- Portada
- Volume 17 (2013)
- numéro 4
- Reusability study of Novozym® 435 for the enzymatic synthesis of mannosyl myristate in pure ionic liquids
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Reusability study of Novozym® 435 for the enzymatic synthesis of mannosyl myristate in pure ionic liquids

Notes de la rédaction
Received on April 7, 2013; accepted on July 30, 2013
Résumé
Stabilité fonctionnelle de la Novozyme® 435 au cours de la synthèse enzymatique du myristate de mannosyle en liquide ionique pur. Lors de l’élaboration d’une voie de synthèse biocatalysée, la réutilisation de l’enzyme est un paramètre important à considérer pour la réduction des couts industriels. Dans ce contexte, la stabilité fonctionnelle de la Novozyme® 435 dans les liquides ioniques (LIs) a été étudiée pour la transestérification du mannose avec le myristate de vinyle. L’enzyme a été réutilisée cinq fois dans trois LIs ([Bmim][BF4], [Bmim][TFO] and [Bmpyrr][TFO]) et dans le tert-butanol (tert-BuOH). [Bmpyrr][TFO] a montré le meilleur rendement après 24 h (η-24 h), avec 68,8 % après le premier cycle et la perte de η-24 h la plus faible (42 %) après cinq cycles (η-24 h de 39,9 %). Comparé à [Bmpyrr][TFO], l’enzyme a présenté la plus importante perte d’activité après cinq réactions dans [Bmim][TFO] (perte de 89 %) en dépit de l’obtention d’un bon η-24 h (60 %). [Bmim][BF4] est le LI le moins intéressant puisqu’il a abouti au η-24 h le plus faible avec 24,5 % après un cycle et d’une importante perte d’activité (77 %) après cinq cycles dont le η-24 h est de 5,6 %. Après cinq cycles, le η-24 h dans [Bmpyrr][TFO] est plus élevé que dans le tert-BuOH et la perte de rendement est plus élevée dans le solvant organique (57 %). En conséquence, ces résultats montrent que le LI basé sur le cation pyrrolidium [Bmpyrr][TFO] est le meilleur LI en termes d’activité enzymatique et de stabilité fonctionnelle pour la Novozyme® 435 dans cette étude.
Abstract
When developing a biocatalyzed synthesis route, the enzyme reusability is an important parameter to consider for the reduction of industrial costs. In this context, the functional stability of Novozym® 435 in ionic liquids (ILs) was studied in the transesterification of mannose with vinyl myristate. The enzyme was re-used five times in three ILs, 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4]), 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([Bmim][TFO]), 1-butyl-1-methylpyrrolidinium trifluoromethanesulfonate ([Bmpyrr][TFO]) and in tert-butanol (tert-BuOH). [Bmpyrr][TFO] showed the best 24 h-yield (24 h-η), with 68.8% after the first cycle and the lowest loss of 24 h-η (42%) after five cycles (24 h-η of 39.9%). In comparison with [Bmpyrr][TFO], Novozym® 435 presented the most prominent loss of activity after five cycles of reaction in [Bmim][TFO] (loss of 89%), despite the good 24 h-η obtained after one cycle (60%). [Bmim][BF4] was the least interesting IL, as it was found to lead to the lowest 24 h-η, with 24.5% after one cycle and a significant loss of activity (77%) after five cycles, with a 24 h-η of 5.6%. After five cycles, the 24 h-η in [Bmpyrr][TFO] was higher than in tert-BuOH and the yield loss was higher for the organic solvent (57%). Consequently, these results reveal that, in the present study, the pyrrolidium-based IL [Bmpyrr][TFO] represented the best IL as it allowed the highest level of enzymatic activity and functional stability of Novozym® 435.
Tabla de contenidos
1. Introduction
1Over the last decade, research exploring the use of ionic liquids (ILs) for biocatalysis has attracted increasing attention (Kim et al., 2001; Sheldon et al., 2002; Park et al., 2003; van Rantwijk et al., 2003; van Rantwijk et al., 2007; Roosen et al., 2008; Habulin et al., 2011). The so-called room temperature ionic liquids (RTILs) are salt-like materials, which have no detectable vapor pressure at room temperature. RTILs also have a high thermal and chemical stability, the capacity to solubilize a large range of polar and apolar compounds, and the ability to improve enzymatic activity and stability (Kaar et al., 2003; Zhao, 2010). Furthermore, the properties of these solvents – such as their viscosity, polarity and hydrophobicity – can be easily adapted through the use of a combination of a large variety of cations and anions (Galonde et al., 2012). All these characteristics make these innovative solvents an excellent “eco-friendly” and benign alternative to the harmful volatile organic solvents (VOSs) traditionally used for the biocatalysis of sugar fatty acid esters (SFAEs). Because SFAEs are widely used in food, pharmaceuticals and cosmetic applications, there is a growing demand for the development of green production routes for these compounds. Indeed, the chemical synthesis of SFAEs, generally carried out in VOSs, requires drastic conditions (high temperature, complex protection/deprotection strategies), which can lead to potentially harmful by-products (Chang et al., 2009). The use of ILs in biocatalysis, by contrast, presents several advantages. However, the obvious drawback for industry is the expensive nature of these solvents. This problem could be overcome if recyclability of the enzymes and ILs used in biocatalytic processes were to be developed (Ward et al., 1997; Selmi et al., 1998; Deng et al., 2003; Itoh et al., 2003; Fehér et al., 2008; Ha et al., 2008; Lee et al., 2008; Vidya et al., 2009).
2In this context, the present study aims to investigate the re-use of Novozym® 435 during five reaction cycles for the synthesis of mannosyl myristate by transesterification both in three ILs (Figure 1), and in a reference organic solvent (tert-BuOH). The yields obtained in each medium after each run of 24 h were compared, and the effect of the IL properties on the enzyme’s operational stability was investigated. The study focuses on the synthesis of an ester of mannose, as mannose vaccines or drug carriers can be useful for increasing immunogenicity (Zhou et al., 2007; Jiang et al., 2008) and tumor targeting (Prakash et al., 2010). The three ILs chosen were highlighted in our earlier study, during which nine ILs (differing by their ion type and their alkyl chain length on the cation) were screened for the synthesis of the same mannose ester (Galonde et al., 2013).
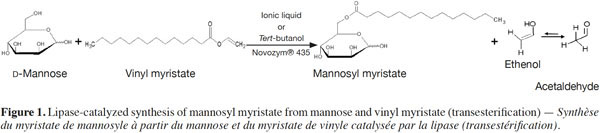
2. Materials and methods
2.1. Materials
3The ILs used (99.5% purity), 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4]), 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([Bmim][TFO]) and 1-butyl-1-methylpyrrolidinium trifluoromethanesulfonate ([Bmpyrr][TFO]), were purchased from Solvionic (Toulouse, France). D-Mannose (> 99%), tetrahydrofurane (THF, ≥ 99.9%), formic acid (> 98%) and tert-butanol (tert-BuOH, ≥ 99%) were purchased from Sigma-Aldrich NV/SA (Bornem, Belgium). Vinyl myristate (> 99%, stabilized with MEHQ) was purchased from TCI Europe NV (Zwijndrecht, Belgium). Lipase B from Candida antarctica, immobilized on acrylic resin (Novozym® 435), was a gift from Novozymes A/S (Bagsværd, Denmark). The HPLC grade acetonitrile (ACN) and methanol (MeOH) were purchased from Scharlab SL (Spain).
2.2. General procedure for enzymatic acylation and recovery of the enzyme
4Enzymatic acylation. A quantity of 0.05 mmol of mannose and 0.30 mmol of vinyl myristate were mixed in 0.5 ml of IL or tert-BuOH in a screw- cap tube, vigorously stirred at 600 rpm and thermostated at 60 °C. The reaction was started by adding 5% (w/v) of Novozym® 435. After 24 h, four volumes of THF were added to the reaction medium.
5Enzyme’s recovery. In order to separate the enzyme from the enzymatic medium for its re-use, the resulting reaction medium with THF was filtered under vacuum. The recovered enzyme was rinsed with 5 ml of THF, dried in an oven at 40 °C overnight and stored in a desiccator until the next run. An aliquot of the reaction medium was filtered on a 0.22 µm pore size nylon membrane, before quantification of the ester by RP-HPLC. In order to evaluate the impact of the recovery process on the enzyme’s activity, 25 mg of Novozym® 435 (5% [w/v] of the reaction medium) was washed, recovered and dried between one and four times before use. After 24 h of reaction (triplicate) and quantitative analysis of the ester by RP-HPLC, the relative 24 h-η was determined using the following equation:
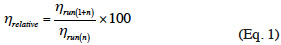
2.3. HPLC analysis method for determination of the product yield
6The quantitative analyses were performed on an Agilent Technologies 1200 series HPLC equipped with an evaporative light scattering detector (ELSD). A Halo® fused-core RP-C18 column (75 x 4.6 mm, 2.6 µm) from Advanced Materials Technology was thermostated at 30 °C. The elution was carried out from a linear gradient of ACN/water (both containing 0.1% of formic acid): the ACN % increased from 60% to 100% in 2 min at a flow rate of 1.5 ml·min-1. The ester was quantified by external calibration. The calibration curve was obtained with a series of mannosyl myristate solutions in a concentration range from 0.1 g·l-1 to 10 g·l-1. The pure standards were obtained after enzymatic esterification of mannose by myristic acid in tert-BuOH, and purification by flash chromatography on a silica gel (60 Å = 40-63 mm) column chromatography as previously described (Nott et al., 2012). The 24 h-η (expressed as a percentage) with mannose as default substrate was determined as follows:
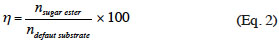
3. Results and discussion
7The influence of the impact of the recovery process on the enzyme’s performance has been tested. The results show that the residual 24 h-η was no lower than 95% after each cycle, no matter what reaction medium was used (data not shown). The recovery process has thus an insignificant influence on the lipase performance.
8Figures 2a and 2b show the 24 h-η and the residual 24 h-η obtained in the three ILs ([Bmim][BF4], [Bmim][TFO] and [Bmpyrr][TFO]), and in tert-BuOH after each step of the five cycles of transesterification. The 24 h-η follows the subsequent decreasing order after the first cycle: [Bmpyrr][TFO] > [Bmim][TFO] ≈ tert-BuOH > [Bmim][BF4]. These results are consistent with those obtained in our previous study (Galonde et al., 2013). As discussed in that article, the use of reasonably hydrophilic ILs (property mainly governed by the anion choice) leads to good reaction yields as the sugar is sufficiently dissolved. The choice of cations with medium chain length (four carbon atoms) is a good compromise since suitable lipase activity is maintained (Mutschler et al., 2009) and the viscosity of the medium is at a reasonable level (van Rantwijk et al., 2003; Zhao et al., 2008).
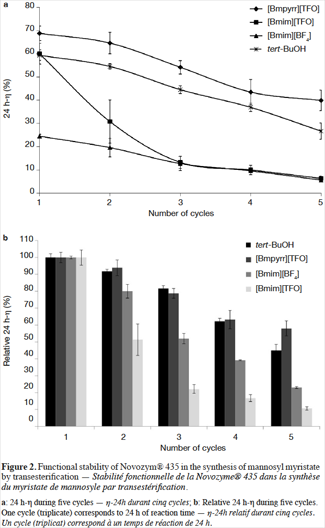
9Figure 2a also shows that for all the solvents tested, the 24 h-η diminishes with the number of reactions. However, as seen on figure 2b the decrease of the 24 h-η is more or less notable depending on the solvent considered. The decrease of 24 h-η observed after five cycles is 42%, 57%, 77% and 89%, for [Bmpyrr][TFO], tert-BuOH, [Bmim][TFO] and [Bmim][BF4] respectively. This result emphasizes the fact that [Bmpyrr][TFO] is the best of the three selected ILs for the mannosyl myristate synthesis.
10Another study into the reusability of CAL-B in tert-BuOH demonstrated a decrease in conversion of 25% after six cycles of reaction, in the case of the synthesis of 6-O-glucose palmitate by esterification. This lower decrease of product yield compared to our study can be explained by the immobilization of CAL-B on polypropylene, which increases the stability of the enzyme in the chosen solvent media (Cao et al., 1999).
11In order to explain the catalytic behavior of enzymes in organic solvents, the log P parameter is utilized. It has been reported that lipases are more stable in hydrophobic solvents (Laane et al., 1987). The log P parameter is an indicator of the hydrophilicity or hydrophobicity of a chemical; it is obtained by measuring the partition coefficient of the given compound between n-octanol and water (Laane et al., 1987; Kaar et al., 2003). It has been stated that solvents with a log P value less than 2 are considered as hydrophilic, and may be less favorable for enzymatic stability than solvents considered as hydrophobic with a log P above 4. The influence of solvents with a log P value between 2 and 4 on the enzymatic activity is unpredictable (Laane et al., 1987). For example, for the synthesis of 6-O-glucose octanoate catalyzed by CAL-B immobilized on polypropylene (Cao et al., 1999), dioxane (log P = -1.1) showed a slightly lower relative activity compared to the less hydrophilic tert-BuOH (log P = 0.37) (He et al., 2012). Conversely, the residual activity is lower in acetone than in dioxane even if acetone is less hydrophilic (log P = 0.23) than dioxane. This confirms the difficulty of predicting the enzyme activity in a solvent solely based on its log P value.
12The same contradictions were observed when the enzyme reusability is compared between ILs and between ILs and VOSs. ILs are in general more hydrophilic than an organic solvent according to their log P value. For the ILs used in this study, the log P value of [Bmpyrr][TFO], [Bmim][TFO] and [Bmim][BF4] are -1.91 (calculated from Molinspiration), -1.61 ± 0.05 (Cho et al., 2011) and -2.51 ± 0.04 respectively (Zhao et al., 2009). According to the log P values, tert-BuOH is more hydrophobic than the ILs tested. However, tert-BuOH does not lead to the best 24 h-η in comparison with [Bmpyrr][TFO]. In addition, this last IL also shows the lowest 24 h-η loss after five cycles (42% vs 57% for tert-BuOH). This result is in correlation with the study of Candida rugosa lipase re-use in n-hexane and [Bmim][PF6] for the enantioselective esterification of (R,S)-2-chloropropanoic acid with butan-1-ol (Gubicza et al., 2003). According to its log P value of 3.5 (Laane et al., 1987), n-hexane is significantly more hydrophobic than [Bmim][PF6] (-2.06) (Ha et al., 2008). However, the loss of activity after five runs in n-hexane is greater (40%) than in [Bmim][PF6] (7%) (Gubicza et al., 2003). The operational stability of Novozym® 435 has been compared in organic solvents and ILs for the kinetic resolution of (R,S)-1-phenylethanol with vinyl acetate, and this comparison has illustrated that the residual activity after six cycles is consistently higher in ILs such as [Emim][TF2N], [Bmim][PF6] (log P value of -1.18 and -2.06 respectively) than in the less hydrophilic toluene and acetone (log P values of 2.5 and -0.23 respectively) (Ha et al., 2008). Some ILs are consequently more efficient as solvents than VOSs despite a lower log P value (correlated to a less hydrophobic aspect).
13The comparison between the three ILs selected in this study shows that the 24 h-η obtained in the reaction catalyzed by Novozym® 435 is least affected in [Bmpyrr][TFO], with a 24 h-η loss after five cycles of 42%. Therefore, this IL is more advantageous than [Bmim][TFO] and [Bmim][BF4] in terms of 24 h-η and the functional stability of Novozym® 435. The most dramatic 24 h-η loss after five cycles is observed in [Bmim][TFO] (89%), and [Bmim][BF4] showed the lowest 24 h-η during these five cycles of reaction (from 24.5% to 5.6%) as well as a very low enzymatic functional stability (77% of 24 h-η loss after five cycles).
14The imidazolium-based ILs, [Bmim][BF4] and [Bmim][TFO] give very different performances in this study. [Bmim][BF4] is the most hydrophilic IL and [Bmim][TFO] the least hydrophilic ILs according to their log P values (-2.51 and -1.61 respectively). Therefore, Novozym® 435 should be less stable in [Bmim][BF4] but, according to figure 2b, the residual 24 h-η (loss of 77%) is much lower than in [Bmim][TFO] (loss of 89%), after five runs. Therefore, the log P value is not the best parameter for predicting the enzymatic activity in ILs and VOSs. Other factors may explain the distinct behavior of the tested ILs:
15– protein-IL interactions leading to a change in the enzyme’s conformation and denaturation;
16– ILs’ viscosity, which increases the mass transfer limitation;
17– substrate’s polarity;
18– degradation of ILs such as [Bmim][BF4], which may release fluorhydric acid during the enzymatic reaction, acidifying the reaction medium (Galonde et al., 2012; Galonde et al., 2013).
19[Bmim][BF4] and [Bmim][TFO] are different due to the nature of their anion, revealing the importance of the IL’s anion on the enzymatic catalysis and stability. Indeed, anions can, depending on their nature, form more or less abundant H-bonds, which are favorable for sugar dissolution, but may disturb the intramolecular H-bonds of proteins, modifying their conformation which is unfavorable for the enzyme (Forsyth et al., 2001; Klahn et al., 2011). Therefore, the TFO- anion seems to be more favorable for the enzymatic stability than BF4-. However, the contradictory results given by the studied TFO-based ILs, [Bmpyrr][TFO] and [Bmim][TFO], show that the influence of ILs not only depends on the anions but also on the cations. Despite the high 24 h-η reached in [Bmim][TFO] after the first cycle (60%), the most drastic decrease of enzymatic activity after five cycles is observed in this IL (89%). Therefore, TFO- based ILs display very different behavior, since the relative 24 h-η after five cycles is much higher in [Bmpyrr][TFO] than in [Bmim][TFO], with a 24 h-η of 39.9% and 5.6% respectively. Previous simulation studies have attempted to explain the behavior of the cation in lipase surroundings (Gorke et al., 2010). Cation components of ILs indirectly influence the interaction strength between the enzymes and the anion parts. The smallest cations lead to stronger Novozym® 435-anion interactions due to the increased number of anions in the enzyme neighborhood (Gorke et al., 2010). Furthermore, the lipase-cation Van der Waals interactions result in the diffusion of cations into the lipase’s active site, while the diffusion of anions is not observed (Klahn et al., 2011). Therefore, the interactions between lipase and the pyrrolidinium cation seem to be more suitable than imidazolium cation for lipase activity (Galonde et al., 2013), as well as for its long-term re-use.
4. Conclusion
20Since the enzyme technology can be an attractive industrial alternative to catalysis, the reusability of the biocatalyst may represent a significant reduction of costs. The reusability study of Novozym® 435 for the synthesis of mannosyl myristate by transesterification in ILs illustrated that this enzyme gave the best 24 h-η in [Bmpyrr][TFO] and was relatively stable in this IL, with a loss of activity of 42% after five utilizations which was better than the loss of 57% found in tert-BuOH. The most significant loss of 24 h-η after five cycles was observed in [Bmim][TFO] (89%) despite the high 24 h-η after the first cycle (60%). Additionally, in [Bmim][BF4] the 24 h-η after one cycle (24.5%) is very low and the decrease percentage of the 24 h-η (77%) very high. Therefore, [Bmpyrr][TFO] appears to be the best compromise between lipase activity and stability compared to the other ILs tested. This observation gives interesting perspectives for pyrrolidinium-based ILs for biocatalysis of SFAEs.
21Abbreviations
22[Bmpyrr][TFO]: 1-butyl-1-methylpyrrolidinium trifluoromethanesulfonate
23[Bmim][TFO]: 1-butyl-3-methylimidazolium trifluoromethanesulfonate
24[Bmim][BF4]: 1-butyl-3-methylimidazolium tetrafluoroborate
25[Bmim][TF2N]:1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide
26[Bmim][PF6]: 1-butyl-3-methylimidazolium hexafluorophosphate
27[Emim][TF2N]:1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide
28tert-BuOH: tert-butanol
29Acknowledgements
30This work was funded through the ARC grant “Superzym”, financed by the Wallonia-Brussels Federation which is gratefully acknowledged for its financial support. Magali Deleu thanks the FNRS (Fonds National de la Recherche Scientifique) from Belgium for her research associate position. Special thanks are addressed to Ketty Pouleur for her technical assistance and interest.
Bibliographie
Cao L., Bornscheuer U.T. & Schmid R.D., 1999. Lipase-catalyzed solid-phase synthesis of sugar esters. Influence of immobilization on productivity and stability of the enzyme. J. Mol. Catal. B: Enzym., 6(3), 279-285.
Chang S.W. & Shaw J.F., 2009. Biocatalysis for the production of carbohydrate esters. New Biotechnol., 26(3-4), 109-116.
Cho C.-W. et al., 2011. Ionic liquids: predictions of physicochemical properties with experimental and/or DFT-calculated LFER parameters to understand molecular interactions in solution. J. Phys. Chem. B., 115(19), 6040-6050.
Deng L., Tan T., Wang F. & Xu X., 2003. Enzymatic production of fatty acid alkyl esters with a lipase preparation from Candida sp. 99-125. Eur. J. Lipid Sci. Technol., 105(12), 727-734.
Fehér E. et al., 2008. Enzymatic production of isoamyl acetate in an ionic liquid-alcohol biphasic system. J. Mol. Catal. B: Enzym., 50(1), 28-32.
Forsyth S., Golding J., MacFarlane D.R. & Forsyth M., 2001. N-methyl-N-alkylpyrrolidinium tetrafluoroborate salts: ionic solvents and solid electrolytes. Electrochim. Acta, 46(10-11), 1753-1757.
Galonde N. et al., 2012. Use of ionic liquids for biocatalytic synthesis of sugar derivatives. J. Chem. Technol. Biotechnol., 87(4), 451-471.
Galonde N. et al., 2013. Study of the influence of pure ionic liquids on the lipase-catalyzed (trans)esterification of mannose based on their anion and cation nature. Curr. Org. Chem., 17(7), 763-770.
Gorke J., Srienc F. & Kazlauskas R., 2010. Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol. Bioprocess Eng., 15(1), 40-53.
Gubicza L., Nemestothy N., Frater T. & Belafi-Bako K., 2003. Enzymatic esterification in ionic liquids integrated with pervaporation for water removal. Green Chem., 5(2), 236-239.
Ha S. et al., 2008. Enhanced stability of Candida antarctica lipase B in ionic liquids. Korean J. Chem. Eng., 25(2), 291-294.
Habulin M., Primožič M. & Knez Ž., 2011. Application of ionic liquids in biocatalysis. In: Kokorin P.A., eds. Ionic liquids: applications and perspectives. InTech Inc., 461-480, DOI: 10.5772/15354.
He W.-S. et al., 2012. Lipase-mediated synthesis of water-soluble plant stanol derivatives in tert-butanol. Bioresour. Technol., 114, 1-5.
Itoh T., Nishimura Y., Ouchi N. & Hayase S., 2003. 1-Butyl-2,3-dimethylimidazolium tetrafluoroborate: the most desirable ionic liquid solvent for recycling use of enzyme in lipase-catalyzed transesterification using vinyl acetate as acyl donor. J. Mol. Catal. B: Enzym., 26(1-2), 41-45.
Jiang H.-L. et al., 2008. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization. Biomaterials, 29(12), 1931-1939.
Kaar J.L. et al., 2003. Impact of ionic liquid physical properties on lipase activity and stability. J. Am. Chem. Soc., 125(14), 4125-4131.
Kim K.-W., Song B., Choi M.-Y. & Kim M.-J., 2001. Biocatalysis in ionic liquids: markedly enhanced enantioselectivity of lipase. Org. Lett., 3(10), 1507-1509.
Klahn M., Lim G.S., Seduraman A. & Wu P., 2011. On the different roles of anions and cations in the solvation of enzymes in ionic liquids. Phys. Chem. Chem. Phys., 13(4), 1649-1662.
Laane C., Boeren S., Vos K. & Veeger C., 1987. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng., 30(1), 81-87.
Lee S.H. et al., 2008. Lipase-catalyzed synthesis of glucose fatty acid ester using ionic liquids mixtures. J. Biotechnol., 133(4), 486-489.
Molinspiration, http://www.molinspiration.com/cgi-bin/properties, (29/03/2013).
Mutschler J. et al., 2009. Ionic liquid-coated immobilized lipase for the synthesis of methylglucose fatty acid esters. Green Chem., 11(11), 1793-1800.
Nott K. et al., 2012. (Trans)esterification of mannose catalyzed by lipase from Candida antarctica in an improved reaction medium using co-solvents and molecular sieve. Prep. Biochem. Biotechnol., 42(4), 348-363.
Park S. & Kazlauskas R.J., 2003. Biocatalysis in ionic liquids – advantages beyond green technology. Curr. Opin. Biotechnol., 14(4), 432-437.
Prakash J. et al., 2010. Tumor-targeted intracellular delivery of anticancer drugs through the mannose-6-phosphate/insulin-like growth factor II receptor. Int. J. Cancer, 126(8), 1966-1981.
Roosen C., Müller P. & Greiner L., 2008. Ionic liquids in biotechnology: applications and perspectives for biotransformations. Appl. Microbiol. Biotechnol., 81(4), 607-614.
Selmi B. & Thomas D., 1998. Immobilized lipase-catalyzed ethanolysis of sunflower oil in a solvent-free medium. J. Am. Oil Chem. Soc., 75(6), 691-695.
Sheldon R.A. et al., 2002. Biocatalysis in ionic liquids. Green Chem., 4(2), 147-151.
van Rantwijk F., Madeira Lau R. & Sheldon R.A., 2003. Biocatalytic transformations in ionic liquids. Trends Biotechnol., 21(3), 131-138.
van Rantwijk F. & Sheldon R.A., 2007. Biocatalysis in ionic liquids. Chem. Rev., 107(6), 2757-2785.
Vidya P. & Chadha A., 2009. The role of different anions in ionic liquids on Pseudomonas cepacia lipase catalyzed transesterification and hydrolysis. J. Mol. Catal. B: Enzym., 57(1-4), 145-148.
Ward O.P., Fang J.W. & Li Z.Y., 1997. Lipase-catalyzed synthesis of a sugar ester containing arachidonic acid. Enzyme Microb. Technol., 20(1), 52-56.
Zhao H., 2010. Methods for stabilizing and activating enzymes in ionic liquids – a review. J. Chem. Technol. Biotechnol., 85(7), 891-907.
Zhao H. et al., 2008. Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem., 10(6), 696-705.
Zhao H. et al., 2009. Effect of ionic liquid properties on lipase stabilization under microwave irradiation. J. Mol. Catal. B: Enzym., 57(1-4), 149-157.
Zhou X. et al., 2007. Enhance immune response to DNA vaccine based on a novel multicomponent supramolecular assembly. Biomaterials, 28(31), 4684-4692.
Para citar este artículo
Acerca de: Nadine Galonde
Univ. Liege - Gembloux Agro-Bio Tech. Department of General and Organic Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: nadine.galonde@ulg.ac.be
Acerca de: Gaëtan Richard
Univ. Liege - Gembloux Agro-Bio Tech. Department of General and Organic Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium) – Univ. Liege - Gembloux Agro-Bio Tech. Department of Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Magali Deleu
Univ. Liege - Gembloux Agro-Bio Tech. Department of Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium) – Univ. Liege - Gembloux Agro-Bio Tech. Center of Numerical Molecular Biophysics. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Katherine Nott
Univ. Liege - Gembloux Agro-Bio Tech. Department of General and Organic Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium) – Univ. Liege - Gembloux Agro-Bio Tech. Department of Biological Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Christine Jerôme
Univ. Liege. Center for Education and Research on Macromolecules. Sart-Tilman, B6a. B-4000 Liege (Belgium).
Acerca de: Marie-Laure Fauconnier
Univ. Liege - Gembloux Agro-Bio Tech. Department of General and Organic Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






