of Campylobacter species in the liver and bile of slaughtered cattle. Int. J. Food Microbiol., 118(3), 259-263.
Fhogartaigh C.N. & Edgeworth J.D., 2009. Bacterial gastroenteritis. Medicine, 37(11), 586-593.
Friedrich A.W. et al., 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis., 185(1), 74-84.
Ge B. et al., 2003. Antimicrobial-resistant Campylobacter species from retail raw meats. Appl. Environ. Microbiol., 69(5), 3005-3007.
Germani Y. et al., 1998. Enterohaemorrhagic Escherichia coli in Ngoila (Cameroon) during an outbreak of bloody diarrhoea. Lancet, 352(9128), 625-626.
Gilbert C., Winters D., O'Leary A. & Slavik M., 2003. Development of a triplex PCR assay for the specific detection of Campylobacter jejuni, Salmonella spp., and Escherichia coli O157:H7. Mol. Cell. Probes, 17(4), 135-138.
Gill C.O., 2007. Microbiological conditions of meats from large game animals and birds. Meat Sci., 77(2), 149-160.
Goualié G.B. et al., 2012. Prevalence and antimicrobial resistance of thermophilic Campylobacter isolated from chicken in Côte d’Ivoire. Int. J. Microbiol., Article ID 150612, 5 pages.
Hilbert F., Smulders F.J.M., Chopra-Dewasthaly R. & Paulen P., 2012. Salmonella in the wildlife-human interface. Food Res. Int., 45(2), 603-608.
Holds G. et al., 2008. Microbial profiles of carcasses and minced meat from kangaroos processed in South Australia. Int. J. Food Microbiol., 123(1-2), 88-92.
Houng H.S. et al., 2001. Development of a ceuE-based multiplex polymerase chain reaction (PCR) assay for direct detection and differentiation of Campylobacter jejuni and Campylobacter coli in Thailand. Diagn. Microbiol. Infect. Dis., 40(1-2), 11-19.
Kaddu-Mulindwa D.H. et al., 2001. Occurrence of Shiga toxin-producing Escherichia coli in fecal samples from children with diarrhea and from healthy zebu cattle in Uganda. Int. J. Food Microbiol., 66(1-2), 95-101.
Karesh W.B. et al., 2012. Ecology of zoonoses: natural and unnatural histories. Lancet, 380, 1936-1945.
Karmali M.A., Gannon V. & Sargeant J.M., 2010. Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol., 140(3-4), 360-370.
Kassa T., Gebre-Selassie S. & Asrat D., 2007. Antimicrobial susceptibility patterns of thermotolerant Campylobacter strains isolated from food animals in Ethiopia. Vet. Microbiol., 119(1), 82-87.
Madsen M., 1996. Prevalence and serovar distribution of Salmonella in fresh and frozen meat from captive Nile crocodiles (Crocodylus niloticus). Int. J. Food Microbiol., 29(1), 111-118.
Membré J.M., Laroche M. & Magras C., 2011. Assessment of levels of bacterial contamination of large wild game meat in Europe. Food Microbiol., 28(5), 1072-1079.
Miko A. et al., 2009. Assessment of Shiga toxin-producing Escherichia coli isolates from wildlife meat as potential pathogens for humans. Appl. Environ. Microbiol., 75, 6462-6470.
Mohammed M.A.M., 2012. Molecular characterization of diarrheagenic Escherichia coli isolated from meat products sold at Mansoura city, Egypt. Food Control, 25, 159-164.
Mrema N., Mpuchane S. & Gasha B.A., 2006. Prevalence of Salmonella in raw minced meat, raw fresh sausages and raw burger patties from retail outlets in Gaborone, Botswana. Food Control, 17, 202-212.
Nzouankeu A. et al., 2010. Multiple contaminations of chickens with Campylobacter, Escherichia coli and Salmonella in Yaounde (Cameroon). J. Infect. Dev. Ctries., 4(9), 583-686.
Ojo O.E. et al., 2010. Potentially zoonotic shiga toxin-producing Escherichia coli serogroups in the faeces and meat of food-producing animals in Ibadan, Nigeria. Int. J. Food Microbiol., 142(1-2), 214-221.
Ologhobo A.D. et al., 2010. Safety of street vended meat products-chicken and beef suya. Afr. J. Biotechnol., 9, 4091-4095.
Onyango C.A., Izumimoto M. & Kutima P.M., 1998. Comparison of some physical and chemical properties of selected game meats. Meat Sci., 49(1), 117-125.
Paton A.W. et al., 1996. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J. Clin. Microbiol., 34(7), 1622-1627.
Piérard D. et al., 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol., 36(11), 3317-3322.
Raji M.A., Minga U. & Machangu R., 2006. Current epidemiological status of enterohaemorrhagic Escherichia coli O157: H7 in Africa. Chin. Med. J., 119(3), 217-222.
Sallam K.I., 2007. Prevalence of Campylobacter in chicken and chicken by-products retailed in Sapporo area, Hokkaido, Japan. Food Control, 18, 1113-1120.
Sanchez S. et al., 2009. Detection and characterization of Shiga toxin-producing Escherichia coli other than Escherichia coli O157:H7 in wild ruminants. Vet. J., 180(3), 384-388.
Sanchez S. et al., 2010. Detection and characterization of O157:H7 and non-O157 Shiga toxin-producing Escherichia coli in wild boars. Vet. Microbiol., 143(2-4), 420-423.
Shelton D.R., Karns J.S. & Park C.H., 2008. A multiple protocol to improve diagnosis and isolation of Shiga toxin-producing Escherichia coli from human stool specimens. Diagn. Microbiol. Infect. Dis., 62(1), 7-10.
Uaboi-Egbeni P.O., Bessong P.O., Samie A. & Obi C.L., 2011. Prevalence and antimicrobial susceptibility profiles of Campylobacter jejuni and coli isolated from diarrheic and non-diarrheic goat faces in Venda region, South Africa. Afr. J. Biotechnol., 10(64), 14116-14124.
van Nierop W. et al., 2005. Contamination of chicken carcasses in Gauteng, South Africa, by Salmonella, Listeria monocytogenes and Campylobacter. Int. J. Food Microbiol., 99(1), 1-6.
van Schalkwyk D.L. et al., 2011. Physico-chemical, microbiological, textural and sensory attributes of matured game salami produced from springbok (Antidorcas marsupialis), gemsbok (Oryx gazella), kudu (Tragelaphus strepsiceros) and zebra (Equus burchelli) harvested in Namibia. Meat Sci., 88(1), 36-44.
Woldemariam E., Molla B., Alemayethu D. & Muckle A., 2005. Prevalence and distribution of Salmonella in apparently healthy slaughtered sheep and goats in abattoir in Debre Zeit area, Ethiopia. Small Rumin. Res., 58(1), 19-24.
Woldemariam T., Asrat D. & Zwede G., 2009. Prevalence of thermophilic Campylobacter species in carcasses from sheep and goats in an abattoir in Debre Zeit area, Ethiopia. Ethiop. J. Health Dev., 23(3), 229-233.
Zweifel C. et al., 2006. Virulence profiles of Shiga toxin 2e-producing Escherichia coli isolated from healthy pig at slaughter. Vet. Microbiol., 117(2-4), 328-332.






