- Startpagina tijdschrift
- Volume 18 (2014)
- numéro 4
- Speciation in Malagasy lemurs: a review of the cryptic diversity in genus Lepilemur
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Speciation in Malagasy lemurs: a review of the cryptic diversity in genus Lepilemur

Nota's van de redactie
Received on March 11, 2013; accepted on November 5, 2014
Résumé
Spéciation des lémuriens de Madagascar : synthèse sur l’énigmatique diversité du genre Lepilemur.
Introduction. Madagascar est considéré comme l’un des plus importants hotspots de biodiversité, mais également comme l’un des pays les plus touchés en termes de dégradation des habitats et des forêts.
Littérature. La faune mammalienne de Madagascar se compose majoritairement de primates, à savoir les lémuriens. Parmi eux, la famille des Lepilemuridae, représentée par un genre unique, Lepilemur, est un groupe d’espèces nocturnes, exclusivement arboricoles et principalement folivores. Ils ont de très petites aires de distribution et sont fortement menacés d’extinction. Différents modèles de mécanismes de spéciation ont été développés à Madagascar, et des auteurs ont analysé la distribution de Lepilemur spp. sur base de deux modèles biogéographiques existants (« Martin model » et « Wilmé model »). La question de la biogéographie des lepilemurs a été particulièrement étudiée dans le Nord-Ouest de l’ile.
Conclusion. Suite à l’impact de la destruction des forêts et des habitats à Madagascar, des stratégies de conservation doivent être mises en place. Cette revue donne un aperçu de l’état actuel des connaissances sur le genre Lepilemur et analyse les processus impliqués dans la spéciation des lémuriens de Madagascar. La compréhension de la répartition des espèces dans les hotspots de biodiversité est un facteur clef pour l’établissement des objectifs de conservation. Nous résumons et comparons trois modèles biogéographiques liés à la distribution des lémuriens afin de tenter de comprendre les raisons de la grande diversité (26 au total) du genre Lepilemur. Nous nous intéressons aux concepts d’espèce dans un contexte de conservation, ainsi qu’à la récente explosion taxonomique pour le genre Lepilemur.
Abstract
Introduction. Madagascar is one of the highest biodiversity hotspots on the planet; however, it is also one of the most heavily impacted countries in the world in terms of forest degradation and general habitat destruction.
Literature. Genus Lepilemur, in family Lepilemuridae, is a genus of small, nocturnal, exclusively arboreal Malagasy folivores. All species in the genus have small ranges of distribution. Fully forest-dependent, they have a high risk of extinction. Various models and theories of speciation mechanisms have been developed for the fauna and flora of Madagascar. For instance, in the northwestern part of the island, some authors used Lepilemur spp. to test two existing models of distribution: the “Martin model” and “Wilmé model”.
Conclusion. Regarding the impact of forest destruction and habitat degradation in Madagascar, conservation strategies for Lepilemur need to be put in place. This paper gives an overview of the current knowledge of the genus Lepilemur and examines speciation for Malagasy lemurs. The understanding of species distribution within biodiversity hotspots is important to identify target for conservation. Therefore, we summarize and compare three biogeography models related to lemurs distribution in order to understand the reasons behind the high diversity (26 species in total) among the genus Lepilemur. Particular attention is also given to the concept of species regarding biodiversity issues and the taxonomic explosion in genus Lepilemur.
Inhoudstafel
1. Introduction
1Most of the plant and animal species found in Madagascar have evolved in long isolation and are found nowhere else. Indeed, the island has been separated from Africa for some 160 million years and from the Indian subcontinent for about 90 million years (Ganzhorn et al., 2006; Vences et al., 2009). This long isolation partly explains why Madagascar is one of the highest biodiversity hotspots on the planet (Myers et al., 2000; Irwin et al., 2010). The tropical and subtropical climate of the island, its topography and its geological history, together with its isolation, have produced a unique level of both species diversity and endemism (Wilmé et al., 2006a; Irwin et al., 2010; Rogers et al., 2010). More than 90% of plants, 92% of reptiles, 44% of birds, 74% of butterflies, and 100% of amphibians and terrestrial mammals native to Madagascar are endemic (Vences et al., 2009). Unfortunately, Madagascar is also one of the world’s most heavily impacted areas in terms of recent habitat destruction (Ganzhorn et al., 2006; Harper et al., 2007; Craul et al., 2009; Mittermeier et al., 2010; Mittermeier, 2013).
2Habitat fragmentation started with human occupation some 2,000 years ago (Craul et al., 2009) but ecosystem destruction and devastation increased enormously during the last decades due to a high rate of human population growth and to frequent political instability (Mittermeier et al., 2010). Nearly 90% of the natural vegetation in Madagascar has already been lost, and erosion on the island is severe (Ganzhorn et al., 2000; Harper et al., 2007). Deforestation on Madagascar is massive, with around 100,000 ha per year lost (Méndez-Cárdenas et al., 2008), and remaining forests have become increasingly vulnerable. Eighty percent of Madagascar’s forests are now located within 1 km of a non-forest edge (Schwitzer et al., 2013a). The dry deciduous forests of western Madagascar are particularly affected (Ganzhorn et al., 2000). The island is now considered one of the most critical global priorities for nature conservation (Myers et al., 2000; Goodman et al., 2005). Obviously, the continuous decline of such forest ecosystem is having a serious impact on all forest-dwelling organisms (Méndez-Cárdenas et al., 2008). Habitat loss and fragmentation are clearly one of the main causes of biodiversity loss worldwide (Fahrig, 2003).
3In recent years, numerous new plant and animal species have been revealed through surveys and analyses on Madagascar. However, the fragile status of both previously known and newly detected species has also been increasingly documented. The mammalian fauna of Madagascar mainly consists of lemurian primates (Lemuroidea) (Randrianambinina et al., 2010). In the early 1980s, 36 species of lemur were recognised on Madagascar (Tattersall, 1982), whereas today 98 species (102 taxa) are recognised (Mittermeier, 2013). An additional three species have been described since the publication of the Handbook, bringing the total of known species to 101, comprising 105 taxa (Schwitzer et al., 2013b). A conservation status assessment conducted in July 2012 for all lemurs by the Primate Specialist Group placed an alarming 94 out of the 103 taxa known at the time into one of the threatened categories of the International Union for Conservation of Nature (IUCN) classification. This is the highest ratio of threatened species ever recorded for a large group of mammals (Mittermeier, 2013; Schwitzer et al., 2013a). The understanding of the diversity of genus Lepilemur, in family Lepilemuridae, has followed the trend observed for Lemuroidea in general. In the last few years, a large number of named subspecies were given full species status (Vences et al., 2009). Up to 2006, only 8 species of Lepilemur were known; 26 species have now been identified using cytogenetic and/or molecular methods (Andriaholinirina et al., 2006; Louis et al., 2006; Rabarivola et al., 2006; Mittermeier et al., 2010).
4Evolutionary mechanisms were the subject of many studies to explain the high species richness in tropical faunas. A variety of speciation modes (e.g. allopatric or sympatric, gradual or instantaneous, non-adaptive or driven by sexual selection or adaptation) are known to generate biotic diversity. Yet, the main drivers of the diversification process often remain unknown (Vences et al., 2009). Biogeographical approaches address fundamental questions of global biodiversity patterns (Wiens et al., 2004). Graham et al. (2005) showed the importance of historical processes in the understanding of local biological diversity patterns, particularly in the study of endemic low-dispersal taxa. Madagascar is well known to be a good model region for the study of species diversification mechanisms (Vences et al., 2009). Indeed, many characteristics of the country make it particularly suitable to test species diversification mechanisms proposed for many tropical regions (Yoder et al., 2006; Vences et al., 2009). Vences et al. (2009) describe five main diversification mechanisms for Madagascar: eco-geographic constraint, western rainforest refuges, riverine barrier, mountain refuges and watersheds. Several biogeographical models have been developed to address this question of high biodiversity of the island. Many studies focused on correlations between lemur speciation and the biogeographical history of Madagascar (Goodman et al., 2004; Ganzhorn et al., 2006; Wilmé et al., 2006; Yoder et al., 2006; Craul et al., 2007; Olivieri et al., 2007).
5The present paper aims to review speciation research in Madagascar, correlated to diversification mechanisms for Malagasy lemurs, to answer the question “Why are there so many species of Lepilemur?”. First, we present an overview of the current knowledge of genus Lepilemur. We summarize three biogeographic models related to Madagascar and Lepilemur diversity. The influence of the choice of species concept on biodiversity evaluation is considered, in particular in relation to the so-called taxonomic inflation in genus Lepilemur and the controversies it has generated. To conduct our review we used databases such as Science Direct, JSTOR, Springer Link, Wiley and BioMed Central. We started the research with terms such as lemurs, biogeography and cryptic species, without any limit on the year of publication. The oldest paper we found was published in 1972 and the more recent one was published in 2013.
2. Species diversification in Madagascar: a case study of genus Lepilemur
2.1. Genus Lepilemur: what do we know?
6Taxonomy. Genus Lepilemur I. Geoffroy, 1851, is the only extant genus in family Lepilemuridae Gray, 1870, one of five families (Cheirogaleidae, Lemuridae, Lepilemuridae, Indriidae, Daubentoniidae) that constitute the infraorder Lemuriformes (suborder Strepsirrhini, order Primates), a monophyletic clade endemic to Madagascar (Groves, 2001; Groves, 2005; Horvath et al., 2007; Mittermeier, 2013; Schwitzer, 2013b). This genus, commonly known as sportive lemurs, received little attention in molecular systematics (Ravaoarimanana et al., 2003). However, in the last few years, several studies on lepilemurs were conducted in the wild and correlated with genetic analysis based on chromosomal rearrangements and mtDNA sequences (cf. section 3.2. of this paper). These studies resulted in the identification of a number of new species, and induced significant changes in the taxonomy of the genus (Ravaoarimanana et al., 2003; Andriaholinirina et al., 2006; Rabarivola et al., 2006; Craul et al., 2007; Mittermeier et al., 2010).
7Morphology-diet. Sportive lemurs are medium-sized nocturnal, folivore and exclusively arboreal animals. These vertical leapers and clingers generally weigh less than 1 kg with, on average, a head-body length of 20 cm and a tail length of 25 cm (Lei et al., 2008; Mittermeier et al., 2010; Mittermeier, 2013). In the wild, the identification of lepilemurs species is limited by the lack of obvious phenotypic differentiation among species (Ravaoarimanana et al., 2003; Andriaholinirina et al., 2006).
8Distribution, home range and density. Lepilemurs are endemic to Madagascar. The 26 currently identified species are widely and discreetly distributed in the country’s low- and mid-altitude evergreen and deciduous forests (Andriaholinirina et al., 2006; Mittermeier et al., 2010). Each species appears to have a very small range. Studies on some species are still too scarce compared to those pertaining to other genera of lemurs, such as genus Lemur (Mittermeier et al., 2010; Mittermeier, 2013). Exact boundaries of the ranges of some species remain unknown, and need further investigation (Louis et al., 2006; Craul et al., 2007; Mittermeier et al., 2010). There is no estimate of population size for any species. The smallest forest fragment occupied by a sportive lemur (i.e. Lepilemur ruficaudatus) seems to be around 6 ha (Ganzhorm et al., 2000; Seiler, 2012). Because sportive lemurs have the lowest metabolic rate known among mammals, their choice of sleeping sites is particularly important (Schmid et al., 1996; Warren et al., 1998; Seiler, 2012). Active during the night, they spend the day hidden in tree holes, tangles of branches and vines, or, as a last resort, in tree forks (Craul et al., 2009; Mittermeier et al., 2010; Seiler, 2012).
9Social structure and behaviour. Lepilemurs are known to be solitary, except two species (L. ruficaudatus and L. edwardsi) that live in pairs (Thalmann, 2001; Zinner et al., 2003). However, social behavior is still unclear and more differences may exist among species (Mittermeier et al., 2010). Reproductive activity of individual species is still poorly known but L. edwardsi shows a seasonal reproduction. This reproductive behavior may be shared by other Lepilemur, with some divergence linked to the latitudinal location of the species range. The reproductive rate is low, with a maximum of one offspring per year. Sexual maturity is reached after two years (Randrianambinina et al., 2007).
10Threats. The main natural predators of lepilemurs are the fossa (Chryptoprocta ferox), the Madagascar harrier-hawk (Polyboroides radius) and snakes (Acrantophis madagascariensis, Acrantophis dumerili and Sanzinia madagascariensis) (Colquhoun, 2006). However, the main threat comes from human activities, particularly hunting and forest disturbance. Because sportive lemurs are exclusively arboreal with very limited distributions, they are particularly affected by deforestation and habitat fragmentation, both of which have intensified in the last 50 years (Ganzhorn et al., 2000; Harper et al., 2007; Schneider et al., 2010). The smaller the forest fragment, and the more disconnected it becomes, the greater the risk that lemur populations become too small and go extinct (Fahrig, 2003; Olivieri et al., 2005; Seiler, 2012). Habitat degradation also means increasing visibility of Lepilemur in the wild and easier access for natural predators and hunters (Olivieri et al., 2005; Seiler, 2012). Forest quality determines food quality and shelter availability for sportive lemurs. Forest fragmentation has a direct impact on population size, home range size and genetic isolation of sub-populations (Randrianambinina et al., 2010). The availability and quality of Malagasy forests is critical for the long-term survival of lepilemurs (Ganzhorn et al., 2000; Craul et al., 2007; Méndez-Cárdenas et al., 2008; Craul et al., 2009; Mittermeier et al., 2010; Randrianambinina et al., 2010).
11Due to all the above factors, sportive lemurs are particularly vulnerable to extinction. Further studies are required to gather information and knowledge on this genus in order to formulate effective conservation programs (Olivieri et al., 2005; Rabarivola et al., 2006; Craul et al., 2007; Olivieri et al., 2007; Randrianambinina et al., 2007; Seiler, 2012).
2.2. Speciation and diversification mechanisms of lemurs in Madagascar
12Tropical regions harbor a large biological diversity, and have long been regarded as ideal locations to investigate general speciation patterns and processes (Vences et al., 2009). The singularities of Madagascar make this island particularly suitable to test hypotheses on species diversification mechanisms developed in other tropical regions, an opportunity that has only recently been fully exploited (Graham et al., 2005; Vences et al., 2009). Madagascar has been isolated from other land masses for a very long time (Irwin et al., 2010; Rogers et al., 2010). The island can be divided into four well-marked bioclimatic and phytogeographic domains:
13– the southwestern subarid spiny forest,
14– the western dry deciduous forest,
15– the eastern and montane rainforests,
16– the central subhumid grassland (Goodman et al., 2004; Vences et al., 2009).
17Highly specific faunas and floras are associated with these major biomes, which are separated by sharp boundaries (Vences et al., 2009). Pleistocene climate vicissitudes and their effects on forest distribution and structure shaped Madagascar’s species distribution and evolutionary history on recent time scales (Ganzhorn et al., 2006; Wilmé et al., 2006; Vences et al., 2009). These factors and the speciation mechanisms that they have flavored have been at the center of many studies aiming at explaining the high degree of microendemism on the island (Goodman et al., 2004; Ganzhorn et al., 2006; Vences et al., 2009). Many of these studies focused on Malagasy primates to test various diversification mechanisms (Vences et al., 2009). In particular, the possible role of the present configuration and past evolution of the hydrographic system, known to have been an important factor in the distribution of primates in the neotropics (Ayeres et al., 1992; Lehman, 2002), has been investigated (Goodman et al., 2004; Vences et al., 2009). Speciation and current distribution of Malagasy lemurs have been studied within the framework of two possible diversification mechanisms, both based on the influence of rivers: the “riverine barrier mechanism” and the “watersheds mechanism” (Goodman et al., 2004; Ganzhorn et al., 2006; Wilmé et al., 2006a; Wilmé et al., 2006b; Craul et al., 2007; Vences et al., 2009). The “riverine barrier mechanism” hypothesis emphasizes the physical division by a river of the continuous range of a species, leading to vicariant divergence (Vences et al., 2009). Width and depth of the river and altitude of its source are significant characteristics (Goodman et al., 2004; Vences et al., 2009). Several studies have shown that large Malagasy rivers act as semi-permanent geographical barriers for lemurs (Goodman et al., 2004; Ganzhorn et al., 2006; Yoder et al., 2006; Craul et al., 2007; Vences et al., 2009). The “watershed mechanism” hypothesis considers river basins as biotic refuges during climatic variation (Wilmé et al., 2006a; Wilmé et al., 2006b). Periods of drier climate lead to a contraction of forests along rivers. Two scenarios exist. The first is applicable to watersheds with sources at high elevation. The upper reaches of these watersheds act as retreat zones, between which connections are maintained or established. These connections may permit the dispersal and range expansion to other basins of species previously restricted to the lower course of a stream. Such retreat-dispersion watersheds are expected to induce low levels of endemism. The second scenario occurs in watersheds with headwaters at low elevation. Forest blocks are reduced to individual riverine forests isolated by arid zones. These arid zones act as barriers to gene flow for forest-dependent species, leading to vicariant divergence and high levels of endemism (Goodman et al., 2004; Wilmé et al., 2006a; Vences et al., 2009).
2.3. Diversity of Malagasy lemurs: biogeographical models
18Three biogeographical models have been developed to address the question of the high diversity of Malagasy lemurs. All three models are based on the hypothesis that microendemism may be explained by diversification mechanisms, involving the past and present hydrographic systems of Madagascar (Martin, 1972; Pastorini et al., 2003; Wilmé et al., 2006a; Wilmé et al., 2006b; Craul et al., 2009). The first model, the “Martin model”, considers large rivers as geographical barriers to gene flow, resulting in allopatric speciation. This model divided Madagascar in eight biogeographic zones, all separated by large rivers (Figure 1A) (Martin, 1972; Pastorini et al., 2003). This model was further refined and used to explain speciation within some lemur genera, such as Eulemur and Propithecus (Pastorini et al., 2003). The second model, the “Wilmé model”, takes into account the effect of quaternary paleoclimatic shifts on patterns of dispersal and vicariance at intra-island level. This model shows the importance of riverine habitats in watersheds acting as buffers for the maintenance of local conditions, and as potential corridors for retreat towards higher altitudinal zones. This model recognises 12 centres of endemism on Madagascar (Wilmé et al., 2006a; Wilmé et al., 2006b). The third model, the “large river model”, was developed by Craul et al. (2007) to test predictions of the two previous models on the distribution of Malagasy mammals. This model focuses on genus Lepilemur in northern and northwestern Madagascar. Indeed, the genus, widely distributed in almost all forested regions of the island, is particularly interesting for testing biogeographic models. This model defined eight “Inter-River-Systems” (IRS): biogeographical zones, which correspond to areas between eight large rivers (Figure 1C). On the one hand, these rivers act as barriers to gene flow leading to cryptic speciation. On the other hand they provide, during periods of aridity, retreat zones in which small isolated populations may become genetically differentiated, later recolonizing surrounding areas (Craul et al., 2007). In northern and northwestern Madagascar, the three models are largely complementary and are founded on similar hypotheses. However, some of the predictions of the three models are different. The “Martin model” predicts four biogeographic zones, which correspond well to the three deepest phylogenetic splits in Craul’s “large river model”, but it fails to represent fully the species diversity in the area. The “Wilmé model” identifies a single centre of endemism between the Betsiboka and Maevarano rivers (Figure 1B), an area in which Craul et al. (2007) locate three species of Lepilemur, all separated by rivers (Figure 1C). Craul’s “large river model” seems to best reflect the complexity of Lepilemur diversity and distribution in the area considered. This effect of large rivers as biogeographic boundaries in northern and northwestern Madagascar has been shown to also apply to mouse lemurs, Microcebus spp. (Olivieri et al., 2007).
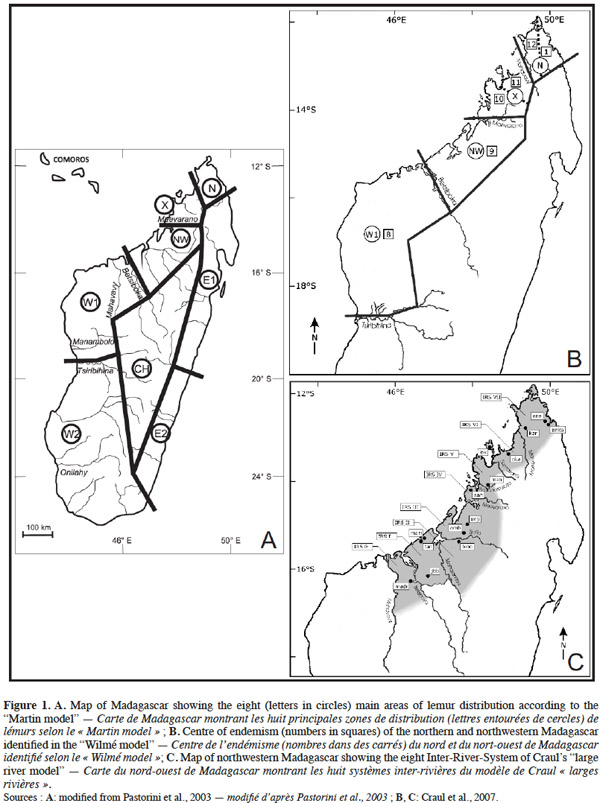
19Complementary research is still needed to confirm the relevance of Craul’s “large river model” and of the findings of Olivieri et al. (2007) to other taxa and areas (Louis et al., 2006; Olivieri et al., 2007; Vences et al., 2009). Validation in the field of such models becomes unfortunately increasingly difficult in the face of the intensive destruction of forested habitats.
3. Recent splitting of species in genus Lepilemur
3.1. The concept of species regarding biodiversity issues
20Species are “the units of the living world” (Groves, 2011) and the perception of “species” is intuitive, central to all popular classifications of living organisms. Yet, a large number of species concepts focusing on different properties, sometimes viewed as mutually exclusive, have been proposed and are in use. They are reviewed by, among others, de Queiroz (1998, 1999, 2007), Mallet (2007), Tattersall (2007), Wilkins (2008), Groves (2011), Groves et al. (2011), Markolf et al. (2011) and Markolf et al. (2013). For primates, the species concepts most frequently invoked in recent years are the Biological Species Concept (BSC), the Recognition Species Concept (RSC), the Genetic Species Concept (GSC), the Phylogenetic Species Concept (PSC) and the General Lineage Concept. The Biological Species Concept (BSC) emphasizes a “harmonious genetic pool” protected from other such pools by reproductive isolating barriers (Alström et al., 2003). The biological species is defined, on the basis of reproductive isolation observed or estimated, as a “group of actually or potentially interbreeding natural populations, which are reproductively isolated from other such groups” (Mayr, 1942; Mayr, 1963; Mayr, 1969). The Recognition Species Concept (RSC) is closely related to the Biological Species Concept through their common emphasis on reproductive isolation mechanisms. Under the RSC, species are recognized by the possession of a unique specific mate recognition system (SMRS). A species is defined as a population, or a group of populations, the members of which share a common mate recognition system (Paterson, 1978; Paterson, 1985).
21Contrary to the Biological Species Concept and related concepts, the Phylogenetic Species Concept (PSC), like the Evolutionary Species Concepts (Simpson, 1961; Wiley, 1978; Wiley, 1981), of which it constitutes one of the variants, places a central emphasis on the evolutionary fate of taxa in the past and in the present. The phylogenetic species is defined as an irreducible (basal) cluster of organisms, diagnosably distinct from other such clusters, and within which there is a parental pattern of ancestry and descent (Cracraft, 1983, 1987, 1989). Examples of consistent use of the Phylogenetic Species Concept in mammal taxonomy and demonstration of its operational advantage over other concepts are offered by Groves (2001, 2011, 2012), Groves et al. (2011), Gippoliti et al. (2012), Gippoliti et al. (2013) and Cotterill et al. (2014). The Genetic Species Concept (GSC) focuses on isolation and divergence of gene pools. The genetic species is defined as “a group of genetically compatible interbreeding natural populations that is genetically isolated from other such groups” (Bradley et al., 2001; Baker et al., 2006). This definition is very close to that of the PSC. Both definitions include genetic independence; both add a criterion of diagnosability to establish evolutionary independence or speciation in progress. Where the PSC allows diagnosability through any characters, the GSC restricts it to genetic markers. Moreover, the PSC only requires diagnosability, without reference to quantification of divergence, while the GSC places much emphasis on genetic distances to distinguish species from similarly-defined infra-specific units. In this, the GSC is similar to the Monophyletic Species Concept (Donoghue, 1985; Alström, 2002; Alström et al., 2003) which resorts to divergence times to rank least-inclusive taxa as either monotypic species or subspecies of a polytypic species (Alström et al., 2003). Groves (2012) argues that ranking processes based on genetic distances are highly subjective. The same is true of the evaluated time of divergence (Alström et al., 2003).
22An entirely new approach to the conceptualization of the species notion is proposed by de Queiroz (1998, 1999, 2005a, 2005b, 2005c, 2007), with the General Lineage Concept of Species or Unified Species Concept. He notes that all contemporary species concepts share a fundamental understanding of species as segments of lineages at the population level of biological organization, and differ only in the secondary properties, which are regarded as necessary for considering lineages to be species. He suggests (de Queiroz, 2005a) that “a unified species concept can be achieved by interpreting the common fundamental idea of being a separately evolving lineage segment as the only necessary property of species and viewing the various secondary properties either as lines of evidence relevant to assessing lineage separation or as properties that define different subcategories of the species category (e.g., reproductively isolated species, monophyletic species, diagnosable species)”. This general lineage concept is not an alternative to the various species concepts, but a more general concept that subsumes all of them. The clarification of de Queiroz (1998) shifts the philosophical controversies over species concepts to methodological differences in the choice of criteria for species recognition (de Queiroz, 2005a; Markolf et al., 2011). In recent years, the General Lineage Concept of Species has been fairly generally adopted in the field of primatology (Weisrock et al., 2010; Markolf et al., 2011; Markolf et al., 2013), the criteria most often used to ascertain or evaluate the separately evolving lineage segments concerned, being those derived from the PSC, the GSC or the BSC.
23A lively controversy subsists on the higher or lower numbers of species that are delineated by use of the PSC, GSC or BSC criteria and on the incidence that such numbers have on the practice of conservation biology. For sympatric taxa, PSC, GSC and BSC criteria, when applied in a context of sufficient data, give essentially identical results for a large spectrum of organisms (e.g. Alström et al., 2003; Groves et al., 2011; Groves, 2011; Gippoliti et al., 2012; Groves, 2013; Devillers et al., 2013; contra Zachos et al., 2013a). The same is largely true of the application to mostly parapatric taxa, hybridising in a zone of contact, of the PSC and the BSC, at least in the forms of the latter amended by Amadon et al. (1992) or Coyne et al. (2004). It is for allopatric taxa that the BSC and the PSC lead to very different appreciations. The PSC does in essence admit that geographical isolation, per se, does separate evolutionary paths while the BSC insists on a conviction that intrinsic separation mechanisms have evolved, ensuring continued distinctness in case of reunion, before accepting that the speciation process is underway. The PSC does therefore identify as species considerably more allopatric and insular taxa then does the BSC. This is not surprising, since, historically, the BSC was introduced with the explicit goal of drastically reducing the number of bird species that had been individualized in the dust of islands of the central Pacific (Mayr, 1942).
24The increase in the number of species, identified by the application of the PSC or GSC-inspired criteria, has induced doubts or criticisms about the conceptual and operational validity of their formulation (e.g. Collar, 1996; Collar, 1997; Agapow et al., 2004; Isaac et al., 2004a; Isaac et al., 2004b; Mace, 2004; Garnett et al., 2007; Tattersall, 2007; Markolf et al., 2011; Zachos et al., 2013b; Zachos et al., 2013c). Some criticisms of this so-called “taxonomic inflation” proceed from a conservative reflex. Thus, much of the argumentation of Zachos et al. (2013a) aims at showing that some species identified under the PSC do not meet the criteria underpinning the BSC, a hardly surprising observation. Many doubts and reservations mainly concern the use of taxonomy in other disciplines. The questioning most relevant to evolutionary science itself is that, as formulated by Tattersall (2007) “even clearly diagnosable populations can be no more than ephemera, at best merely potential actors on the evolutionary ... stage”. That even distinctive isolates, which are bound by barriers that fluctuate rapidly at an evolutionary time-scale, might be soon reabsorbed is a valid argument, though it hardly applies to ones which are separated by barriers that change in geological time. That isolates may have a limited life-expectancy is a more general observation, but their chances of survival are not related to whether or not they have been, in Tattersall's (2007) terms, “historically validated—individuated—by speciation”, a property he seems to equate with the development of presumed intrinsic isolation mechanisms.
25Several critics have argued that the elevation to species level, through the application of the PSC criteria, of a substantial number of previously known populations or infraspecific taxa is detrimental to conservation (e.g. Collar, 1996; Isaac et al., 2004a; Garnett et al., 2007; Zachos et al., 2013a; Zachos et al., 2013b; Zachos et al., 2013c). The objections of many of these authors seem to proceed more from the all-too-widespread acceptance that “the resources available for conservation are insufficient to prevent the loss of much of the world’s threatened biodiversity” (Collen et al., 2011) than from a necessarily relentless effort to seek support to augment those resources. A more preoccupying concern is raised by Zachos et al. (2013b), that of fine genetic discriminations hindering restoration efforts. Examples of such mishaps exist (e.g. Zink et al., 1995), but they may equally happen in a context of species-level over-lumping or over-splitting, and usually result from genetic rigorism unwittingly serving the vested interests of economic or financial growth. Groves et al. (2011), Groves (2012) and Gippoliti et al. (2012) convincingly argue the advantages to conservation of emphasizing species-level diversity.
3.2. Recent species-level taxonomic explosion in genus Lepilemur
26Lemurs are a prime subject for the study of the evolutionary and biogeographic mechanisms that have led to the high species richness and megadiverse biota of Madagascar (Martin, 1972; Wilmé et al., 2006; Weisrock et al., 2010). In the last decades, the number of lemur populations that have been individualized at species-level has considerably increased (Groeneveld et al., 2009; Mittermeier et al., 2010). Genus Lepilemur is one of the clades for which this explosion has been particularly marked. As lepilemurs are very homogeneous in pelage coloration and other morphological characters, and their vocalisations and chemical signals are still very poorly known. The genus is thus likely to include many cryptic species (Ravaoarimanana et al., 2003), the term “cryptic species” being taken in the sense of Bickford et al. (2007), as “two or more distinct species that are erroneously classified (and hidden) under one species name”. The term is often used in a more restrictive sense to designate species that are difficult to distinguish visually, although the animals themselves may use quite different signals, auditive or olfactive for instance, so that their differences are “cryptic” only to humans. In 1977, genus Lepilemur was thought to include seven species, one of which was polytypic (Petter et al., 1977). Since 2001, the taxonomy of the genus has been frequently revised, mostly through use of constantly improving molecular techniques, an increasingly powerful and valuable tool for the detection of cryptic species (Ravaoarimanana et al., 2003; Andriaholinirina et al., 2006; Louis et al., 2006; Bickford et al., 2007; Groves, 2011). In 2001, one of the subspecies of the most northerly Lepilemur, L. septentrionalis, was raised to species level as L. ankaranensis, after identification of the karyotypes (Rumpler et al., 2001). In 2006, three new species were proposed: L. aeeclis, L. randrianasoli, L. sahamalazensis (Andriaholinirina et al., 2006). In the same year, Louis et al. (2006) identified 11 new species, L. fleuretae, L. saeli, L. betsileo, L. wrightae, L. jamesorum, L. ahmansonorum, L. hubbardorum, L. pettri, L. grewcockorum, L. tymerlachsonorum and L. milanoiin, on the basis of mitochondrial DNA (D-loop, 12s RNA) analysis, and Rabarivola et al. (2006) defined L. mittermeieri on the basis of karyotipic and mtDNA criteria. In 2007, L. otto and L. manasamody were described (Craul et al., 2007); the latter was however later synonymized with L. grewcockorum (Zinner et al., 2007). Finally, L. scottorum and L. hollandorum were described in 2008 and 2009 (Lei et al., 2008; Ramaromilanto et al., 2009). Thus, a total of 26 species of Lepilemur are currently recognised (Mittermeier et al., 2010; Mittermeier, 2013).
27As for other groups of organisms that display such a trend, and, notably, for lemurs in general, this multiplication (by more than 3 in 10 years) of the number of recognized species of Lepilemur has sparked a debate on whether this increase reflects the biological reality or a biased taxonomic subdivision (Tattersall, 2007; Groeneveld et al., 2009; Weisrock et al., 2010; Markolf et al., 2011; Thiele et al., 2013). Thus, Tattersall (2007) asks if “this recent increase of recognised lemur species [is] due to previous unnoticed cryptic diversity, or to taxonomic inflation?”. Although valid arguments have been advanced on both sides of the “inflation” controversy, at least the assertion that the increase in the number of species-level taxa is caused by the recourse to criteria inspired by the Phylogenetic Species Concept is, in the case of Lepilemur, unfounded. Many of the recently described species (e.g. Louis et al., 2006) have been identified through analysis of mitochondrial DNA in rather small samples and reliance on thresholds in genetic distance to ascertain species status (Tattersall, 2007; Markolf et al., 2011). This approach constitutes a fairly restrictive usage of criteria derived from the Genetic Species Concept, and does not guarantee that the entities circumscribed meet the criteria that define phylogenetic species, genetic independence and diagnosability (e.g. Groves, 2011). Differences in mtDNA of small samples indicate distinct maternal lineages, but do not preclude male-mediated gene flow, ancestral polymorphism or delays in lineage sorting. Diagnosability is not achieved if it cannot be shown that intertaxon differences exceed intrataxon variability, a condition that is difficult to meet with small samples. One can however recognize, as Groves (2011), that “the use of GSC-inspired concepts has been enormously valuable in uncovering cryptic diversity in nocturnal Malagasy lemurs" and that, in particular, the collecting of mitochondrial sequence data for the identification of phylogenetic relationships within the genus Lepilemur has helped to solve systematic and taxonomic issues (Andriaholinirina et al., 2006). Since the increase in research effort and the fact that remote forests have been visited make the detection of previously cryptic species predictable (Groeneveld et al., 2009; Mittermeier et al., 2010; Markolf et al., 2011), it appears best to apply the principle of precaution, and treat the entities identified as species until, or unless, such an hypothesis is falsified. The collection of ecological, geographical, ethological, morphological and molecular data concerning more characters and much larger samples of Lepilemur populations are clearly needed to comfort or infirm the inferences drawn from mitochondrial DNA analyses. Studies aimed at evaluating the congruence in the distribution of several characters and their convergence in defining species-level taxa have been conducted for several other genera, in particular Cheirogaleus (Groeneveld et al., 2009; Thiele et al., 2013), Microcebus (Weisrock et al., 2010; Rasoloarison et al., 2013) and Eulemur (Markolf et al., 2013). They mostly confirm the divisions detected through mtDNA analysis, or increase their number. They find no sign of unwarranted “taxonomic inflation”. Partial investigations on genus Lepilemur yield similar results (Méndez-Cárdenas et al., 2009), but more research remains necessary.
4. Conclusion
28This review underlines the biodiversity richness and high endemism of Madagascar (Myers et al., 2000; Irwin et al., 2010). These characteristics, which spring from long isolation, low rates of colonization and unique natural history, make the island a perfect environment for studying species diversification (Vences et al., 2009). The mammalian fauna of Madagascar consists mainly of lemurian primates (Lemuroidea) and models and theories of mechanisms of speciation have been frequently developed on lemurs (Ganzhorn et al., 2006; Wilmé et al., 2006; Vences et al., 2009). Three current biogeographical models developed to explain the high diversity of Malagasy primates mainly differ in the number of biogeographical zones recognised (Martin, 1972; Wilmé et al., 2006a; Wilmé et al., 2006b; Craul et al., 2009). All three attribute the high level of endemism to the hydrographic system and its historical variations. The refinements incorporated by successive models over the years have resulted in the identification of a growing number of biogeographical areas (Martin, 1972; Wilmé et al., 2006a; Wilmé et al., 2006b; Craul et al., 2009). In the context of these models, the biogeography and distribution of genus Lepilemur has been most precisely analyzed in northwestern Madagascar (Rumpler et al., 2001; Andriaholinirina et al., 2006; Louis et al., 2006; Craul et al., 2007). The “large river model” defines eight “Inter-River-Systems” and seems particularly pertinent to explain Lepilemur distribution (Craul et al., 2007). The number of species-level taxa recognized in the infra-order Lemuriformes, and in genus Lepilemur in particular, has undergone a considerable recent increase. This trend, shared with a number of other groups of organisms, is the subject of a continuing debate on whether it reflects the uncovering of previously undetected (cryptic) diversity or constitutes an artefact attributable to species concepts, criteria for species identification or faulty application of these criteria. In the case of lemurs, doubts on the reality of the proposed diversity are kindled by the frequent recourse, for its detection, to a single criterion, the genetic distance measured in terms of mitochondrial DNA, and the small samples used to assess this parameter (Tattersall, 2007; Markolf et al., 2011). However, studies conducted on several genera, specifically designed to test, through consideration of several characters, the validity of the mtDNA-based conclusions, have confirmed them. Preliminary results on Lepilemur (e.g. Méndez-Cárdenas et al., 2009) suggest the same may apply in this genus. Accepting the 26 presently described species is, thus, a valid working hypothesis to initiate crucially needed further studies. Most of these species are threatened with extinction because of forest destruction and hunting (Méndez-Cárdenas et al., 2008; Mittermeier et al., 2010; Randrianambinina et al., 2010). As sportive lemurs are nocturnal and difficult to detect and arduous to follow in the wild, precise estimations of population ranges, sizes, densities and requirements are quite difficult (Méndez-Cárdenas et al., 2008; Randrianambinina et al., 2010). Nevertheless, it is evident that Lepilemur populations, restricted in range and fully forest-dependent, have a high-risk of extinction. The rate of deforestation and forest degradation in Madagascar is extremely high. The impact of such a threat, not only for lepilemurs, but for all forest-dependent species is such that measures to prevent further destruction must be urgently implemented. To develop such conservation strategies with the support of local communities, more data are still required. Additional information on range boundaries, on population densities, on minimum viable populations, and on minimum size of forest patches are vitally needed for all Lepilemur.
29Acknowledgements
30This work is essentially funded by FNRS-FRIA. The authors wish to thank Jean Devillers-Terschuren for the revision of the manuscript.
Bibliographie
Agapow P.-M. et al., 2004. The impact of species concept on biodiversity studies. Quaterly Rev. Biol., 79, 161-179.
Alström P., 2002. Species limits and systematics in some passerine birds. Comprehensive summaries of Uppsala dissertations from the Faculty of Science and Technology, 726, 1-31.
Alström P. & Mild K., 2003. Pipits and wagtails of Europe, Asia and North America. Identification and systematics. London: Christopher Helm.
Amadon D. & Short L.L., 1992. Taxonomy of lower categories - suggested guidelines. Bull. B.O.C., Centenary Suppl. 112a, 11-38.
Andriaholinirina N. et al., 2006. Molecular phylogeny and taxonomic revision of the sportive lemurs (Lepilemur, Primates). BMC Evol. Biol., 6, 17.
Ayeres J.M. & Clutton-Brock T.H., 1992. River boundaries and species range size in Amazonian primates. Am. Nat., 140(3), 531-537.
Baker R.J. & Bradley R.D., 2006. Speciation in mammals and the Genetic Species Concept. J. Mammalogy, 87, 643-662.
Bickford D. et al., 2007. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol., 22(3), 148-155.
Bradley R.D. & Baker R.J., 2001. A test of the Genetic Species Concept: cytochrome-b sequences and mammals. J. Mammalogy, 82, 960-973.
Collar N.J., 1996. Species concepts and conservation: a response to Hazevoet. Bird Conserv. Int., 6, 197-200.
Collar N.J., 1997. Taxonomy and conservation: chicken and egg. Bull. Br. Ornithology Club, 117, 122-136.
Collen B. et al., 2011. Investing in evolutionary history: implementing a phylogenetic approach for mammal conservation. Philos. Trans. R. Soc. London, Ser. B, 366, 2611-2622.
Colquhoun I.C., 2006. Predation and cathemerality. Comparing the impact of predators on the activity patterns of lemurids and ceboids. Folia Primatologica (Basel), 77(1-2), 143-165.
Cotterill F.P.D. et al., 2014. Why one century of phenetics is enough: response to “are there really twice as many bovid species as we thought ?” Syst. Biol., 63(5), 819-832.
Coyne J.A. & Orr H.A., 2004. Speciation. Sunderland, MA, USA: Sinauer Associates, Inc., Publishers.
Cracraft J., 1983. Species concepts and speciation analysis. Curr. Ornithology, 1, 159-187.
Cracraft J., 1987. Species concepts and the ontology of evolution. Biol. Philosophy, 2, 63-80.
Cracraft J., 1989. Speciation and its ontology: the empirical consequences of alternative species concepts for understanding patterns and processes of differentiation. In: Otte D. & Endler J.A., eds. Speciation and its consequences. Sunderland, MA, USA: Sinauer Associates, Inc.
Craul M. et al., 2007. Unexpected species diversity of Malagasy primates (Lepilemur spp.) in the same biogeographical zone: a morphological and molecular approach with the description of two new species. BMC Evol. Biol., 7(83).
Craul M. et al., 2009. Influence of forest fragmentation on an endangered large-bodied lemur in northwestern Madagascar. Biol. Conserv., 142, 2862-2871.
de Queiroz K., 1998. The general lineage concept of species, species criteria, and the process of speciation: a conceptual unification and terminological recommendations. In: Howard D.J. & Berlocher S.H., eds. Endless forms: species and speciation. Oxford, UK: Oxford University Press, 57-75.
de Queiroz K., 1999. The general lineage concept of species and the defining properties of the species category. In: Wilson R.A., ed. Species: new interdisciplinary essays. Cambridge, MA, USA: MIT Press, 49-89.
de Queiroz K., 2005a. Ernst Mayr and the modern concept of species. Chapter 13. In: Hey J., Fitch W.M. & Ayala F.J., eds. Systematics and the origin of species: on Ernst Mayr’s 100th Anniversary. Washington: National Academies Press, 243-263.
de Queiroz K., 2005b. A unified species concept and its consequences for the future of taxonomy. Proc. California Acad. Sci., 56(suppl.1)(18), 196-215.
de Queiroz K., 2005c. Different species problems and their resolution. BioEssays, 27, 1263-1269.
de Queiroz K., 2007. Species concepts and species delimitation. Syst. Biol., 56, 879-886.
Devillers P. & Devillers-Terschuren J., 2013. Orchidées et concepts modernes de l'espèce. Naturalistes Belg., 94(Orchid. 26), 61-74.
Donoghue M.J., 1985. A critique of the biological species concept and recommendations for a phylogenetic alternative. Bryologist, 88, 172-181.
Fahrig L., 2003. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst., 34, 487-515.
Ganzhorn J.U. et al., 2000. Effects of fragmentation and assessing minimum viable population of lemurs in Madagascar. Bonner Zool. Monographien, 46, 265-272.
Ganzhorn J.U., Goodman S.M., Nash S. & Thalmann U., 2006. Lemur biogeography. Primate biogeography. In: Lehman S.M. & Fleagle J.G., eds. Primate biogeography. Developments in primatology: progress and prospects. New York, USA: Springer, 229-254.
Garnett S.T. & Christidis L., 2007. Implications of changing species definitions for conservation purposes. Bird Conserv. Int., 17, 187-195
Gippoliti S. & Groves C.P., 2012. “Taxonomic inflation” in the historical context of mammalogy and conservation. Hystrix, 23, 8-11.
Gippoliti S., Cotterill F.P.D. & Groves C.P., 2013. Mammal taxonomy without taxonomists: a reply to Zachos and Lovari. Hystrix, 24, 145-147.
Goodman M.S. & Ganzhorn J.U., 2004. Biogeography of lemurs in the humid forests of Madagascar: the role of elevation distribution and rivers. J. Biogeogr., 31, 47-55.
Goodman M.S. & Benstead J.P., 2005. Update estimates of biotic diversity and endemism for Madagascar. Oryx, 39, 73-77.
Graham C.H., Moritz C. & Williams S.E., 2005. Habitat history improves prediction of biodiversity in rainforest fauna. Proc. Natl. Acad. Sci. U.S.A., 103, 632-636.
Groeneveld L.F. et al., 2009. Species delimitation in lemurs: multiple genetic loci reveal low levels of species diversity in the genus Cheirogaleus. BMC Evol. Biol., 9, 30.
Groves C.P., 2001. Primate taxonomy. Washington: Smithsonian Institution Press.
Groves C.P., 2005. Order Primates. In: Wilson D.E. & Reeder D.M. Mammal species of the world: a taxonomic and geographic reference. Baltimore, MD, USA: Johns Hopkins University Press, 111-184.
Groves C., 2011. Introduction to primate taxonomy and species concepts. In: Rowe N. & Myers M., eds. All the world’s primates. Charlestown, RI, USA: Primate Conservation Inc.
Groves C. & Grubb P., 2011. Ungulate taxonomy. Baltimore, MD, USA: Johns Hopkins University Press.
Groves C.P., 2012. Species concepts in primates. Am. J. Primatology, 74, 687-691.
Groves C.P., 2013. The nature of species: a rejoinder to Zachos et al. Mammalian Biol., 78, 7-9.
Harper G.J. et al., 2007. Fifty years of deforestation and forest fragmentation in Madagascar. Environ. Conserv., 34, 1-9.
Horvath J.E. & Willard H.F., 2007. Primate comparative genomics: lemur biology and evolution. Trends Genet., 23(4), 173-182.
Irwin M.T. et al., 2010. Patterns of species change in anthropogenically disturbed forests of Madagascar. Biol. Conserv., 143, 2351-2362.
Isaac N.J.B., Mallet J. & Mace G.M., 2004a. Taxonomic inflation: its influence on macroecology and conservation. Trends Ecol. Evol., 19, 464-469.
Isaac N.J.B. & Purvis A., 2004b. The ‘species problem’ and testing macroevolutionary hypotheses. Divers. Distrib., 10, 275-281.
Lehman S.M., 2002. Distribution and diversity of primates in Guyana: species-area relationships and riverine barriers. Int. J. Primatol., 25(1), 73-95.
Lei R. et al., 2008. Nocturnal Lemur diversity at Masoala National Park. Spec. Publ. Mus. Texas Tech Univ., 53, 1-41.
Louis E.E. Jr. et al., 2006. Molecular and morphological analyses of the sportive lemurs (Family Megaladapideae: Genus Lepilemur) reveals 11 previously unrecognized species. Spec. Publ. Mus. Texas Tech Univ., 49, 1-47.
Mace G.M., 2004. The role of taxonomy in species conservation. Philos. Trans. R. Soc. London, Ser. B, 359, 711-719.
Mallet J., 2007. Species, concepts of. In: Levin S., ed. Encyclopedia of biodiversity. Vol. 5. Oxford, UK: Academic Press, 427-440.
Markolf M., Brameier M. & Kappeler P.M., 2011. On species delimitation: yet another lemur species or just genetic variation? BMC Evol. Biol., 11, 216.
Markolf M. et al., 2013. True lemurs…true species - species delimitation using multiple data sources in the brown lemur complex. BMC Evol. Biol., 13, 233.
Martin R.D., 1972. Adaptive radiation and behavior of the Malagasy lemurs. Philosophical Philos. Trans. R. Soc. London., Ser. B., 246, 295-352.
Mayr E., 1942. Systematics and the origin of species. New York, USA: Columbia University Press.
Mayr E., 1963. Animal species and evolution. Cambridge, UK: Harvard University Press.
Mayr E., 1969. Principles of systematic zoology. New York, USA: McGraw-Hill.
Méndez-Cárdenas M. et al., 2008. Geographic variation in loud calls of sportive lemurs (Lepilemur ssp.) and their implications for conservation. Am. J. Primatology, 70, 1-11.
Méndez-Cárdenas M. & Zimmermann E., 2009. Duetting - A mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi)? Am. J. Phys. Anthropol., 139, 523-532.
Mittermeier R.A. et al., 2010. Lemurs of Madagascar. 3d ed. Washington: Conservation International.
Mittermeier R.A., 2013. Introduction. In: Mittermeier R.A., Rylands A.B. & Wilson D.E., eds. Handbook of the mammals of the world. 3. Primates. Barcelona, Spain: Lynx Editions, 13-26.
Myers N. et al., 2000. Biodiversity hotspots for conservation priorities. Nature, 403, 853-858.
Olivieri G., Craul M. & Radespiel U., 2005. Inventaires des lémuriens dans 15 fragments de forêt de la province de Mahajanga. Lemur News, 10, 11-16.
Olivieri G. et al., 2007. The ever-increasing diversity in mouse lemurs: three new species in north and northwestern Madagascar. Mol. Phylogenet. Evol., 43, 309-327.
Pastorini J., Thalmann U. & Martin R.D., 2003. A molecular approach to comparative phylogeography of extant Malagasy lemurs. Proc. Natl. Acad. Sci. U.S.A., 100, 5879-5884.
Paterson H.E.H., 1978. More evidence against speciation by reinforcement. S. Afr. J. Sci., 74, 369-371.
Paterson H.E.H., 1985. The recognition concept of species. In: Vrba E.S., ed. Species and speciation. Transvaal Museum Monograph, 4. Pretoria: Transvaal Museum.
Petter J.J, Albignac R. & Rumpler Y., 1977. Mammifères lémuriens (Primates Prosimiens). Faune de Madagascar, 44. Paris: ORSTOM/CNRS.
Rabarivola C. et al., 2006. Cytogenetic and molecular characteristics of a new species of sportive lemur from northern Madagascar. Lemur News, 11, 45-49.
Ramaromilanto B. et al., 2009. Sportive lemur diversity at Mananara-Nord Biosphere Reserve, Madagascar. Occasional Pap. Mus. Texas Tech Univ., 286, 1-22.
Randrianambinina B. et al., 2007. Seasonality in reproduction of Lepilemur edwardsi. Int. J. Primatology, 28, 783-790.
Randrianambinina B. et al., 2010. Abundance and conservation status of two newly described lemur species in northwestern Madagascar (Microcebus danfossi, Lepilemur grewcockorum). Madagascar Conserv. Dev., 5(2), 95-102.
Rasoloarison R.M. et al., 2013. Two new species of mouse lemurs (Cheirogaleidae: Microcebus) from eastern Madagascar. Int. J. Primatology, 34, 455-469.
Ravaoarimanana I.B., Tiedemann R., Montagnon D. & Rumpler Y., 2003. Molecular and cytogenetic evidence for cryptic speciation within a rare endemic Malagasy lemur, the Northern Sportive Lemur (Lepilemur septentrionalis). Mol. Phylogenet. Evol., 31, 440-448.
Rogers H.M., Glew L., Honzák M. & Hudson M.D., 2010. Prioritizing key biodiversity areas in Madagascar by including data on human pressure and ecosystem services. Landscape Urban Plann., 96, 48-56.
Rumpler Y., Ravoarimanana B., Hauwy M. & Water S., 2001. Cytogenetic arguments in favour of taxonomic revision Lepilemur septentrionalis. Folia Primatologica, 72, 308-315.
Schmid J. & Ganzhorn J., 1996. Resting metabolic rates of Lepilemur mustelinus ruficaudatus. Am. J. Primatology, 38, 169-174.
Schneider N., Chikhi L., Currat M. & Radespiel U., 2010. Signals of recent spatial expansions in the grey mouse lemur (Microcebus murinus). BMC Evol. Biol., 10, 105.
Schwitzer C. et al., 2013a. Lemurs of Madagascar: a strategy for their conservation 2013-2016. Bristol, UK: IUCN SSC Primate Specialist Group, Bristol Conservation and Science Foundation, and Conservation International.
Schwitzer C., Mittermeier R.A., Louis E.E. Jr. & Richardson M.C., 2013b. Family Lepilemuridae (sportive lemurs). In: Mittermeier R.A., Rylands A.B. & Wilson D.E., eds. Handbook of the mammals of the world. 3. Primates. Barcelona, Spain: Lynx Editions, 66-88.
Seiler M., 2012. The impact of habitat degradation and fragmentation on ecology and behaviour of the Sahamalaza sportive lemur, Lepilemur sahamalazensis, in Northwest-Madagascar. PhD thesis: Bristol University, Bristol (England).
Simpson G.G., 1961. Principles of animal taxonomy. New York , USA: Columbia University Press.
Tattersall I., 1982. The primates of Madagascar. New York, USA: Columbia University Press.
Tattersall I., 2007. Madagascar's lemurs: cryptic diversity or taxonomic inflation? Evol. Anthropol., 16, 12-23.
Thalmann U., 2001. Food resource characteristics in two nocturnal lemurs with different social behavior: Avahi occidentalis and Lepilemur edwardsi. Int. J. Primatology, 22(2), 287-324.
Thiele D., Razafimahatratra E. & Hapke A., 2013. Discrepant partitioning of genetic diversity in mouse lemurs and dwarf lemurs – Biological reality or taxonomic bias? Mol. Phylogenet. Evol., 69, 593-609.
Vences M., Wollenberg K.C., Vieites D.R. & Lees D.C., 2009. Madagascar as a model region of species diversification. Trends Ecol. Evol., 24(8), 456-465.
Warren R.D. & Cromton R.H., 1998. Diet, body size and the energy costs of locomotion in saltatory primates. Folia Primatologica, 69, 86-100.
Weisrock D.W. et al., 2010. Delimiting species without nuclear monophyly in Madagascar’s mouse lemurs. PLoS One, 5(3), e9883.
Wiens J.J. & Donoghue M.J., 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol., 19(12), 639-644.
Wiley E.O., 1978. The evolutionary species concept reconsidered. Syst. Zool., 27, 17-26.
Wiley E.O., 1981. Remarks on Willis’ species concept. Syst. Zool., 30, 86-87.
Wilkins J., 2008. Species concepts in modern literature: summary of 26 species concepts. Addendum to Wilkins J., 2006. Species, kinds, and evolution. Rep. Natl. Center Sci. Educ., 26(4), 36-45.
Wilmé L., Goodman M.S. & Ganzhorn J.U., 2006a. Biogeographic evolution of Madagascar’s microendemic biota. Science, 312, 1063-1065.
Wilmé L. & Callmander M.W., 2006b. Les populations reliques de primates : les propithèques. Lemur News, 11, 24-31.
Yoder A.D. & Heckman K., 2006. Mouse lemurs phylogeography revises a model of ecogeographic constraint in Madagascar. In: Fleagle J. & Lehman S.M., eds. Primate biogeography: progress and prospects. New York, USA: Kluwer, 255-268.
Zachos F.E. et al., 2013a. Species inflation and taxonomic artefacts – A critical comment on recent trends in mammalian classification. Mamm. Biol., 78, 1-6.
Zachos F.E. et al., 2013b. Species splitting puts conservation at risk. Nature, 494, 35.
Zachos F.E. & Lovari S., 2013c. Taxonomic inflation and the poverty of the Phylogenetic Species Concept – a reply to Gippoliti and Groves. Hystrix, 24, 142-144.
Zink R.M. & Kale H.W., 1995. Conservation genetics of the extinct dusky seaside sparrow Ammodramus maritimus nigrescens. Biol. Conserv., 74, 69-71.
Zinner D. et al., 2003. Social organization of Lepilemur ruficaudatus. Int. J. Primatology, 24(4), 869-888.
Zinner D. et al., 2007. Disputed taxonomy classification of sportive lemurs (Lepilemur) in NW Madagascar. Lemur News, 12, 53-56.






