- Portada
- Volume 18 (2014)
- Numéro 3
- Morphological, physiological and biochemical responses to soil water deficit in seedlings of three populations of wild pear tree (Pyrus boisseriana)
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Morphological, physiological and biochemical responses to soil water deficit in seedlings of three populations of wild pear tree (Pyrus boisseriana)

Notes de la rédaction
Received on October 24, 2013; accepted on May 6, 2014
Résumé
Réponses morphologiques, physiologiques et biochimiques au déficit en eau chez les jeunes plants de trois populations de poiriers sauvages (Pyrus boisseriana). En régions arides et semi-arides, la disponibilité en eau est le facteur limitant des vergers de production, comme en culture de poirier, par exemple. À cet égard, l'évaluation d'une collection de poiriers sauvages pourrait mettre en évidence du matériel potentiellement utilisable comme porte-greffe et tolérant à la sécheresse dans ces régions. Des poiriers sauvages (Pyrus boisseriana) originaires de trois populations différentes selon leur répartition en altitude (populations « semi-aride 1 000 m », « semi-humide 1 350 m » et « semi-humide 1 600 m ») ont été évalués pour leur tolérance à la sécheresse dans un essai en serre. Des semis cultivés en pots ont été soumis à 18 jours de sécheresse, puis à une reprise de sept jours de croissance en conditions normales d'irrigation. La répartition et l'accumulation de la biomasse, les paramètres physiologiques (conductance stomatique, photosynthèse, transpiration, potentiel hydrique du xylème) et biochimiques (teneurs en pigments des feuilles, proline libre et malondialdéhyde ; production de peroxyde d'hydrogène) ont été évalués par rapport à des témoins non soumis au stress hydrique. Bien que toutes les populations de poirier aient pu se rétablir après la période sans irrigation et présentent de ce fait une certaine tolérance à la sécheresse, certaines différences de comportement entre celles-ci ont été détectées pour ce qui concerne les échanges gazeux, l'accumulation de la biomasse et la concentration de la proline dont les valeurs étaient en faveur de la population établie en zone semi-aride à une altitude de 1 000 m. Celle-ci a d'ailleurs montré une reprise plus rapide et plus complète de son activité physiologique (conductance stomatique et fixation du carbone). À noter que le taux de caroténoïdes dans les feuilles a augmenté dans toutes les populations soumises au stress hydrique. De manière générale, nous avons montré que les plantes appartenant à la population établie en zone semi-aride à 1 000 m d'altitude présentaient une plus grande tolérance à la sécheresse que celles appartenant aux populations des altitudes plus élevées (populations semi-humides). Ces plantes constituent donc un matériel prometteur comme candidats porte-greffe pour les greffons commerciaux de poiriers destinés à la culture dans les régions soumises fréquemment à des déficits hydriques.
Abstract
Water shortage limits the production of fruit orchards, such as pear, in arid and semi-arid regions. The identification of wild pear germplasm for potential use as rootstock would be valuable for pear cultivation in semi-arid regions. The relative drought tolerance of wild pear germplasm (Pyrus boisseriana) from three different populations distributed along an elevational gradient (‘semi-arid 1,000’, ‘semi-wet 1,350’ and ‘semi-wet 1,600’ populations) was evaluated in a greenhouse trial. Established container-grown seedlings were exposed to 18 days of simulated drought, or not, followed by a seven day recovery period. Biomass allocation and accumulation, physiological (stomatal conductance, photosynthesis, transpiration, xylem water potential) and biochemical parameters (leaf pigments, free proline, malondialdehyde and hydrogen peroxide production) were evaluated. Although all populations were able to recover from water shortage, thereby proving to be relatively drought tolerant, some differences between populations were detected for gas exchange parameters, biomass accumulation and proline concentration in favor of the ‘semi-arid 1,000’ elevation population, which was more drought tolerant. This population showed the most rapid and complete recovery of physiological activity (stomatal conductance and carbon fixation). In addition, all populations showed an increase in carotenoid content in the leaves. Overall, we showed that plants from the ‘semi-arid 1,000’ elevation had greater tolerance to drought than those from the higher elevations (semi-wet populations). It therefore appears that plants from the ‘semi-arid 1,000’ elevation represent a promising source of material to be tested as rootstock for commercial scions of pear in field conditions in areas prone to suffer from water deficit.
Tabla de contenidos
1. Introduction
1Alteration of historical precipitation patterns and soil-water availability are some of the tangible effects of global warming. It is predicted that the percentage of droughty terrestrial areas will double by the end of the current century (Deeba et al., 2012). Water is commonly the most limiting resource for fruit production worldwide (Sircelj et al., 2007). Besides developing new thrifty and novel irrigation schedules (Jones, 2004), the use of less water-demanding or more drought resistance genotypes is a promising solution for fruit tree culture in arid and semi-arid regions (Cruz et al., 2012). Wild germplasm in natural arid ecosystems evolved in response to a plethora of stressful conditions, such as extreme temperatures, salinity and drought (Frankel, 1989; Zhou et al., 2013). In addition, neighboring populations from different elevations may represent locally specialized ecotypes (Snaydon et al., 1982; Mollard et al., 2010; Chapolagh et al., 2013). For that reason, evaluating wild germplasm from several local sites can be useful in discovering locally adapted populations.
2Pear (Pyrus spp.) is the third most important fruit produced in temperate regions after grapes and apples (Chevreau et al., 1992). The mountains of Iran provide varied habitats occupied by wild pear germplasm (Vavilov, 1994), with lower elevation sources being better adapted to stressful arid environmental conditions, and thus, be an important resource for pear breeding programs seeking drought resistant traits (Sisko et al., 2009).
3Drought is a major stress that disrupts metabolic processes and constrains plant growth (Pustovoitova et al., 1996; Chaves et al., 2003). Woody plants have developed various mechanisms to cope with water deprivation (Gholami et al., 2012). The negative effects of drought include reduced plant growth (Delgado et al., 1992; Ohashi et al., 2000), photosynthesis (Boyer, 1970; Ogen et al., 1985), cell growth (Bohnert et al., 1995; Nonami et al., 1997) and hormone production (Munns et al., 1996). The active accumulation of solutes (such as proline) allows plants to maintain positive turgor pressure, a requirement for maintaining stomata aperture and gas exchange (White et al., 2000). Drought stress often leads to the accumulation of reactive oxygen species (ROS), which might initiate destructive oxidative processes such as lipid peroxidation, chlorophyll bleaching and protein oxidation (Terzi et al., 2006). Plants have evolved both enzymatic and non-enzymatic defense systems for scavenging and detoxifying ROS, resulting in antioxidant defense capacity that is a useful criterion for the screening of resistant genotypes (Faize et al., 2011). Besides the non-enzymatic antioxidants (e.g. ascorbic acid and glutathione), carotenoids are pigments with a protective role for dissipating the excess of energy necessary to avoid ROS generation (Sircelj et al., 2007). Thus, there are three general types of responses to drought stress including (Sircelj et al., 2005):
4– mechanisms to avoid water loss (e.g. osmotic adjustment),
5– mechanisms for protection of cellular components (e.g. qualitative and quantitative changes of pigments),
6– mechanisms of repairing against oxidative damage (e.g. antioxidant systems).
7Some researchers have placed the wild pear species among xerophytic woody plants according to their relative low demand for soil moisture (Bouček, 1954). Nevertheless, there are no comprehensive studies of drought tolerance and the presumably intra-specific variation of responses in populations of a wild pear species. On the other hand, most researchers have focused the study of plant responses to drought during the period of stress, while there has been much less attention to the recovery process after the stress (Miyashita et al., 2005; Striker, 2012).
8The relative responses of three wild Pyrus boisseriana populations to water deficit were explored. The two objectives were to examine the presumable existence of locally adapted ecotypes of P. boisseriana populations along an elevational gradient, a surrogate for aridity, and to identify the traits and mechanisms of tolerance to soil water deficit.
2. Materials and methods
2.1. Plant material
9In autumn 2011, fruits of P. boisseriana were collected from a natural forest in northeastern Iran (Khorasan province, near the city of Boojnord) where the species is extensively distributed from 1,000 to about 2,000 m.a.s.l. We set an elevational gradient in this range and collected the seeds from three different populations (see table 1 for details about populations). Collected seeds from each population were cold stratified at 4° C for three months. After stratification, most of the seeds began to germinate. Germinated seeds were sown in black nylon pots when the radicle reached 1-2 cm length. After four months, 100 uniformly sized seedlings per population were transplanted to plastic pots (4 l) containing a mixture of forest brown soil, river sand and clay (2:1:1, v/v/v). So, a total of 300 seedlings were prepared for this experiment and the pots were moved to the main greenhouse (Tarbiat Modares University, Faculty of Natural Resources, Mazandran, Noor, Iran). All the seedlings were equally irrigated (500 ml per pot) three times per week until the middle of summer 2012, when half of the plants in each population were subjected to drought stress by suspending irrigation.
2.2. Treatments and experimental set up
10A factorial experiment was carried out following a fully randomized design with two fixed factors: source (three levels: sources named as “semi-arid 1,000”, “semi-wet 1,350” and “semi-wet 1,600”) and irrigation treatment (two levels: control well irrigated, and no-irrigation followed by a recovery period). The experiment started on July 22nd, 2012, when 100 seedlings of each three populations were subjected to two following water treatments (Echevarría-Zomeño et al., 2008):
11– irrigated (control): seedlings were irrigated and maintained near field capacity during the 25 days of experiment,
12– non-irrigated: seedlings were watered to field capacity on day 0, and then maintained unirrigated for 18 days until leaf rolling occurred. From day 19 of experiment, seedlings were irrigated similarly to the control plants for seven days to assess the degree of recovery for physiological and biochemical parameters. This procedure simulated a short sudden drought (Poorter et al., 2008).
2.3. Measurements of physiological parameters
13Net photosynthesis (A, µmol·m-2·s-1), stomatal conductance (gs, mmol·m-2·s-1) and transpiration (E, mmol·m-2·s-1) were measured during the drought stress period (at days 7, 10, 13 and 18) and during the recovery period on same plants using three randomly selected mature leaves per plant (at days 19, 22 and 25) using a portable infrared gas analyzer (Model LCpro+, ADC BioScientific Ltd., Hertfordshire, UK). Xylem predawn stem potential (ψstem, MPa) was measured between 04:00 and 06:00 on day 18 and day 25 with a pressure chamber system (Skye, SKPM 1400, UK). Leaf relative water content (RWC) was determined according to following formula:
14RWC= (Mf - Md) / (Mt - Md) ×100,
15where Mf is leaf fresh mass, Mt, turgid mass and Md, dry mass (Munns et al., 2010). To estimate the electrolyte leakage, fresh leaf samples were rinsed 3 times (2-3 min) with distilled water and leaf discs of 0.5 cm2 were floated in 10 ml of distilled water for 24 h and electrical conductivity of the solution was measured using a conductimeter (EC meter- PC 300, Eutech instrument Pte Ltd/ Oakton instruments, USA). Total conductivity was obtained after boiling the samples in a bath (90 °C) for 2 h and results expressed as a percentage of the total conductivity (Campos et al., 2003) after adjusting for the EC value of the distilled water.
2.4. Assessment of biomass and morphology
16Half the plants from each population and treatment combination were randomly selected and harvested on day 18 with the rest harvested on day 25. Samples of leaf tissue were taken at days 18 and 25 from randomly selected plants within each treatment combination for biochemical analysis (see below). At harvest, individual plants were separated into leaves, stems and roots and oven-dried at 70 °C for 72 h, and weighed to obtain their dry weight.
2.5. Measurements of biochemical parameters
17On days 18 and 25, leaf areas of leaf samples were determined as described before and then covered with aluminum foil, frozen in liquid nitrogen and stored at -85 °C until used for biochemical analysis. Chlorophylls and carotenoids were extracted from leaf samples in 80% acetone and their concentrations were determined by spectrophotometry according to Gholami et al. (2012). Free proline content in leaves was quantified following the procedure proposed by Bates et al.1 (1973), which was cited by Nikolaeva et al. (2010). Soluble carbohydrates were estimated by the anthrone reagent method (Yemm et al., 1954). The lipid peroxidation was measured in terms of malondialdehyde (MDA) concentration (Dhindsa et al., 1981) according to the original methodology Heath and Packer method2 (1968) as cited in Bian et al. (2009). Hydrogen peroxide was assessed through spectrophotometric analysis after reaction with potassium iodide (KI) (Velikova et al., 2005).
18All physiological, biochemical and morphological parameters utilized to compare the responses of the populations of P. boisseriana to water deficit are summarized in table 2.
2.6. Statistical analysis
19At each harvest, equal numbers of irrigated and non-irrigated plants were randomly selected for harvest. Biochemical and physiological data were analyzed using a two-way ANOVA, where “population” and “water stress” were the fixed factors. Variations in leaf gas exchange parameters during the experiment were evaluated by two-way repeated measures ANOVA with “population” and “water stress” as the between-subject main factors, and time as the within-subject factor. Mauchly's test of sphericity was performed to verify the hypothesis of sphericity of the covariance matrices (Von Ende, 1993). As the assumption about the covariance matrix was met (Mauchly’s test was no significant, P > 0.05), we used the “Sphericity Assumed test” to analyze the within-subjects effects. Before analyses, normality and homoscedasticity of the data were checked to satisfy ANOVA assumptions. These statistical analyses were performed with SPSS 16.0 (SPSS Inc., Chicago, IL). In addition, Standardized Major Axis regressions (SMA) were performed to study if there were allometric changes in the relationships of biomass between shoots and roots caused by water stress (see Falster et al., 2006). Slope tests of the fitted regression between treatments were executed for each population by using “smatr” package (Warton et al., 2012) for R 2.10.0 statistical platform (R Development Core Team 2011).
3. Results
3.1. Effects of drought on gas exchange parameters
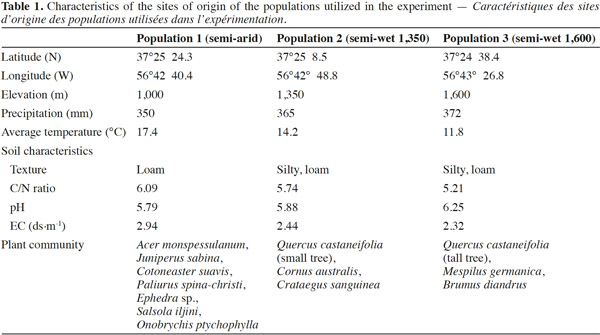
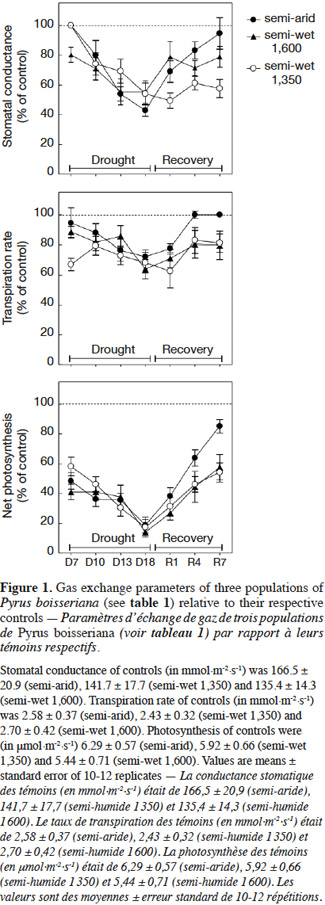
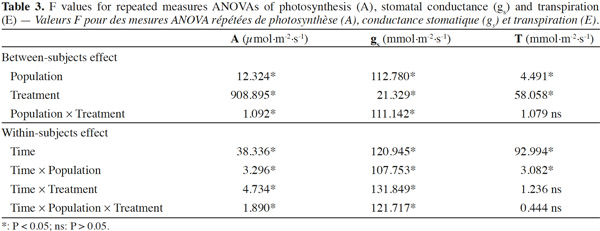
20Stomatal conductance (gs), transpiration (E) and net photosynthesis (A) of unirrigated seedlings for all populations, relative to control seedlings, during the 25 day experiment are shown in figure 1. The interaction “treatment” × “population” × “time” was significant for all gas exchange variables excepting E (Table 3), indicating that physiological behavior of plants when facing drought depended on the origin of the plants and varied during the course of the experiment. The effects of “population” and “treatment” as single factors were significant for all variables: stomatal conductance (gs), photosynthesis rate (A) and transpiration (E) (Figure 1; Table 3). Irrigated seedlings for the semi-arid population had highest average rate of stomatal conductance, and photosynthesis during days 0 to 18, while seedlings of the “semi-wet 1,600 m” provenance had the highest transpiration rates (see values in caption of figure 1). For all populations, relative stomatal conductance of unirrigated seedlings decreased during the imposed stress period (days 0 to 18) and increased during the recovery period (days 19 to 25). The decrease ranged from 40 to 50% with respect to control (P < 0.01 in all cases). By day 25, stomatal conductance of seedlings from the semi-arid provenance recovered to 98%, relative to irrigated seedlings, while those from the “semi-wet 1,350” population recovered only slightly from the lowest rate (45%) which occurred on day 18. Relative transpiration rates declined and were similar for all populations through day 18, except for seedlings from “semi-wet 1,350” population, which were slightly lower than those of the other provenances (P < 0.07). By day 22, relative transpiration recovered for all populations, with significantly greater recovery exhibited by seedlings from the semi-arid population (P < 0.01). Relative net photosynthesis followed a pattern similar to that of relative transpiration, decreasing equally among the population as irrigation was withheld and recovering greatest for seedlings from semi-arid population as irrigation was restored (higher A in comparison to the other origins; P < 0.01).
3.2. Effects of drought on biomass and morphology
21The wild pear seedlings treated with drought stress showed no significant changes in leaf area and specific leaf area (P > 0.4). There was a significant “population” × “irrigation” treatment interaction for stem, leaf and root dry mass at the end of the drought period (P = 0.021, P = 0.033 and P = 0.036, respectively); therefore comparisons were made between treatments within populations. For the semi-arid source, the unirrigated seedlings showed the same leaf, stem and root dry mass as irrigated ones (P = 0.49; Figure 2, right panel). For the “semi-wet 1,350” and “semi-wet 1,600” populations, unirrigated seedlings had lower leaf, stem and root dry mass than their respective controls (P < 0.05). Interestingly, the control seedlings from the three provenances had similar leaf, stem and root dry weights, whereas root dry mass was decreased by approximately 25% and leaf dry mass by 30% in unirrigated seedlings of the “semi-wet 1,350” and “semi-wet 1,600” populations (P < 0.01 in all cases). Stem dry mass decreased in seedling subjected to drought by 43% and 31% for “semi-wet 1,350” and “semi-wet 1,600” populations, respectively (P < 0.05 in all cases; figure 2, right panels).
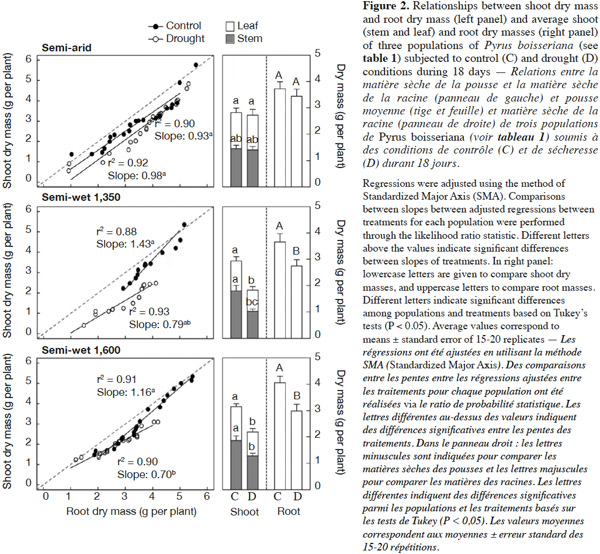
22Allometric analyses through Standardized Major Axis (SMA) regressions showed important differences among populations for the relationships between shoot and root dry mass (Figure 2, left panels). Plants belonging to the semi-arid population maintained the shoot to root biomass ratio unchanged and near to 1 irrespective of the treatment applied (slope test P = 0.56, slopes not different from 1; P = 0.33 and P = 0.78 for plants under control and drought treatments, respectively). By contrast, plants from both semi-wet populations showed a decrease in the slope of the relationship between shoot and root dry mass when subjected to drought stress (slopes under control conditions 1.16-1.43 vs slopes under drought conditions 0.70-0.79; slope test between treatments: P < 0.001 for both cases), which means that for a same root mass a low shoot mass was obtained under water deficit as symptom of stress (compare slopes of the adjusted regressions in figure 2, left panels).
3.3. Effects of drought on xylem water potential, relative water content and electrolyte leakage
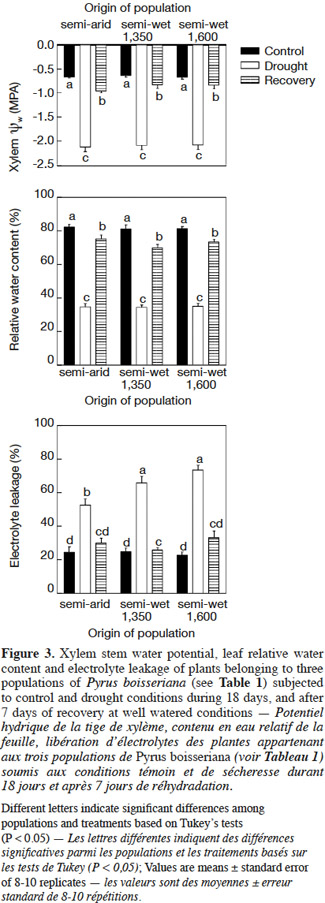
23On day 18, unirrigated plants had lower leaf water potential than control plants (Figure 3, upper panel) irrespective of the origin-population of the plants (interaction “population” × “treatment”: P > 0.25). After the recovery period, the leaf water potential in previously unirrigated plants increased significantly, but it was still lower than for irrigated plants (P < 0.05 in all cases). There was no difference among populations in the degree of leaf water potential recovery or in the leaf water potential of the irrigated plants (Figure 3, upper panel). The observed pattern in relative water content (RWC) of leaves was similar to that of leaf water potential. On day 18 irrigated plants had higher leaf RWC above 80% while unirrigated plants had only 35-40% of RWC but with a significant recovery (RWC ranging from 75 to 80%) at day 25 (“treatment”: P < 0.001; “population”: P > 0.05; Figure 3, middle panel). In all cases no differences among populations were detected for RWC (“treatment” × “population”: P = 0.23). Similarly, leaf electrolyte leakage was lower in irrigated control plants of all populations in comparison to unirrigated plants (“treatment”: P < 0.01; Figure 3, lower panel). Interestingly, electrolyte leakage was lower in plants from the semi-arid origin than the registered for plants from both semi-wet origins (“semi-wet 1,350” and “semi-wet 1,600”) as it was indicated by the significant “population” × “treatment” interaction (P < 0.01; Figure 3, lower panel). After seven days of recovery of plants previously subjected to water deficit, the differences in electrolyte leakage registered on day 18 were no more apparent when comparing with those of plants growing under control well-watered conditions (P > 0.05). Also, no differences were detected among populations for this parameter after the recovery period (“population”: P = 0.14).
3.4. Effects of drought on biochemical parameters
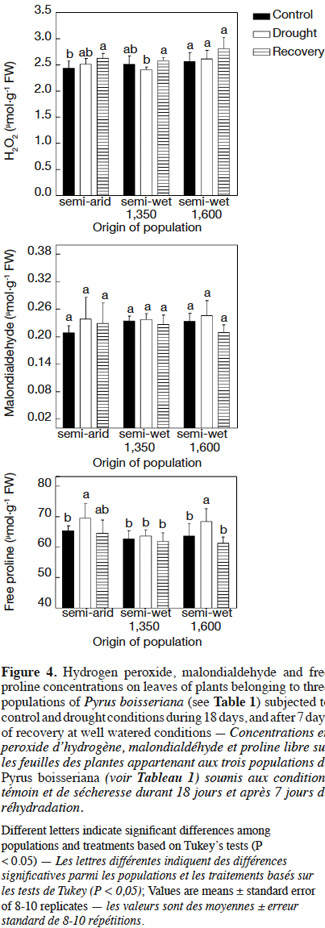
24There were no statistical differences in the biochemical parameters between the control plants at the two harvests on days 18 and 25 (P > 0.2). Therefore, only the data of irrigated (control) plants harvested on day 25 were included in figures 4 and 5. Hydrogen peroxide concentration in leaves was quite similar in plants under either treatment (control, drought, or those allowed to recover from the stress) and irrespective of the origin of plants (Figure 4, upper panel). The slightly lower H2O2 concentration in leaves registered in control plants of the semi-arid and “semi-wet 1,350” (2.25 vs 2.5-2.7 µmol·g-1 of fresh weight), although were statistically different with respect to all other treatment-population combinations, did not appear to have biological significance. Similarly, there were no differences in malondialdehyde concentration among populations or irrigation treatments (P > 0.05 in all cases; Figure 4, middle panel). Free proline concentration in leaves was higher in unirrigated plants from semi-arid and “semi-wet 1,600” populations at day 18 after drought (P < 0.05) without differences between irrigation treatments in plants from the “semi-wet 1,350” origin (P = 0.4) (Figure 4, lower panel). After the recovery period, free proline concentration of previously water-stressed plants was similar to those observed in control irrigated plants (P > 0.05 in all cases) without evidence of differences among populations (Figure 4, lower panel).
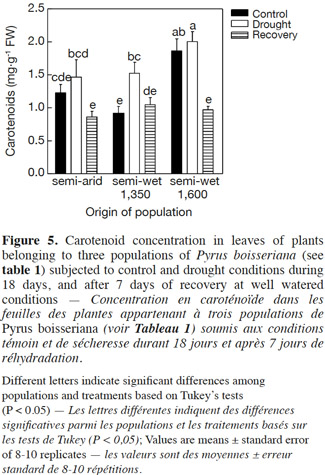
25Total chlorophyll (TC) in leaves ranged between 70.5 μg·g-1 FW and 119.4 μg·g-1 FW. This parameter was not affected by the origin of plants (“population”: P = 0.24) or when control plants and plants subjected to water deficit were compared at day 18 (“treatment”: P > 0.05). However, in plants of all populations there was an unexpected trend towards low values of TC when evaluated after their recovery from drought (data not shown). Interestingly, carotenoids concentration in leaves differed among populations and treatments as indicated a significant interaction between these factors (P < 0.05; Figure 5). Under drought stress all plants tended to increase the leaf carotenoids concentration with respect to control although in a different magnitude according to their origin: increases of 16% were observed in plants from semi-arid origin, 40% in plants from the “semi-wet 1,350” origin and of 7% in plants from the “semi-wet 1,600” origin (Figure 5). The magnitude of the increase in carotenoids concentration was opposite to their constitutive values registered at control conditions (compare control values of “semi-wet 1,350” and “semi-wet 1,600”).
4. Discussion
26In mountainous habitats, sharp changes in ecological conditions can occur over short distances, leading to major changes in the selection pressures acting on plant morphology and physiology (Vitasse et al., 2009). Therefore, along an altitudinal gradient, intra-specific variation among populations of a same species can be found in a small area (Still et al., 2005). In this study, we found inter-population variation in plant performance among wild pear plants from three different origins. At first glance, it was observed that plants from the semi-arid origin were the most tolerant ones to drought stress than plants from both semi-wet populations (1,350 and 1,600). Interestingly, the differences among populations were not related with their physiological behavior registered during the stress period but during the recovery after drought. In this respect, all populations showed progressive reductions in stomatal conductance under water deficit, which were effective in decreasing water loss by transpiration but with coupled costs associated with decreases in photosynthesis. These responses are in line with previously reports on this topic, where stomatal closure is considered the primary short-term mechanism used by plants under drought stress to reduce water loss by transpiration, with the concomitant decrease in photosynthesis (Chaves, 1991). Importantly, the differential tolerance to drought among populations was evident during the recovery period where the semi-arid population showed the highest recovery of their physiological parameters after re-watering, while the plants from both semi-wet populations (1,350 and 1,600) showed relatively low recovery ability from water deficit (Figure 1). This highlights the importance of considering a recovery period after the stress to assess the true drought tolerance of plants of each population. In addition, the variation in photosynthetic performance registered among our wild pear populations could be related to their different positions along the elevational gradient. The inter-population variation in photosynthetic rates in tree species is associated with their geographical-altitudinal distribution (e.g. Benowicz et al., 2000; Soolanayakanahally et al., 2009).
27As discussed before, gas exchange limitations during drought did not lead only to a reduced water loss, but also to a reduction in whole-plant carbon assimilation, and consequently, a reduced growth and biomass accumulation could be expected (Bañon et al., 2006). In this regard, we showed that under drought stress all wild pear plants from semi-wet origins had lower shoot and root biomass than the well-watered plants (controls), but this response was not significant for plants belonging from semi-arid origin (Figure 2). These results again confirm our claim that populations of semi-arid are more tolerant to drought than those from semi-wet origins. Interestingly, dry mass partitioning (between shoots and roots) was differentially affected by drought when comparing populations. The semi-arid population was able to maintain unaltered the shoot to root ratio when analyzed through an allometric approach, while in plants of both semi-wet populations this parameter was reduced (see slope values in figure 2, left panels). Accordingly, shoot biomass (leaf and stem) of populations of semi-wet origin was much more depressed than root biomass compared to the semi-arid population. In this respect, Gibson et al. (1995) suggested that plants from low rainfall regions are able to allocate greater proportion of dry matter to roots than plants from high rainfall regions when water is not limiting. Although drought stress is able to reduce both root and shoot growth (Liu et al., 2004), the roots had some mechanisms – like osmolyte accumulation in root tips – that might led to less negative effect of water deficit (Sharp et al., 1979) as we observed in plants of all wild pear populations in this experiment. Moreover, plants belonging to the semi-arid origin did not registered any detrimental effect on root dry mass accumulation.
28Leaf water potential is a primary indicator of the degree of plant’s stress under water deficit (McCutchan et al., 1992). Taking into consideration that leaf water potential reflects soil moisture levels (Elfving et al., 1972; Sellin, 1996), the highest (-0.66 MPa) and lowest (-2.12 MPa) values of xylem water potential were recorded in well-irrigated and stressed wild pear plants, as expected (Figure 3). According to Hsiao’s classification, the wild pear plants in our experiment – irrespective of the origin of the plants (populations) – were under moderate drought stress (i.e. when the difference in leaf water potential between stressed and control plants ranges within 0.5 and 1.5 MPa sensu Hsiao, 1973). For the plant to acclimate to water deficit and survive drought, its roots have to maintain a flow of water along the xylem (Costa e Silva et al., 2004), so undoubtedly there is a threshold value for each species to maintain an adequate water absorption. For apple, this threshold value ranges between -1.8 and -2.2 MPa (Lakso, 1979). Our data showed that after a drought period of 18 days, xylem water potential was slightly lower than -2.1 MPa, but that after 7 days of re-watering, plants of all populations reached a well degree of recovery of their water status (Figure 3, upper panel), although it was not a full recovery if the values are compared with the ones of never-stressed control plants. Such water status recovery is in line with the increases in stomatal conductance, and presumably high cell turgor related to recovered values for RWC (Figure 3, middle panel), and recovery of photosynthesis. Nevertheless, the plants of semi-arid origin exhibited a high recovery in physiological variables that was not linked to a better water status of the plant according to the measurements we made (water potential, RWC and osmotic adjustment inferred from proline accumulation). So, the mechanisms by which this population manifested a better physiological behavior when subjected to drought deserve further experimental investigation.
29Under water deficit, cell membranes are subjected to changes such as increase in permeability and decrease in selectivity, which can be viewed through the increase in electrolyte leakage (Blokhina et al., 2003). On this note, we found a general increase of electrolyte leakage under water deficit for all three populations, which suggest the occurrence of damage to cell membranes (see also Campos et al., 2003). However, a less damage to cell membranes – viewed through a lower value of electrolyte leakage – was registered on the more drought-tolerant population of semi-arid origin (Figure 3, lower panel). Importantly, all populations showed an almost complete recovery of membrane function-integrity as evaluated by the electrolyte leakage values that returned to the ones of control plants. In addition, the accumulation of osmolytes, like proline and soluble sugars, is considered as part of the suite of adaptive mechanisms that plants develop when dealing with drought. This is because such osmolyte accumulation allows plants to continue with water absorption through osmotic adjustment, and thereby helps to maintain (or to have less impact on) cell turgor (Sofo et al., 2004; Munns et al., 2008). In this regard, as we did not register differences between treatments/populations for soluble leaf sugar concentration (data not shown), we focused our attention in the concentration of free proline in leaves (Figure 4, lower panel). Small but significant accumulations of proline were found in leaves of stressed plants of semi-arid and “semi-wet 1,350” origins that might be related with a certain degree of osmotic adjustment capacity of these populations (see also Errabii et al., 2006; Monreal et al., 2007 for sugarcane and sugarbeet species).
30Previous research has shown that drought stress eventually provokes oxidative stress (Larson, 1995), so that the oxygen scavenging system is a very important defense response to cope with water deficit (Arndt et al., 2001; Pinheiro et al., 2001). Injury of plants as a result of oxidative stress is driven by the overproduction of reactive oxygen species (ROS) such as H2O2 (Wise et al., 1987). Our results suggested that the wild pear plants did not experience evident oxidative stress during the imposed drought stress period of 18 days taking into account that the levels of H2O2 were not modified, a response that was unaffected by the origin of the plants (Figure 4, upper panel). This idea was also supported by the lack of increase in malondialdehyde (MDA) as it is a good indicator of oxidative damage to membrane lipids (Ozkur et al., 2009). Finally, the typical decrease in the chlorophyll content as a symptom of oxidative stress (Egert et al., 2002) did not occur for any population, which added further evidence to suggest that the antioxidative defense system was effective in preventing the oxidative damage under drought stress. On the other hand, carotenoids as recognized pigments for their antioxidant activity (Niyogi, 1999) significantly increased their concentration in all populations under drought stress (Figure 5), which suggest that these pigments might play an important role in the mechanisms of ROS detoxification in wild pear.
5. Conclusions
31Fruit tree culture in arid and semi-arid areas must be directed towards the use of less water-demanding and more drought-tolerant plant materials (Cruz et al., 2012). On the other hand, it is known that the use of wild material is a useful way to improve drought tolerance in different crops (Ashraf, 2010). In this study, we focused on wild pear tree (P. boisseriana) as a drought-tolerant promising rootstock by evaluating the responses of three populations from an elevational gradient when subjected to water deficit. The results indicated that pear trees conserve water through early stomatal closure, to reduce water loss through transpiration during the drought period (water conservative behavior). Moreover, there is a fast recovery of the physiological activity (stomatal conductance and photosynthesis) after the stress. In addition, we found that increasing the carotenoid content appears to be an effective antioxidant system for this species (Figure 5). We proved that plants from semi-arid origin, which experience harsh environmental conditions, had more resistance to drought stress than those distributed in semi-wet and wet habitats. The differences were particularly notorious for the physiological responses (Figure 1), final biomass and carbon allocation (Figure 2). In the last case, plants from semi-arid origin were the only ones that did not change the shoot to root ratio (compare slopes among treatments/populations in figure 2, left panel) when subjected to water deficit. Finally, we conclude that the use of P. boisseriana, in particular plant material belonging to semi-arid origin, appears as a promising rootstock for commercial pear in areas suffering from water deficit and/or for orchards where the irrigation schedule restricts the frequency of plant watering (e.g. every two weeks). However, future experimental investigation on grafting studies at field conditions is desirable in order to confirm our results at experimental garden as well as to test the behavior of the promissory plant material from semi-arid origin as rootstock of commercial scions of pear trees.
32Acknowledgements
33We thank Dr. Cecilia Casas (Chair of Soil Science, University of Buenos Aires) for her advice and help with statistical analyses related to SMT regressions performed in R statistical platform.
342 Heath R.L. & Packer L., 1968. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125, 189-198.
Bibliographie
Arndt S.K. et al., 2001. Physiological and morphological adaptations of the fruit tree Ziziphus rotundifolia in response to progressive drought stress. Tree Physiol., 21, 705-715.
Ashraf M., 2010. Inducing drought tolerances in plants: recent advances. Biotechnol. Adv., 28, 169-183.
Bañon S. et al., 2006. Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ. Exp. Bot., 56, 36-43.
Benowicz A., Guy R.D. & El-Kassaby Y.A., 2000. Geographic pattern of genetic variation in photosynthetic capacity and growth in two hardwood species from British Columbia. Oecologia, 123, 168-174.
Bian S. & Jiang Y., 2009. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. Amsterdam, 120, 264-270.
Blokhina O., Virolainen E. & Fagerstedt K.V., 2003. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot., 91, 179-194.
Bohnert H.J., Nelson D.E. & Jensen R.G., 1995. Adaptations to environmental stresses. Plant Cell, 7, 1099-1111.
Bouček B., 1954. Hrušeň. Lesnícka Práce, 33, 57-62.
Boyer J.S., 1970. Differing sensitivity of photosynthesis to low leaf water potentials in corn and soybean. Plant Physiol., 46, 236-239.
Campos P.S., Quartin V., Ramalho J.C. & Nunes M.A., 2003. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol., 160, 283-292.
Chapolagh I. et al., 2013. Leaf macro- and micro-morphological altitudinal variability of Carpinus betulus in the Hyrcanian forest (Iran). J. For. Res., 24(2), 301-307.
Chaves M.M., 1991. Effects of water deficits on carbon assimilation. J. Exp. Bot., 42, 1-16.
Chaves M.M., Maroco J.P. & Pereira J.S., 2003. Understanding plant responses to drought — from genes to the whole plant. Funct. Plant Biol., 30, 239-264.
Chevreau E. & Skirvin R.M., 1992. Pear. In: Hammerschlag F.A. & Litz R.E., eds. Biotechnology of perennial fruit crops. Wallingford, UK: C.A.B. International, 263-276.
Costa e Silva F., 2004. Responses to water stress in two Eucalyptus globulus clones differing in drought tolerance. Tree Physiol., 24, 1165-1172.
Cruz Z.N. et al., 2012. Leaf mechanisms for drought resistance in Zizyphus jujuba trees. Plant Sci., 197, 77-83.
Deeba F. et al., 2012. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem., 53, 6-18.
Delgado E. et al., 1992. Effect of water stress on photosynthesis, leaf characteristics and productivity of field-grown Nicotiana tabacum L. genotypes selected for survival at low CO2. J. Exp. Bot., 43, 1001-1008.
Dhindsa R.S., Dhindsa P.P. & Thorpe T.A., 1981. Leaf senescence: correlation with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot., 32, 93-101.
Echevarría-Zomeño S. et al., 2008. Changes in the protein profile of Quercus ilex leaves in response to drought stress and recovery. J. Plant Physiol., 166, 233-245.
Egert M. & Tevini M., 2002. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ. Exp. Bot., 48, 43-49.
Elfving D.C., Kauffmann M. & Hall A.E., 1972. Interpreting leaf water potential measurements with a model of the soil-plant-atmosphere continuum. Physiol. Plant., 27, 161-168.
Errabii T. et al., 2006. Growth, proline and ion accumulation in sugarcane callus cultures under drought-induced osmotic stress and its subsequent relief. Afr. J. Biotechnol., 5, 1488-1493.
Faize M. et al., 2011. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J. Exp. Bot., 62, 2599-2613.
Falster D.S., Warton D.I. & Wright I.J., 2006. SMATR: standardised major axis estimation and testing routines. R package version 2.1., http://web.maths.unsw.edu.au/~dwarton, (13/10/13).
Frankel O.H., 1989. Principles and strategies of evaluation. In: Brown A.H.D., Frankel O.H., Marshall D.R. & Williams J.T., eds. The use of plant genetic resources. Cambridge, UK: Cambridge University Press, 245-259.
Gholami M., Rahemi M., Kholdebarin B. & Rastegar S., 2012. Biochemical responses in leaves of four fig cultivars subjected to water stress and recovery. Sci. Hortic. Amsterdam, 148, 109-117.
Gibson A., Bachelard E.P. & Hubick K.T., 1995. Relationship between climate and provenance variation in Eucalyptus camaldulensis Dehnh. Aust. J. Plant Physiol., 22, 453-460.
Hsiao T.C., 1973. Plant responses to water stress. Annu. Rev. Plant Physiol., 24, 519-570.
Jones H.G., 2004. Irrigation scheduling: advantages and pitfalls of plant-based methods. J. Exp. Bot., 55, 2427-2436.
Lakso A.N., 1979. Seasonal changes in stomatal response to leaf water potential in apple. J. Am. Soc. Hortic. Sci., 104, 58-60.
Larson R.A., 1995. Plant defenses against oxidative stress. Biochem. Physiol., 29(2), 175-186.
Liu F. & Stützel H., 2004. Biomass partitioning, specific leaf area and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci. Hortic. Amsterdam, 102, 15-27.
McCutchan H. & Shackel K.A., 1992. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci., 117(4), 607-611.
Miyashita K., Tanakamaru S., Maitani T. & Kimura K., 2005. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ. Exp. Bot., 53, 205-214.
Mollard F.P.O., Striker G.G., Ploschuk E.L. & Insausti P., 2010. Subtle topographical differences along a floodplain promote different plant strategies among Paspalum dilatatum subspecies and populations. Austral Ecol., 35, 189-196.
Monreal J.A. et al., 2007. Proline content of sugar beet storage roots: responses to water deficit and nitrogen fertilization at field conditions. Environ. Exp. Bot., 60, 257-267.
Munns R. & Gramer G.R., 1996. Is coordination of leaf and root growth mediated by abscisic acid? Plant Soil, 185, 33-49.
Munns R. & Tester M., 2008. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol., 59, 651-681.
Munns R. & Prometheus Wiki contributors, 2010. Plant water content and relative water content, http://prometheuswiki.publish.csiro.au/tiki-index.php?page=Plant+water+content+and+relative+water+content, (June 20, 2014).
Nikolaeva M.K., Maevskaya S.N., Shugaev A.G. & Bukhov N.G., 2010. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ. J. Plant Physiol., 57, 87-95.
Niyogi K.K., 1999. Photoprotection revisited: genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol., 50, 333-359.
Nonami H., Wu Y. & Boyer J.S., 1997. Decreased growth induced water potential: a primary cause of growth inhibition at low water potentials. Plant Physiol., 114, 501-509.
Ogen E. & Öquist G., 1985. Effects of drought on photosynthesis, chlorophyll fluorescence and photoinhibition susceptibility in intact willow leaves. Planta, 166, 380-388.
Ohashi Y., Saneoka H. & Fujita K., 2000. Effect of water stress on growth, photosynthesis, and photoassimilate translocation in soybean and tropical pasture legume siratro. Soil Sci. Plant Nutr., 46, 417-425.
Ozkur O., Ozdemir F., Bor M. & Turkan I., 2009. Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environ. Exp. Bot., 66, 487-492.
Pinheiro C., Chaves M.M. & Ricardo C.P.P., 2001. Alterations in carbon and nitrogen metabolism induced by water deficit in the stem and leaves of Lupinus albus L. J. Exp. Bot., 52, 1063-1070.
Poorter L. & Markesteijn L., 2008. Seedling traits determine drought tolerance of tropical tree species. Biotropica, 40, 321-331.
Pustovoitova T.N. et al., 1996. Drought resistance, recovery capacity, and phytohormone content in polyploid plum leaves. Russ. J. Plant Physiol., 43, 232-235.
Sellin A., 1996. Base water potential of Picea abies as a characteristic of soil water status. Plant Soil, 184, 273-280.
Sharp R.E. & Davies W.J., 1979. Solute regulation and growth by roots and shoots of water-stressed maize plants. Planta, 147, 43-49.
Sircelj H., Tausz M., Grill D. & Batic F., 2005. Biochemical responses in leaves of two apple tree cultivars subjected to progressing drought. J. Plant Physiol., 162, 1308-1318.
Sircelj H., Tausz M., Grill D. & Batic F., 2007. Detecting different levels of drought stress in apple trees (Malus domestica Borkh.) with selected biochemical and physiological parameters. Sci. Hortic. Amsterdam, 113, 362-369.
Sisko M., Javornik B., Siftar A. & Ivancic A., 2009. Genetic relationships among Slovenian pears assessed by molecular markers. J. Am. Soc. Hortic., 134, 97-108.
Snaydon R.W. & Davies T.M., 1982. Rapid divergence of plant populations in response to recent changes in soil conditions. Evolution, 36, 289-297.
Sofo A., Dichio B., Xiloyannis C. & Masia A., 2004. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci., 166, 293-302.
Soolanayakanahally R.Y. et al., 2009. Enhanced assimilation rate and water use efficiency with latitude through increased photosynthetic capacity and internal conductance in balsam. Plant Cell Environ., 32(12), 1821-1832
Still D.W., Aoyama N. & Kim D.H., 2005. Genetic variation in Echinacea angustifolia along a climatic gradient. Ann. Bot., 96, 467-477.
Striker G.G., 2012. Time is on our side: the importance of considering a recovery period when assessing flooding tolerance in plants. Ecol. Res., 27, 984-987.
Terzi R. & Kadıoğlu A., 2006. Drought stress tolerance and the antioxidant enzyme systems in Ctenanthe setosa. Acta Biol. Cracow Ser. Bot., 48(2), 89-96.
Vavilov V., 1994. Origin and geography of cultivated plants. Cambridge, UK: Cambridge University Press.
Velikova V. & Loreto F., 2005. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperature and during the recovery from a heat stress. Plant Cell Environ., 28, 318-327.
Vitasse Y. et al., 2009. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res., 39, 1259-1269.
Von Ende C.N., 1993. Repeated-measures analysis: growth and other time-dependent measures. In: Scheiner S.M. & Gurevitch J., eds. Design and analysis of ecological experiments. New York, NY, USA: Chapman and Hall, 113-137
Warton D.I., Duursma R.A., Falster D.S. & Taskinen S., 2012. Smatr 3–an R package for estimation and inference about allometric lines. Methods Ecol. Evol., 3(2), 257-259.
White D.A., Turner N.C. & Galbraith J.H., 2000. Leaf water relations and stomatal behavior of four allopatric Eucalyptus species planted in Mediterranean southwestern Australia. Tree Physiol., 20, 1157-1165.
Wise R.R. & Naylor A.W., 1987. Chilling-enhanced peroxidation: the peroxidative destruction of lipids during chilling injury to photosynthesis and ultrastructure. Plant Physiol., 83, 272-277.
Yemm E.W. & Willis A.J., 1954. The estimation of carbohydrates in plants extracts by anthrone. Biochem. J. Colchester, 57, 508-514.
Zhou Y., Lambrides C.J. & Fukai S., 2013. Drought resistance of bermudagrass (Cynodon spp.) ecotypes collected from different climatic zones. Environ. Exp. Bot., 85, 22-29.
Notes
1 Bates L.S., Waldran R.P. & Teare I.D., 1973. Rapid determination of free proline for water stress studies. Plant Soil, 39, 205-208.






