- Portada
- Volume 19 (2015)
- Numéro 1
- Prevalence reduction of pathogens in poultry fed with Saccharomyces cerevisiae
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Prevalence reduction of pathogens in poultry fed with Saccharomyces cerevisiae

Notes de la rédaction
Received on November 3, 2014; accepted on November 5, 2014
Résumé
Réduction de la prévalence de pathogènes en poulets de chair supplémentés avec Saccharomyces cerevisiae
Description du sujet. Le développement de nouvelles souches de bactéries pathogènes résistantes aux antibiotiques constitue un enjeu majeur pour la filière volaille. Il est établi que la levure vivante peut efficacement contribuer à la protection contre plusieurs bactéries pathogènes pour les oiseaux et représente un risque de santé publique pour l’homme. À ce jour, peu d’informations, parfois contradictoires, sont disponibles sur ce sujet.
Objectifs. L’objectif de cette étude était d’étudier l’effet d’une levure vivante sur la présence de Salmonella enteritidis et Campylobacter jejuni dans les fientes, le caecum et sur la peau des poulets de chair.
Méthode. La levure vivante utilisée est une Saccharomyces cerevisiae (Levucell® SB20, type boulardii I-1079, Lallemand, France), incorporée au taux de 1 x 106 UFC·g-1 aliment. À 10 jours d’âge, les animaux étaient soumis à une infection par sollicitation orale de S. enteritidis (1 x 105 UFC/poulet) et C. jejuni (3 x 105 UFC/poulet). Les performances zootechnique et l’énumération des coliformes, levures et lactobacilles étaient évalués à 0, 10, 20 et 38 jours. Dix et dix huit jours après infection (AI), 10 animaux par réplicat ont été abattus et le contenu cécal composite était analysé pour la population de levures, Salmonelles et Campylobacter ainsi que leur fréquence d’apparition. La détection de présence et l’énumération de Salmonelles et Campylobacter sur la peau du cou et des blancs étaient réalisées sur un sujet par réplicat.
Résultats. L’ajout de S. cerevisiae dans l’aliment des poulets de chair a entrainé une augmentation des comptages des levures et lactobacilles (p = 0,01), tandis que la population et fréquence de détection des Salmonelles étaient significativement réduites sur la peau du cou (p = 0,03) et tendaient à être diminuées dans le caecum (p = 0,06), les fientes (p = 0,06) et les blancs de poulet (p = 0,08). Dix jours AI, la présence de Campylobacter était significativement réduite dans le caecum (p = 0,01), les fientes (p < 0,01), les blancs (p = 0,04) et la peau du cou (p < 0,01), alors que l’énumération était significativement plus basse dans les fientes (p < 0,01) et sur la peau du cou (p = 0,05). À la fin de l’essai, la fréquence de détection de ce pathogène était significativement diminuée dans les fientes (p < 0,01) et sur la peau du cou (p = 0,03), tandis que la population était significativement réduite dans le caecum (p < 0,05) et les fientes (p < 0,05).
Conclusions. Cette étude démontre que l’inclusion de Levucell® SB20 peut significativement contrôler le transport de Campylobacter dans les poulets de chair et la présence de Salmonelles, contribuant ainsi à réduire la contamination de la carcasse à l’abattage et prévenir les intoxications alimentaires.
Abstract
Description of the subject. The growth of new antibiotic-resistant strains of pathogens represents a huge problem in poultry rearing. There is evidence that dietary yeast could be effective in the protection against a variety of pathogens that can affect poultry health and cause foodborne diseases in humans. Since still few or contradictory information are available for this topic.
Objectives. The objective of this study was to investigate the effects of live yeast supplementation in broiler chickens on Salmonella enteritidis and Campylobacter jejuni content in feces, cecum, and skin.
Method. Supplemented yeast consisted of Saccharomyces cerevisiae (Levucell® SB20, type boulardii I-1079, Lallemand, France) and was administered at a rate of 1 x 106 CFU·g-1 of feed. On day ten of life, birds were orally challenged with S. enteritidis (1 x 105 CFU/bird) and C. jejuni (3 x 105 CFU/bird). Growth performance, and coliforms, yeasts and lactobacilli enumeration were evaluated on day 0, 10, 20 and 38. Ten and eighteen days post infection (PI), 10 animals per replicate were slaughtered and pooled ceca content were analyzed for yeast enumeration and Salmonella and Campylobacter frequency and enumeration. The presence and the enumeration of Salmonella and Campylobacter in neck and breast skin were performed on one subject per replicate.
Results. Dietary S. cerevisiae increased yeast and lactobacilli (p = 0.01) count, while Salmonella enumeration and frequency significantly decreased in neck (p = 0.03) and tended to decrease in cecum (p = 0.06), feces (p = 0.06), and breast (p = 0.08). On 10d PI Campylobacter presence was decreased in cecum (p = 0.01), feces (p < 0.01), breast skin (p = 0.04) and neck skin (p < 0.01), while the enumeration was found to be lower in feces (p < 0.01) and neck skin (p = 0.05). At the end of the trial the frequency of this pathogen was decreased in feces (p < 0.01), and breast skin (p = 0.02), while the enumeration was diminished in cecum (p < 0.05) and feces (p < 0.05).
Conclusions. The present study shows that the inclusion of Levucell® SB20 can significantly control Campylobacter carriage in chickens with some positive effects also on Salmonella presence, thus reducing the contamination of carcasses at slaughtering and preventing human foodborne diseases.
Tabla de contenidos
1. Introduction
1The use of sub-therapeutic antibiotics has been a cornerstone to prevent the microorganism infection and promote growth in poultry industry for many years. As new antibiotic-resistant strains of pathogens emerge and since the European ban of non-therapeutic antibiotic use in feed, poultry rearing is searching for new strategies to prevent and contrast bacterial infections that are often common. During past years probiotics have been studied in different animal species such as ruminants (Ripamonti et al., 2009; Ripamonti et al., 2011), horses (Agazzi et al., 2011) and laying hens (Quarantelli et al., 2008) with positive results on performance and health status. This kind of feed additive has been shown to be involved in the protection against a variety of pathogens in chicken, including Escherichia coli (Chateau et al., 1993), Salmonella and Campylobacter (Stern et al., 2001), Clostridium and Eimeria (Dalloul et al., 2005), that can affect poultry health and cause foodborne diseases in humans. The mechanism of action in poultry is based on positive alterations in intestinal microflora population by competitive exclusion, enhancement of growth of nonpathogenic facultative anaerobic and Gram+ bacteria forming lactic acid and hydrogen peroxide, suppression of growth of intestinal pathogens, and enhancement of digestion and utilization of nutrients (Yeo et al., 1997). In addition probiotics have shown also to interact with the host by influencing the immune response (Delcenserie et al., 2008; Tellez et al., 2012), or producing components able to positively affect mucosa development or the metabolism of the host’s intestinal cells (Johnson-Henry et al., 2008). Therefore, the major outcomes using probiotics include the improvement in growth (Yeo et al., 1997), the reduction in mortality (Kumprecht, 1998), the improvement in feed conversion rate (Yeo et al., 1997; Jin et al., 2000; Yoon et al., 2004; Schneitz, 2005; Awad et al., 2009; Kizerwetter-Swida et al., 2009), and the contribution in preventing human foodborne diseases form Salmonella and Campylobacter (Stern et al., 2001). The aim of this trial was to investigate the effects of live yeast supplementation in broiler chickens on Salmonella enteritidis and Campylobacter jejuni content in feces, cecum, breast skin and neck skin.
2. Materials and methods
2.1. Experimental animals and housing conditions
2The trial was performed at the Animal Production Research and Teaching Centre of Università degli Studi di Milano. A total number of 480 one-day-old Hubbard female chickens were randomly allotted to one of the two experimental groups on the basis of dietary treatment for a period of 38 days. Each experimental group was composed by 12 replicates (5 x 3 m2) of 20 subjects reared on wood shavings. Both groups were allocated in the same room with controlled environmental conditions and free access to water. Initial room climate program considered a temperature equal to 33 °C under the brooder and 30 °C in the living area with a humidity of 60%, and a ventilation of 1 m3·kg-1 body weight (BW) until day 21. From day 22 of age until 35 the experimental room had 26 °C under the brooder, 23 °C in the living area, 65% humidity and a ventilation of 3.4 m3·kg-1 BW. In the last three days of the trial room temperature was maintained at 19 °C with 65% humidity and a ventilation of 3.4 m3·kg-1 BW.
2.2. Diet composition and trial design
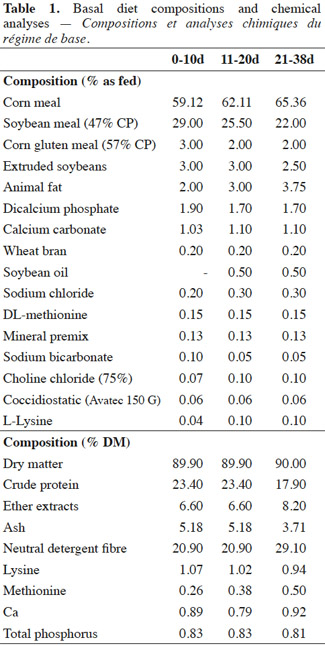
3The experimental groups were submitted to one of the following dietary treatments: C) fed a basal diet; Y) fed the same basal diet with the supplementation of Saccharomyces cerevisiae boulardii (Levucell® SB20, strain I-1079, Lallemand, France) at a concentration of 1 x 106 CFU·g-1 feed. A Prestarter (0-10d), a starter (11-20d) and a grower (21-38d) basal diet were adopted during the trial with decreasing crude protein (CP) (from 23.40% to 17.90% as fed) and increasing ether extracts (EE) (from 6.60% to 8.20% as fed) content (Table 1). Yeast was directly included in the diet of Y group subtracting the respective amount of feed. At the beginning of the trial, samples of each experimental diet for the different rearing phases were collected and analyzed for S. cerevisiae I-1079 content. On day 10 from the arrival, all experimental chickens were challenged by oral gavage (Line et al., 1998) with S. typhimurium (1 x 105 CFU per bird), and C. jejuni (3 x 105 CFU per bird).
2.3. Growth performance and microbiological assays
4Individual BW and feed intake (FI) per pen were recorded on day 0, 10 and 20 with 20 birds per replicate and on days 21 and 38 with 10 birds per pen after first slaughtering, thus average daily gain (ADG) and feed conversion rate (FCR) per pen were calculated. Pooled pen fresh droppings (20 g) were collected at the beginning of the trial and at the end of each feeding phase for enumeration of coliforms, yeasts, and total lactic acid bacteria. On days 20 and 38 from the beginning of the trial pooled fecal samples were analyzed for Salmonella and Campylobacter frequency and enumeration.
5At 10 days post infection (PI), 10 animals for each replicate were slaughtered. Cecum content was analyzed for yeast enumeration and Salmonella and Campylobacter frequency and enumeration, while neck and breast skin frequency and enumeration of Salmonella and Campylobacter were determined on one slaughtered chicken per pen. At the end of the trial, all the remaining chickens were slaughtered and analyzed as described for day 10 PI. Lactobacilli enumeration was performed accordingly with ISO 15214:1998, while Coliforms were enumerated on VRBA (Violet Red Bile Agar; 37 °C, 24 h). For both microorganisms serial 10-fold dilutions were obtained and plated into the different media.
6The enumeration of yeasts in feces and cecum was performed according to ISO 21527-1:2008 protocol with the same sample dilution rates as for Coliforms and Lactobacilli. Yeast growth media and 100 µl solution were then incubated for 5 days (25 °C). Salmonella frequency and enumeration were performed according to with the most probable number (MPN) method (ISO 6579-1,2:2012). Briefly, the samples were weighed and homogenized in nine parts of Buffered Peptone Water (BPW) and incubated at 37 °C (18 h). BPW cultures (100 µl) were then seeded on semisolid modified Rappaport-Vassiliadis Agar (MSRV) plates and incubated (41.5 °C, 48 h). The lapful of growth on a MRSV plate was streaked onto XLD (Xylose-Lysine-Deoxycholate Agar) and BGA (Brilliant Green Agar) plated and hence incubated at 37 °C overnight.
7Campylobacter frequency and enumeration were performed according to OIE (2008) using PBS solution (1:10 sample/solution rate) and blood agar plates with filter (porosity 45 µm). The plates were then incubated for 30 min (37 °C) and 44 h (41.5 °C, 10% CO2).
2.4. Statistical analysis
8The experimental data were split according to the day of challenge in two separate periods from 1 to 20 and from 21 to 38 days and analyzed applying a Mixed procedure of SAS package (SAS/STAT, Version V8, SAS Inst., Inc., NC, USA, 2006) in a randomized block design. The model included treatment as fixed effect and the replicate was considered the experimental unit.
3. Results
3.1 Growth performance
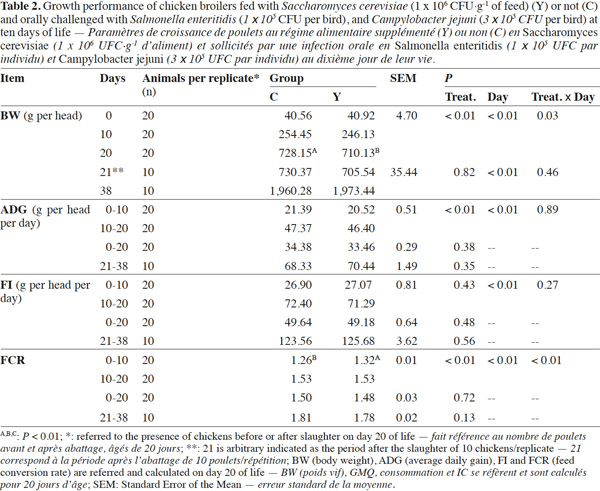
9Body weight did not differ at the beginning of the trial (40.56 g vs 40.92 g; p = 0.94) nor at the end (1960.28 g vs 1973.44 g; p = 0.71) between the two experimental groups, although a significant higher BW was found in C group than Y group on day 20 (728.15 g vs 710.15 g; p = < 0.01) (Table 2). As a result, C chickens showed a tendency to an increased ADG from 10 to 20 days of the trial (47.37 g·head-1·d-1 vs 46.40 g·head-1·d-1; p = 0.07), but no differences were detected on the whole-trial period (p = 0.35). In both 0-20 d or 21-38 d periods of the trial FI (p > 0.50) and FCR (p > 0.05) were not influenced by the dietary treatment.
3.2. Microbiological assays
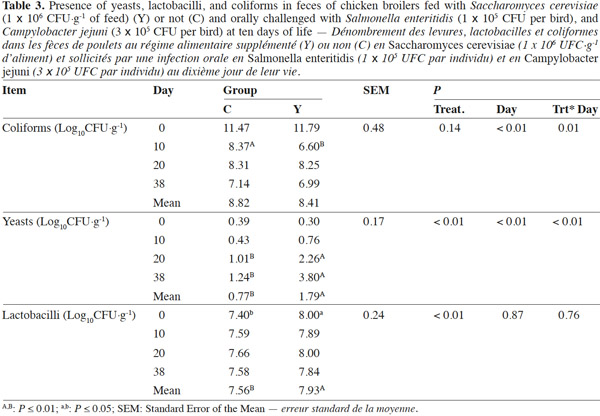
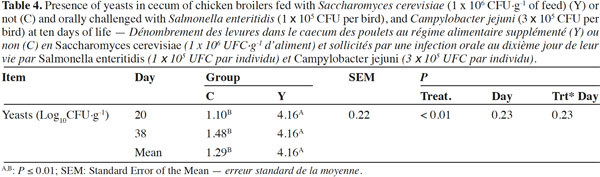
10Coliforms fecal content was not influenced by the administration of dietary yeast during the trial except for lower values in group Y than in group C on day 10 (6.60 log10CFU·g-1 vs 8.37 log10CFU·g-1; p < 0.01, respectively), while total yeasts content was improved in treated chickens on the overall the trial period starting from day 20 (p < 0.01) (Table 3). Mean Lactobacilli content in feces was higher in group Y than in group C (7.93 log10CFU·g-1 vs 7.56 log10CFU·g-1; p < 0.01) although a significant difference was detected at the beginning of the trial only. Yeast enumeration in cecum was found to be higher in group Y than in group C for both trial periods (4.16 log10CFU·g-1 vs 1.29 log10CFU·g-1; p = 0.01) (Table 4).
11No differences were detected in the frequency of Salmonella detection on day 20 of the trial between the two experimental groups for the different sampled anatomical districts, while Salmonella enumeration was lower in breast skin in Y group than C group (2.70 log10CFU·g-1 vs 3.22 log10CFU·g-1; p < 0.05) (Figure 1).
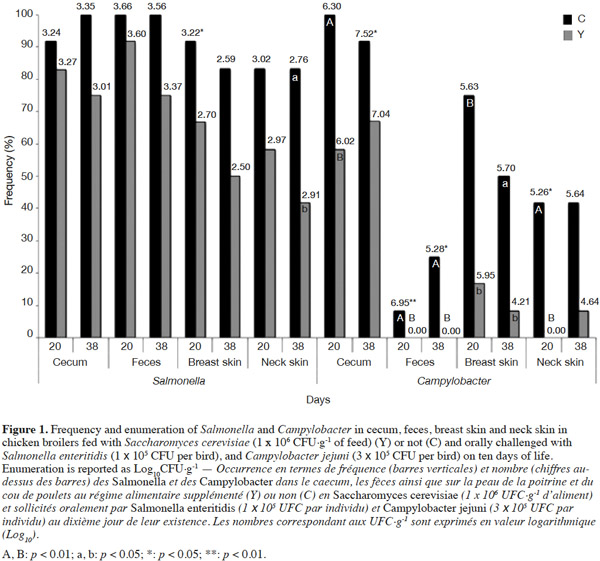
12A significant decrease in contamination frequency with Salmonella in yeast-fed chickens was found in neck skin (-41%; p = 0.03), and tended to decrease in feces (-25%; p = 0.06), cecum (-25%; p = 0.06), and breast skin (-33%; p = 0.08) at the end of the trial, but no differences were detected on Salmonella enumeration between the two experimental groups.
13At first slaughtering time, Campylobacter colonization was significantly lower in yeast-fed birds in cecum (-42%; p = 0.01), and breast skin (-58%; p = 0.04).
14The presence in feces (p = 0.01) and neck skin (p = 0.01) was not detected on day 10 PI in Y group as opposed to C birds that were Campylobacter positive, thus significant lower enumeration (p < 0.01; p = 0.05, respectively) was evidenced in both sampled districts.
15At the end of the trial, the presence of Campylobacter in feces (p < 0.01) was not detected in Y group contrary to C group, thus significant lower enumeration (p < 0.05) was found. Yeast-supplemented chickens evidenced also a reduction in breast skin Campylobacter frequency (-42%; p = 0.02) and a tendency to decreased values in cecum (-25%; p = 0.13) and neck skin (-33%; p = 0.06) (Figure 1). Campylobacter enumeration was lower in Y group than C in cecum (7.04 log10CFU·g-1 vs 7.52 log10CFU·g-1; p < 0.05).
4. Discussion
16The protection of the gut microflora is an important element in the health status of the host that ensures the local immunity, and its balance depends on the contact with environmental antigens, as competitive exclusion products, probiotics, or other immunostimulants that can contribute to immune stimulation and/or exclusion and prevention of pathogen colonization. The enhancement of colonization resistance and/or indirect inhibitory effects against pathogens are important factors where probiotics compounds could be effective in reducing the incidence and duration of diseases. Moreover a possible reduction of pathogen microorganisms that are responsible of foodborne diseases in humans, such as Salmonella and Campylobacter, is a cornerstone to increase food safety of poultry products. The competition in attachment sites and/or for nutrients between yeast and pathogen microorganisms are popular hypotheses to explain the action of probiotics (Patterson et al., 2003; Leser et al., 2008). Many authors proposed that competitive exclusion could be used as a method to prevent Salmonella infection; numerous researchers have reported the ability of probiotic organisms to also reduce colonization of opportunistic pathogens in the gastrointestinal tract (Stern et al., 2001; Mountzouris et al., 2007; Al-Zenki et al., 2009).
17In the present study the positive effects of yeast administration seems to be linked to with the microbial carriage reduction that could lead to a beneficial impact on food safety rather than increased performance.
18Our data show that dietary treatment did not have any significant effect on overall growth performance confirming findings of some other authors (Zhang et al., 2005; Mountzouris et al., 2007; Al-Zenki et al., 2009).
19On the contrary obtained positive results over fecal lactobacilli content and decreased pathogen frequency and enumeration in fecal samples confirm that dietary live yeast administration in poultry can manage with the microbial population of the gut being involved in the protection against a variety of pathogens including Escherichia coli (Chateau et al., 1993), Salmonella and Campylobacter (Line et al., 1998; Stern et al., 2001). Few studies have characterized microbial communities changes in poultry fed probiotic diets. In this study, we showed that the inclusion of S. cerevisiae increased the number of lactobacilli in the feces. Coliforms presence in feces, although not statistically significant, was found to be lower in yeast-fed group confirming the findings of Chateau et al. (1993). Many authors proposed that competitive exclusion could be used as a method to prevent Salmonella infection; numerous researchers have reported the ability of probiotic organisms to also reduce colonization of opportunistic pathogens in the gastrointestinal tract (Stern et al., 2001; Mountzouris et al., 2007; Al-Zenki et al., 2009). In our study yeast administration reduced the enumeration of Salmonella on day 20 in breast skin and the frequency of colonization in neck skin at the end of the trial, but the mean log numbers of Salmonella in feces, cecum, and neck skin were unaffected by the dietary treatment. The lack of wide decreased Salmonella enumeration differs from results reported in previous studies (Line et al., 1998; Al-Zenki et al., 2009) that revealed lower CFU·g-1 when using yeast as experimental treatment. However, Line et al. (1998) reported a similar reduction of this pathogen colonization in yeast-treated birds compared with the positive control. In our study, the reduction of Salmonella frequency, but not enumeration, could outline a positive effect of yeast inclusion in the diet not on severity of contamination, but on the reduction of the total number of animals affected. Campylobacter is one of the most common bacterial causes of foodborne illness, and a few studies have shown that probiotics may be able to reduce the amount of bacteria in chickens (Stern et al., 2001). In the present study the frequency of Campylobacter colonization was significantly reduced in cecum, feces, neck skin and breast skin, and the mean log numbers of Campylobacter were likewise reduced by yeast treatment confirming the positive effect of yeast inclusion in poultry diet over these pathogen bacteria (Willis et al., 2008).
5. Conclusion
20The results of this study showed that feeding live yeast to chickens challenged with pathogenic microorganisms like Salmonella and Campylobacter is able to reduce the frequency of these same pathogens in feces and on body surface. Controlling Campylobacter carriage and Salmonella contamination in chickens could lead to a reduced contamination of carcasses with both foodborne pathogens, resulting in safer foods for consumers.
Bibliographie
Agazzi A. et al., 2011. Evaluation of the effects of live yeast supplementation on apparent digestibility of high-fiber diet in mature horses using the acid insoluble ash marker modified method. J. Equine Vet. Sci., 31(1), 13-18.
Al-Zenki S.F. et al., 2009. Effects of using a chicken-origin competitive exclusion culture and probiotic cultures on reducing Salmonella in broilers. J. Appl. Poult. Res., 18, 23-29.
Awad W.A. et al., 2009. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci., 88, 49-56.
Chateau N. et al., 1993. Distribution of pathogen inhibition in the Lactobacillus isolates of commercial probiotic consortium. J. Appl. Bacteriol., 74, 36-40.
Dalloul R.A. et al., 2005. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comp. Immunol. Microbiol. Infect. Dis., 28, 351-361.
Delcenserie V. et al., 2008. Immunomodulatory effects of probiotics in the intestinal tract. Mol. Biol., 10, 37-54.
ISO (International Organization for Standardization), 1998. Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of mesophilic lactic acid bacteria. Colony-count technique at 30 °C. ISO 15214:1998. Geneva, Switzerland: ISO.
ISO (International Organization for Standardization), 2008. Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of yeasts and moulds. Part 1: Colony count technique in products with water activity greater than 0,95. ISO 21527-1:2008. Geneva, Switzerland: ISO.
ISO (International Organization for Standardization), 2012. Microbiology of food and animal feed stuffs. Horizontal method for the detection, enumeration, and serotyping of Salmonella. Part 1: detection method. ISO/TS 6579-2:2012. Geneva, Switzerland: ISO.
ISO (International Organization for Standardization), 2012. Microbiology of food and animal feed stuffs. Horizontal method for the detection, enumeration, and serotyping of Salmonella. Part 2: enumeration by a miniaturized most probable number technique. ISO/TS 6579-2:2012. Geneva, Switzerland: ISO.
Jin L.Z., 2000. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poult. Sci., 79, 886-891.
Johnson-Henry K.C. et al., 2008. Lactobacillus rhamnosus strain GG prevents enterohemorragic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect. Immun., 76, 1340-1348.
Kizerwetter-Swida M. et al., 2009. Protective effect of potentially probiotic Lactobacillus strain on infection with pathogenic bacteria in chickens. Pol. J. Vet. Sci., 12, 15-20.
Kumprecht I. et al., 1998. The effect of probiotic preparations containing Saccharomyces cerevisiae and Enterococcus faecium in diets with different levels of B-vitamins on chicken broiler performance. Czech J. Anim. Sci., 43, 63-70.
Leser T.D. et al., 2008. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J. Appl. Microbiol., 104, 1025-1033.
Line J.E. et al., 1998. Effect of yeast supplemented feed on Salmonella and Campylobacter populations in broilers. Poult. Sci., 77, 405-410.
Mountzouris K.C. et al., 2007. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci., 86, 309-317.
OIE, 2008. Campylobacter jejuni and Campylobacter coli diagnostic techniques. In: Manual of diagnostic tests and vaccine for terrestrial animals. Paris: OIE.
Patterson J.A. et al., 2003. Application of prebiotics and probiotics in poultry production. Poult. Sci., 82, 627-631.
Quarantelli A. et al., 2008. Effects of the administration of Pediococcus acidilactici to laying hens on productive performance. Vet. Res. Commun., 32(suppl.1), 359-361.
Ripamonti B. et al., 2009. Administration of Bacillus coagulans in calves: recovery from faecal samples and evaluation of functional aspects of spores. Vet. Res. Commun., 33, 991-1001.
Ripamonti B. et al., 2011. Screening of species-specific lactic acid bacteria for veal calves multi-strain probiotic adjuncts. Anaerobe, 17, 97-105.
Schneitz C., 2005. Competitive exclusion in poultry – 30 years of research. Food Control, 16, 657-667.
Stern N.J. et al., 2001. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter sp. colonization in broiler chickens. Poult. Sci., 80, 156-160.
Tellez G. et al., 2012. Probiotics/direct fed microbials for Salmonella control in poultry. Food Res. Int., 45, 628-633.
Willis W.L. et al., 2008. Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poult. Sci., 87, 606-611.
Yeo J. et al., 1997. Effect of feeding diets containing an antibiotic, a probiotic, or yucca extract on growth and intestinal urease activity in broiler chicks. Poult. Sci., 76, 381-385.
Yoon C. et al., 2004. Effect of feeding multiple probiotics on performance and fecal noxious gas emission in broiler chicks. Kor. J. Poult. Sci., 3, 229-235.
Zhang A. et al., 2005. Effect of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult. Sci., 84, 1015-1021.






