- Portada
- Volume 26 (2022)
- Numéro 2
- Impacts of copper on photosynthetic pigments and anatomy of Alcantarea imperialis (Bromeliaceae) under in vitro conditions
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Impacts of copper on photosynthetic pigments and anatomy of Alcantarea imperialis (Bromeliaceae) under in vitro conditions

Documento adjunto(s)
Version PDF originaleRésumé
Impacts du cuivre sur les pigments photosynthétiques et l'anatomie d'Alcantarea imperialis (Bromeliaceae) dans des conditions in vitro
Description du sujet. La connaissance de l'état morphophysiologique des plantes et de leurs réponses aux excès de métaux, tels que le cuivre (Cu), permet de déterminer leur utilisation potentielle en biosurveillance. Les techniques in vitro sont prometteuses dans les études impliquant la physiologie et l'anatomie, car elles peuvent isoler les effets des oligo-éléments sur les caractéristiques morphophysiologiques et d'autres facteurs de stress possibles.
Objectifs. L'objectif était de vérifier les changements morphophysiologiques et les ajustements d'Alcantarea imperialis induits par l'excès de Cu dans des conditions in vitro.
Méthode. Des plants d'A. imperialis ont été transférés dans des milieux de culture in vitro contenant un gradient de concentration de Cu (0, 25, 50, 100 ou 200 μM). Après 90 jours, la teneur en pigments photosynthétiques et en Cu a été mesurée de même que la croissance et les caractéristiques anatomiques.
Résultats. Les plantes cultivées avec des concentrations de Cu supérieures à 50 µM de Cu présentaient des signes évidents de toxicité, tels que la chlorose. Les plantes exposées à 200 µM de Cu ont montré un niveau de toxicité sévère et étaient mortes après 90 jours. Une réduction drastique de tous les pigments photosynthétiques ainsi que leurs ratios ont été vérifiés avec une exposition à 100 µM Cu. Les plantes exposées à ce niveau de cuivre présentaient la plus petite surface de stomates, le plus petit nombre d’éléments du xylème ainsi que les éléments vasculaires les plus minces. La teneur en Cu des plantes augmente linéairement en fonction des concentrations en Cu dans le milieu. Les plantes d'A. imperialis peuvent bioaccumuler de grandes quantités de Cu.
Conclusions. Les plantes d'A. imperialis ont un bon potentiel de biosurveillance dans les zones urbaines en raison de leur forte capacité de bioaccumulation de Cu et de changements morphophysiologiques clairs. Cependant, ils ont une faible tolérance à des niveaux de Cu très élevés.
Abstract
Description of the subject. Knowledge of morphophysiological status of plants and their responses to excess metals, such as copper (Cu), allows determining their potential use as bio-indicators. In vitro techniques are promising in studies that involve physiology and anatomy because they can isolate the effects of trace elements on morphophysiological features from other possible stress factors.
Objectives. The aim was to verify the morphophysiological changes and adjustments of Alcantarea imperialis induced by excess Cu under in vitro conditions.
Method. Alcantarea imperialis plants were transferred to in vitro culture media containing a concentration gradient of Cu (0, 25, 50, 100, or 200 μM). After 90 days, the contents of photosynthetic pigments and Cu were analyzed along with growth and anatomical features.
Results. Plants cultured with Cu concentrations higher than 50 µM Cu had clear signs of toxicity, such as chlorosis. Plants exposed to 200 µM Cu showed a severe level of toxicity and were dead after 90 days. A drastic reduction of all photosynthetic pigments, as well as their ratios, was verified with exposure to 100 µM Cu. Plants exposed to that copper level presented the smallest stomata area, the lowest xylem number, as well as the thinnest vessel elements. The Cu content in the plants increased linearly as a function of Cu concentrations in the medium. Alcantarea imperialis plants can bioaccumulate high amounts of Cu.
Conclusions. Alcantarea imperialis plants have good potential for bio-indication in urban areas due to their high Cu bioaccumulation capacity and clear morphophysiological changes. However, they have a low tolerance to very high Cu levels.
Tabla de contenidos
Received 19 July 2021, accepted 23 February 2022, available online 17 March 2022
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1The accumulation of trace elements in the soil is an environmental concern nowadays. Road dust contains pollutants, such as trace elements, which can cause public health problems and damage to natural ecosystems (Trujillo-González et al., 2016). The presence and accumulation of trace elements in road dust are mainly due to brake lining and tire wear (Adamiec et al., 2016). Brake dust can contain significant amounts of trace elements, such as copper (Cu), zinc (Zn), nickel (Ni), chrome (Cr), and lead (Pb) (Hjortenkrans et al., 2007).
2In plants, a number of trace elements are essential micronutrients to assure normal growth by taking part in important metabolic processes, such as Cu, Zn and, Ni (Morkunas et al., 2018). Copper acts as a cofactor of numerous metalloproteins and is associated with several biochemical and physiological processes. However, at high levels, Cu can affect the morphophysiological features, in turn affecting water loss via leaves. In addition, excess Cu can induce oxidative stress in plants via enhanced production of reactive oxygen species (ROS) (Rucińska-Sobkowiak, 2016; Shabbir et al., 2020). Thus, knowledge of the specific morphological, biochemical and physiological status of plants of particular species and their responses to excess metals allows determining their potential use as bio-indicators (Rai, 2016).
3Monitoring the level of pollutants by living organisms (bio-indicators) can measure and identify trace elements that are of potential health risks (Severoglu et al., 2015). This technique allows continuous observation, enabling integrated response with a high spatial and temporal resolution. Biomonitoring and bio-indication carried out with plants is a simple, cost‐effective, and reliable method compared with conventional physicochemical techniques (Izquierdo‐Díaz et al., 2019). Furthermore, the accumulated trace element in the aerial part of plants can provide information about the bioavailable fraction of an element in the environment, which can also indicate the degradation level (Bonanno et al., 2017).
4Plants grown in urban gardens can be used as bio-indicators of atmospheric pollution (Izquierdo‐Díaz et al., 2019). The use of ornamental plants, as well as other plant species, as bio-indicators of trace elements has already been reported (Sorrentino et al., 2017; Khan et al., 2019), including bromeliads (Giampaoli et al., 2016; Martins et al., 2016; Martins et al., 2021). Among the ornamental bromeliads is Alcantarea imperialis (Carrière) Harms, a rupicolous and endemic species with showy inflorescence widely used in landscaping and gardening (Andrade-Santos et al., 2021).
5Several studies have been performed under in vitro conditions. This is because in vitro techniques can isolate the effects of trace elements on morphophysiological features from other possible stress factors (Martins et al., 2016; Rodrigues et al., 2017; Martins et al., 2021). Physiological analyses, such as chlorophyll content, can assess the influence of different pollutants on plant metabolism since they are highly sensitive to changes in the environment (Jaskulak et al., 2018; Oladele et al., 2019). Nevertheless, more important than quantifying the content of photosynthetic pigments is analyzing their ratios (Gitelson, 2020; Zhang et al., 2020; Martins et al., 2021). Changes in anatomical traits are also frequently used for monitoring the influence of trace elements (Martins et al., 2016; Vezza et al., 2018; Martins et al., 2020b). These changes in morphophysiology may be fundamental for the adjustment of plants to trace element stress.
6The aim of this study was to verify the morphophysiological changes and adjustments of A. imperialis plants induced by excess Cu under in vitro conditions. Moreover, we also investigated the species’ potential as a bio-indicator of Cu.
2. Materials and methods
2.1. Plant material, culture conditions, and copper exposure
7Alcantarea imperialis plants, previously established in vitro, were multiplied in a stationary liquid MS culture medium (Murashige & Skoog, 1962) with no growth regulator (Martins et al., 2020a). After 90 days, the obtained side shoots with approximately 3 cm length were individualized with the aid of a scalpel and transferred to 280 ml glass containers holding 25 ml of Cu-free (with no added Cu) MS medium solidified with 5 g·l-1 agar, supplemented with 30 g·l-1 sucrose and 0, 25, 50, 100 or 200 µM Cu (CuSO4·5H2O). The experiment was performed with 12 glass containers, and each container received five side shoots. Before the procedures of plant material inoculation in the culture medium, the pH of all media was adjusted to 5.8 before autoclaving at 120 ºC for 20 min. After inoculation in a laminar flow cabinet, the plant material was kept in a growth room for 90 days at 25 ± 2 °C and 16:8 h light:dark photoperiod, under fluorescent tube lamps (Empalux FT8 HO, 36W/6400K, Empalux, Paraná, Brazil), which provided 90 μmol·m-2·s-1 of photosynthetically active radiation (PAR).
2.2. Growth traits
8To infer the growth of plants, 40 plants from each treatment were collected randomly, mixed and divided into eight samples, and weighed on a precision scale. The fresh weights (FW) of the shoots (aerial part) and roots (n = 8) were determined separately (milligrams per plant – mg FW·plant-1).
2.3. Photosynthetic pigments content
9The photosynthetic pigments were quantified using 0.04 g of plant material. The analysis was conducted according to Arnon (1949). The preparation and pigment extraction procedures were according to Martins et al. (2019). The absorbances were read at λ = 480, 665 and, 645 nm for carotenoids (Car), chlorophyll a (Chl a) and, chlorophyll b (Chl b), respectively, using a Genesys™ 10S UV–Vis spectrophotometer (Thermo Fisher Scientific, West Palm Beach, FL, USA). The photosynthetic pigment contents were expressed in μg·g-1 FW of leaf tissue (n = 8).
2.4. Anatomical analysis
10To characterize the anatomical changes of the leaves grown under the imposed conditions, four A. imperialis plants from each treatment were used. All the samples were randomly collected after growth for 90 days and fixed/stored in 50% ethanol. The anatomical characterization was performed by examination of paradermal and cross-sections of leaves and all procedures were performed according to Martins et al. (2019). All the sections were viewed using a light microscope (Bioval, L-2000AFluor), and images were captured with a Leica EC3 camera (Wetzlar, Germany). The software UTHSCSA-Imagetool® was used to measure the anatomical characteristics shown in the photomicrographs. Two cross-sections per slide were photographed and analyzed per sample. The density of stomata (mm-2) and trichomes (mm-2), stomatal area (mm2), thickness of the chlorenchyma (μm), and hydrenchyma (μm) (abaxial and adaxial sides), as well as the number and diameter of vessel elements, were determined in leaves (n = 4).
11The characterization of roots was also performed to visualize the lignin and/or suberin distribution in the tissues (Brundrett et al., 1988). The cross-sections were first stained with a 0.1% berberine hemisulfate solution (w/v) for 1 h, followed by 0.5% aniline blue (w/v) for 30 min. Then, 50% glycerin (v/v) containing 0.1% (w/v) FeC13 was used to assemble the slides. The sections were observed using a fluorescence microscope (Bioval, L-2000A-Fluor) and images were captured with a Leica EC3 camera, using UV light with an excitation/emission spectrum of 358/461 nm.
2.5. Analysis of Cu content and bioaccumulation factor
12The Cu content in A. imperialis plants was analyzed after 90 days of growth. Only the aerial part was used (previously washed in distilled water and dried by forced circulation at 70 °C). The dried plant material was ground in a Wiley mill with 20 mesh sieve in order to obtain small particles. Then, the plant material was digested in nitro-perchloric solution 2:1 (v/v) to determine Cu content. This content was determined by atomic absorption spectroscopy (AA-7000, Shimadzu, Tokyo, Japan). The analytic procedures were according to Malavolta et al. (1997) and performed with three repetitions per treatment (n = 3). In addition, the bioaccumulation factor (BF) of Cu was also verified according to the element’s concentration in the leaves in relation to its concentration in the culture medium. The BF analysis (n = 3) was performed as described by Martins et al. (2020b), and this protocol was adapted from the methods described in Fan et al. (2011).
2.6. Statistical analysis
13The experimental design was completely randomized and the resulting data were submitted to analysis of variance (ANOVA), and the averages were compared using the Tukey test at 5% significance. All analyses were performed using the SISVAR software.
3. Results
3.1. Biomass accumulation
14Under the imposed conditions, the plants cultured with higher Cu concentrations than 50 µM Cu showed clear signs of toxicity, such as chlorosis. In addition, all the plants cultivated in the medium supplemented with 200 µM Cu showed a severe level of toxicity and were dead after 90 days. For this reason, no anatomical and physiological analyses were performed on the plant material of this treatment.
15Plants grown in the medium with 100 µM Cu were shorter and had the lowest fresh weight. Likewise, those plants also had a reduced number and length of roots, which negatively influenced the biomass accumulation (Figure 1).
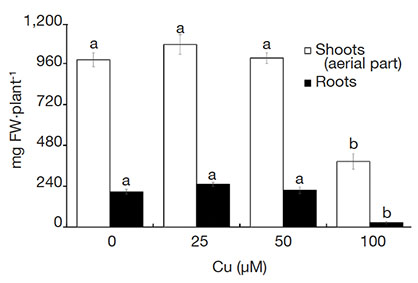 Figure 1. Fresh weight (FW) of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Poids frais de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
Figure 1. Fresh weight (FW) of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Poids frais de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
Means (± SE) followed by the same letter do not differ significantly by the Tukey test at 5% probability — Les moyennes (± ES) suivies de la même lettre ne diffèrent pas significativement par le test de Tukey avec une probabilité de 5 %.
3.2. Content of photosynthetic pigments
16A drastic reduction of all photosynthetic pigments was verified in plants grown with 100 µM Cu (Figure 2A). Similarly, the ratios of pigment contents also declined when plants were exposed to 100 µM Cu, except for the significant increase of Car/(Chl a + Chl b) in plants cultured with high Cu concentrations (Figure 2B).
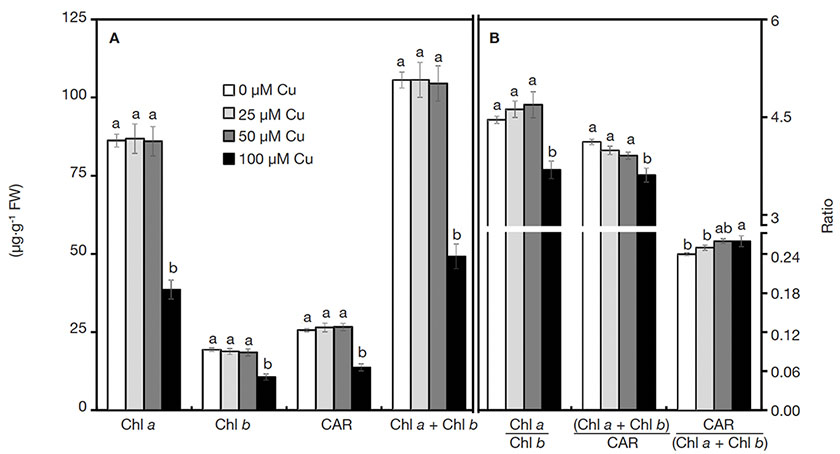 Figure 2. Contents of photosynthetic pigments of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Teneur en pigments photosynthétiques de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
Figure 2. Contents of photosynthetic pigments of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Teneur en pigments photosynthétiques de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
a, b: see figure 1 — voir figure 1.
3.3. Anatomic characterization
17On the abaxial epidermis surface, plants presented similar density of trichomes and stomata in all treatments. However, the stoma size was influenced by the Cu concentrations. Plants exposed to 100 µM Cu presented the smallest stoma size (Figure 3A-D and Figure 4).
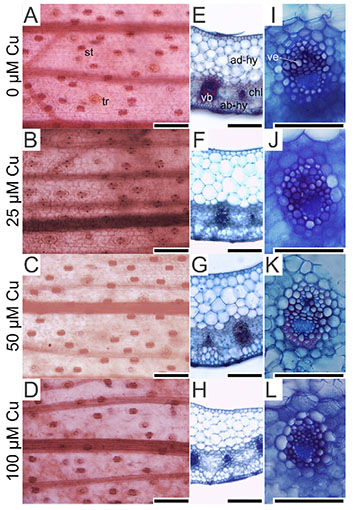 Figure 3. Paradermal- and cross-sections of Alcantarea imperialis leaves at 90 days of growth in a medium containing 0, 25, 50 or 100 μM Cu during in vitro culture — Coupes paradermiques et transversales de feuilles d’Alcantarea imperialis à 90 jours de croissance dans un milieu contenant 0, 25, 50 ou 100 μM Cu lors d’une culture in vitro.
Figure 3. Paradermal- and cross-sections of Alcantarea imperialis leaves at 90 days of growth in a medium containing 0, 25, 50 or 100 μM Cu during in vitro culture — Coupes paradermiques et transversales de feuilles d’Alcantarea imperialis à 90 jours de croissance dans un milieu contenant 0, 25, 50 ou 100 μM Cu lors d’une culture in vitro.
ab-hy: abaxial hydrenchyma — hydrenchyme abaxial; ad-hy: adaxial hydrenchyma — hydrenchyme adaxial; ch: chlorenchyma — chlorenchyme; st: stoma — stomie; tr: trichome — trichome; vb: vascular bundle — faisceau vasculaire; ve: vessel element — élément vasculaire; bars — barres: 100 μm.
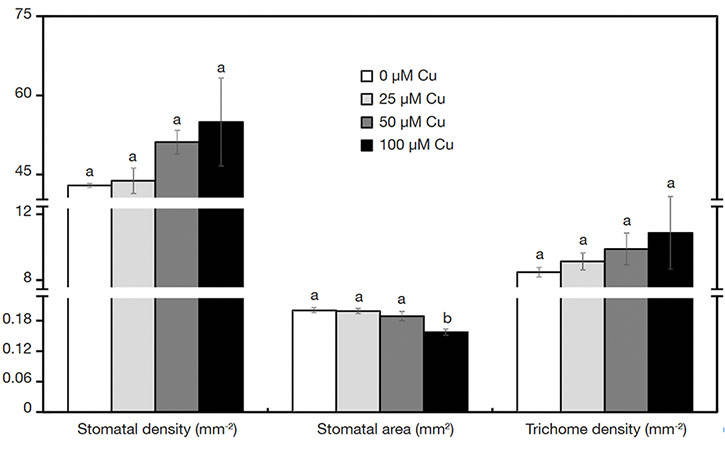 Figure 4. Characteristics of stomata and trichomes of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Caractéristiques des stomates et trichomes de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
Figure 4. Characteristics of stomata and trichomes of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Caractéristiques des stomates et trichomes de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
a, b: see figure 1 — voir figure 1.
18In the cross-sections of A. imperialis’s leaves, differences in the anatomical traits were verified. The water storage tissues (abaxial and adaxial hydrenchyma) showed increased thickness in the function of Cu concentrations. However, the thickness of those tissues decreased sharply at 100 μM Cu. The Cu concentrations also influenced the xylem traits. The plants exposed to 100 μM Cu presented the lowest xylem number as well as thinnest vessel elements (Figures 3I-L and 5).
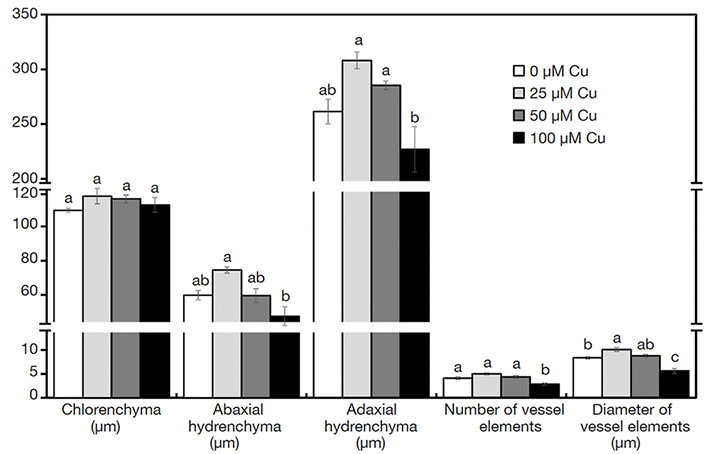 Figure 5. Anatomical structures of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Structures anatomiques de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
Figure 5. Anatomical structures of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Structures anatomiques de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
a, b: see figure 1 — voir figure 1.
19The cross-sections of roots stained by berberine hemisulfate and aniline blue solutions had different levels of emitted fluorescence in the cell walls. In the absence of Cu, the exodermis cell walls did not show intense fluorescence (Figure 6A). In contrast, plants exposed to Cu presented intense fluorescence of the exodermis cell walls (Figure 6B-D). All treatments showed intense fluorescence of endodermis cell walls (Figure 6).
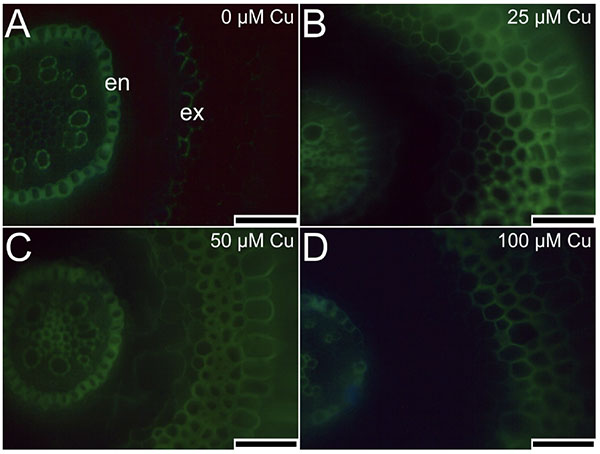 Figure 6. Cross-sections of roots (stained by berberine hemisulfate and aniline blue solutions) of Alcantarea imperialis plants at 90 days of growth in a medium containing 0, 25, 50, or 100 μM Cu during in vitro culture — Coupes transversales de racines (colorées par des solutions d’hémisulfate de berbérine et de bleu d’aniline) de plantes d’Alcantarea imperialis à 90 jours de croissance dans un milieu contenant 0, 25, 50 ou 100 µM de Cu pendant la culture in vitro.
Figure 6. Cross-sections of roots (stained by berberine hemisulfate and aniline blue solutions) of Alcantarea imperialis plants at 90 days of growth in a medium containing 0, 25, 50, or 100 μM Cu during in vitro culture — Coupes transversales de racines (colorées par des solutions d’hémisulfate de berbérine et de bleu d’aniline) de plantes d’Alcantarea imperialis à 90 jours de croissance dans un milieu contenant 0, 25, 50 ou 100 µM de Cu pendant la culture in vitro.
en: endodermis — endoderme; ex: exodermis — exoderme; bars — barres: 100 μm.
3.4. Cu content
20The treatments influenced the content of Cu in the aerial part of plants. The content of Cu increased linearly (R2 = 0.83) as a function of Cu concentrations in the medium (Figure 7).
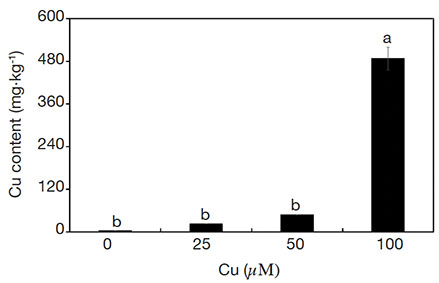 Figure 7. Cu contents of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Teneurs en Cu de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
Figure 7. Cu contents of Alcantarea imperialis plants grown in vitro as a function of concentrations of Cu (µM) in the medium — Teneurs en Cu de plantes d’Alcantarea imperialis cultivées in vitro en fonction des concentrations de Cu (µM) dans le milieu.
a, b: see figure 1 — voir figure 1.
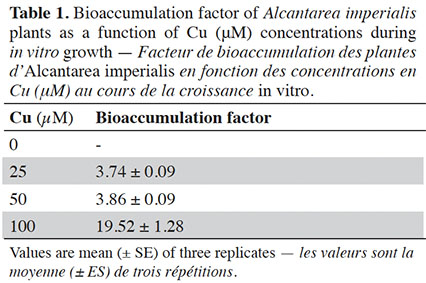
21The bioaccumulation factor (BF) presented values equal to or higher than 3.74 when plants were treated with Cu. The highest BF values were observed in plants exposed to 100 µM (Table 1).
4. Discussion
22In the present work, A. imperialis plants presented clear signs of stress and sensitivity to excess Cu. Under Cu-free or intermediate concentrations (25 and 50 μM Cu), no visual symptoms indicating toxicity were observed, while chlorotic symptoms and reduced biomass accumulation were perceived at 100 μM Cu. The toxicity of high Cu concentrations was evidenced by the senescence of plants cultured with 200 μM Cu.
23The first sign of morphophysiological disturbances of A. imperialis plants caused by Cu consisted of differences in growth and biomass accumulation. A noticeable reduction in shoot and/or root growth under trace element stress is directly related to physiological changes in plants, as already verified by Martins et al. (2016), Gong et al. (2019), and Zaouali et al. (2020). Exposure to Cu at high concentrations can cause oxidative stress in plants, which in turn induces extensive damage to membranes and macromolecules. In addition, those effects can be perceived by different symptoms, such as chlorosis and stunted growth (Ghori et al., 2019).
24The physiological alterations were verified by the photosynthetic pigment contents of plants. The content of photosynthetic pigments can provide a rapid method to detect and quantify damage to leaves’ photosynthetic apparatus due to stress conditions in toxicity studies (Jaskulak et al., 2018; Oladele et al., 2019). In this work, a sharp decline in the pigment contents was observed, possibly associated with alterations of the antioxidant system or by Chl biosynthesis inhibition due to excess Cu (Mao et al., 2018; Oladele et al., 2019; Hossain et al., 2020). A reduction of the total content of Chl followed by alterations in pigment ratio can indicate disturbances in the photosynthetic apparatus of plants. A coordinated decrease of Chl a and Chl b (without any changes in the Chl a/b ratio) indicates that physiological adjustments were effective against the deleterious effects of excess trace elements, such as Cu (Martins et al., 2021). In contrast, changes in the Chl a/b ratio denote physiological disturbances. Plants cultured with 100 μM Cu presented a diminished Chl a/b ratio, indicating larger degradation of Chl a compared to Chl b (Ranjbarfordoei et al., 2006). This outcome can impair the photosystem II performance (Martins et al., 2020b).
25Still concerning the pigments, the Car content declined when plants were exposed to high Cu levels. Besides the direct role in photosynthesis, Car also acts as a low-molecular antioxidant, whose biosynthesis in plants can increase in response to stress to reduce ROS production (Taran et al., 2017). Therefore, a decrease in Car content in A. imperialis plants indicated low tolerance of excess Cu and suggested that flaws in the physiological mechanisms that control oxidative stress may have occurred. The total Chl-to-Car ratio in plants is a sensitive indicator of photosynthetic activity and stress responses (Gitelson, 2020). In this work, plants cultured with 100 µM Cu showed decreased (Chl a + Chl b)/Car, a result of faster degradation of total Chl relative to Car and reduced photosynthetic activity (Gamon et al., 2016). In addition, the increased Car/(Chl a + Chl b) ratio verified in the same conditions can indicate disturbances, which is related to protection from photooxidation by increasing heat dissipation (Zhang et al., 2020; Huang et al., 2021). Thus, both ratios demonstrated a severe decline in the eco-physiological performance of the photosynthetic apparatus and disorders in the photoprotective mechanisms that occurred under excess Cu.
26The alterations of anatomical traits induced by the treatments suggested modifications in the water status of plants. Even though the stomata and trichome density were similar in all treatments, the reduction of stomatal area observed in plants exposed to 100 μM Cu suggests lower stomatal conductance. This anatomical adjustment is also known to be a response to water deficit (Oliveira et al., 2019; Lobato et al., 2021). Smaller stomatal guard cells can confer a greater stomatal functioning due to their faster response (open/close) than larger ones (Rucińska-Sobkowiak, 2016; Rouphael et al., 2017; Lawson & Vialet‐Chabrand, 2019). Stomatal conductance is associated with controlling the water flux in plants (Tardieu et al., 2017). In metal-stressed plants, the water content status is regulated by anatomical traits such as stomata density and/or size (Rucińska-Sobkowiak, 2016). In A. imperialis plants cultured with excess Cu, the water uptake and delivery from the roots to shoots may have been reduced as an effect of lower stomatal conductance and greater stomatal functionality, among other anatomical changes (e.g., traits of vessel elements).
27The transversal sections of leaves evidenced the changes in the water content status. A reduction of the xylem traits (diameter and/or number of vessel elements) can indicate lower hydraulic conductance (Rucińska-Sobkowiak, 2016; Martins et al., 2019). The lower number and thinner diameter of vessel elements of leaves grown under high Cu concentrations can impair the water flow in the whole plant. Changes in the water flow dynamics can be considered an adaptive strategy to adjust the uptake and translocation of metals, but this can affect the water supply and consequently the water content, which jeopardizes plants’ survival (Vezza et al., 2018). The diminished water content was confirmed by the reductions in the water-storage tissues. Environmental conditions like water deficit or excess trace element exposure can modulate the formation and thickness of bromeliads’ hydrenchyma (Martins et al., 2016; Vieira et al., 2017; Mollo et al., 2019). In our study, the number of cell layers was similar in all treatments (adaxial side = 4-5 cell layers and abaxial side = 2-3 cell layers), so the thinner hydrenchyma tissue was due mainly to a reduction in the size of its cells. This can be linked to alterations in the water flow during tissue formation, which may have modified the turgor that is essential for cell expansion.
28The cross-sections of roots also showed the influence of Cu concentrations. The emitted fluorescence of the endodermis denoted higher deposition of lignin and/or suberin in cell walls in all treatments. Likewise, the roots that grew in the medium supplemented with Cu (25, 50, and 100 μM) also presented higher fluorescence emission of the exodermis. In addition, the exodermis cell walls of roots grown in the medium supplemented with Cu (25, 50, and 100 μM) were thicker. Under stress conditions (e.g., salt or trace element), plants’roots can present endodermis and exodermis with thicker cell walls and impregnated with lignin and/or suberin (Martins et al., 2016; Benáková et al., 2017; Byrt et al., 2018). These ultra-structures present in the roots act as apoplastic barriers and are responsible for protecting vascular tissues against abiotic stresses (Líška et al., 2016). Lignin and suberin biosynthesis can be used by plants as a strategy to control the free access and absorption of Cu (Martins et al., 2016; Kováč et al., 2018).
29Although the exposure to Cu induced stress in the A. imperialis plants, this species also showed high bioaccumulation capacity. Khan et al. (2020) mentioned that BF values higher than 1 indicate effective Cu storage of plants. However, this high capacity for transport and bioaccumulation of Cu can be interpreted as a low ability to control the Cu uptake under high levels. This probably led to a breakdown of the physiological mechanisms, which failed to deal with the toxic effects of Cu, especially at the beginning of the exposure.
5. Conclusions
30The excess Cu induced changes in the morphophysiological features of A. imperialis plants. The alterations in the photosynthetic pigment ratios demonstrated a decline in the eco-physiological performance of the photosynthetic apparatus and physiological disorders under excess Cu. The anatomical adjustment showed that treatments influenced the water status of plants. Plants of this species can bioaccumulate high amounts of Cu. Alcantarea imperialis plants presented a potential for bio-indication in urban areas due to their high Cu bioaccumulation capacity and clear morphophysiological changes. However, they have a low tolerance to very high Cu levels.
Acknowledgements
31The authors would like to acknowledge the scholarship awarded by the CNPq (Brazilian National Council for Scientific and Technological Development) and the FAPES (Espírito Santo State Research Foundation).
Bibliographie
Adamiec E., Jarosz-Krzemińska E. & Wieszała R., 2016. Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ. Monit. Assess., 188, 1-11, doi.org/10.1007/s10661-016-5377-1
Andrade-Santos S.V. et al., 2021. Effect of nitrate accumulation in the ornamental bromeliad Alcantarea imperialis. J. Plant Nutr., 44, 1-15, doi.org/10.1080/01904167.2020.1806300
Arnon D.I., 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol., 24, 1-15, doi.org/10.1104/pp.24.1.1
Benáková M. et al., 2017. Effects of Cd and Zn on physiological and anatomical properties of hydroponically grown Brassica napus plants. Environ. Sci. Pollut. Res., 24, 20705-20716, doi.org/10.1007/s11356-017-9697-7
Bonanno G., Borg J.Á. & Martino V., 2017. Levels of heavy metals in wetland and marine vascular plants and their biomonitoring potential: a comparative assessment. Sci. Total Environ., 576, 796-806, doi.org/10.1016/j.scitotenv.2016.10.171
Brundrett M.C., Enstone D.E. & Peterson C.A., 1988. A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma, 146, 133-142, doi.org/10.1007/BF01405922
Byrt C.S. et al., 2018. Root cell wall solutions for crop plants in saline soils. Plant Sci., 269, 47-55, doi.org/10.1016/j.plantsci.2017.12.012
Fan K.C. et al., 2011. Cadmium accumulation and tolerance of mahogany (Swietenia macrophylla) seedlings for phytoextraction applications. J. Environ. Manage., 92, 2818-2822, doi.org/10.1016/j.jenvman.2011.06.032
Gamon J.A. et al., 2016. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. PNAS, 113, 13087-13092, doi.org/10.1073/pnas.1606162113
Ghori N.H. et al., 2019. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol., 16, 1807-1828, doi.org/10.1007/s13762-019-02215-8
Giampaoli P., Wannaz E.D., Tavares A.R. & Domingos M., 2016. Suitability of Tillandsia usneoides and Aechmea fasciata for biomonitoring toxic elements under tropical seasonal climate. Chemosphere, 149, 14-23, doi.org/10.1016/j.chemosphere.2016.01.080
Gitelson A., 2020. Towards a generic approach to remote non-invasive estimation of foliar carotenoid-to-chlorophyll ratio. J. Plant Physiol., 252, 153227, doi.org/10.1016/j.jplph.2020.153227
Gong Q. et al., 2019. Effects of copper on the growth, antioxidant enzymes and photosynthesis of spinach seedlings. Ecotoxicol. Environ. Saf., 171, 771-780, doi.org/10.1016/j.ecoenv.2019.01.016
Hjortenkrans D.S.T., Bergbäck B.G. & Häggerud A.V., 2007. Metal emissions from brake linings and tires: case studies of Stockholm, Sweden 1995/1998 and 2005. Environ. Sci. Technol., 41, 5224-5230, doi.org/10.1021/es070198o
Hossain M.S. et al., 2020. Insights into acetate-mediated copper homeostasis and antioxidant defense in lentil under excessive copper stress. Environ. Pollut., 258, 113544, doi.org/10.1016/j.envpol.2019.113544
Huang W.T. et al., 2021. Growth, mineral nutrients, photosynthesis and related physiological parameters of Citrus in response to nitrogen deficiency. Agronomy, 11, 1859, doi.org/10.3390/agronomy11091859
Izquierdo‐Díaz M. et al., 2019. Urban allotment gardens for the biomonitoring of atmospheric trace element pollution. J. Environ. Qual., 48, 518-525, doi.org/10.2134/jeq2018.06.0232
Jaskulak M., Rorat A., Grobelak A. & Kacprzak M., 2018. Antioxidative enzymes and expression of rbcL gene as tools to monitor heavy metal-related stress in plants. J. Environ. Manage., 218, 71-78, doi.org/10.1016/j.jenvman.2018.04.052
Khan A.H.A. et al., 2019. Combined application of selected heavy metals and EDTA reduced the growth of Petunia hybrida L. Sci. Rep., 9, 1-12, doi.org/10.1038/s41598-019-40540-7
Khan Z.I. et al., 2020. Copper bioaccumulation and translocation in forages grown in soil irrigated with sewage water. Pak. J. Bot., 52, 111-119, doi.org/10.30848/PJB2020-1(12)
Kováč J., Lux A. & Vaculík M., 2018. Formation of a subero-lignified apical deposit in root tip of radish (Raphanus sativus) as a response to copper stress. Ann. Bot., 122, 823-831, doi.org/10.1093/aob/mcy013
Lawson T. & Vialet‐Chabrand S., 2019. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol., 221, 93-98, doi.org/10.1111/nph.15330
Líška D., Martinka M., Kohanová J. & Lux A., 2016. Asymmetrical development of root endodermis and exodermis in reaction to abiotic stresses. Ann. Bot., 118, 667-674, doi.org/10.1093/aob/mcw047
Lobato S.M.S. et al., 2021. Protective mechanism triggered by Pigeonpea plants exposed to water deficit: modifications linked to paraheliotropism, stomatal characteristics and antioxidant enzymes. J. Plant Growth Regul., 40, 20-36, doi.org/10.1007/s00344-020-10077-5
Malavolta E., Vitti G. & Oliveira A.S., 1997. Avaliação do estado nutricional das plantas: princípios e aplicações. Piracicaba, Brazil: Potafos.
Mao F. et al., 2018. The metal distribution and the change of physiological and biochemical process in soybean and mung bean plants under heavy metal stress. Int. J. Phytorem., 20, 1113-1120, doi.org/10.1080/15226514.2017.1365346
Martins J.P.R. et al., 2016. Anatomical and physiological responses of Billbergia zebrina (Bromeliaceae) to copper excess in a controlled microenvironment. Plant Cell Tissue Organ Cult., 126, 43-57, doi.org/10.1007/s11240-016-0975-8
Martins J.P.R. et al., 2019. Sources and concentrations of silicon modulate the physiological and anatomical responses of Aechmea blanchetiana (Bromeliaceae) during in vitro culture. Plant Cell Tissue Organ Cult., 137, 397-410, doi.org/10.1007/s11240-019-01579-6
Martins J.P.R. et al., 2020a. Modulation of the anatomical and physiological responses of in vitro grown Alcantarea imperialis induced by NAA and residual effects of BAP. Ornam. Hortic., 26, 283-297, doi.org/10.1590/2447-536X.v26i2.2138
Martins J.P.R. et al., 2020b. Morphophysiological responses, bioaccumulation and tolerance of Alternanthera tenella Colla (Amaranthaceae) to excess copper under in vitro conditions. Plant Cell Tissue Organ Cult., 143, 303-318, doi.org/10.1007/s11240-020-01917-z
Martins J.P.R. et al., 2021. Morphophysiological responses of Aechmea blanchetiana (Bromeliaceae) to excess copper during in vitro culture. Plant Biosyst., 155, 447-456, doi.org/10.1080/11263504.2020.1756976
Mollo L. et al., 2019. Drought survival strategies of juvenile bromeliads of Alcantarea imperialis (Carrière) Harms. Plant Cell Tissue Organ Cult., 139, 295-304, doi.org/10.1007/s11240-019-01682-8
Morkunas I. et al., 2018. The role of heavy metals in plant response to biotic stress. Molecules, 23, 2320, doi.org/10.3390/molecules23092320
Murashige T. & Skoog F., 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant., 15, 473-497, doi.org/10.1111/j.1399-3054.1962.tb08052.x
Oladele E.O., Adewumi O.O., Yahaya T. & Taiwo I.A., 2019. Response of bambara groundnut (Vigna subterranean L.) and maize (Zea mays L.) to heavy metal stress. Beni-Suef Univ. J. Basic Appl. Sci., 8, 19, doi.org/10.1186/s43088-019-0024-x
Oliveira V.P. et al., 2019. Brassinosteroids confer tolerance to salt stress in Eucalyptus urophylla plants enhancing homeostasis, antioxidant metabolism and leaf anatomy. J. Plant Growth Regul., 38, 557-573, doi.org/10.1007/s00344-018-9870-3
Rai P.K., 2016. Impacts of particulate matter pollution on plants: implications for environmental biomonitoring. Ecotoxicol. Environ. Saf., 129, 120-136, doi.org/10.1016/j.ecoenv.2016.03.012
Ranjbarfordoei A., Samson R. & Van-Damme P., 2006. Chlorophyll fluorescence performance of sweet almond [Prunus dulcis (Miller) D. Webb] in response to salinity stress induced by NaCl. Photosynthetica, 44, 513-522.
Rodrigues L.C.A. et al., 2017. Tolerance and potential for bioaccumulation of Alternanthera tenella Colla to cadmium under in vitro conditions. Plant Cell Tissue Organ Cult., 130, 1-13, doi.org/10.1007/s11240-017-1241-4
Rouphael Y. et al., 2017. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol., 29, 459-470, doi.org/10.1007/s10811-016-0937-x
Rucińska-Sobkowiak R., 2016. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant., 38, 257, doi.org/10.1007/s11738-016-2277-5
Severoglu Z. et al., 2015. The usability of Juniperus virginiana L. as a biomonitor of heavy metal pollution in Bishkek City, Kyrgyzstan. Biotechnol. Biotechnol. Equip., 29, 1104-1112, doi.org/10.1080/13102818.2015.1072478
Shabbir Z. et al., 2020. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere, 259, 127436, doi.org/10.1016/j.chemosphere.2020.127436
Sorrentino M.C., Capozzi. F., Giordano S. & Spagnuolo V., 2017. Genotoxic effect of Pb and Cd on in vitro cultures of Sphagnum palustre: an evaluation by ISSR markers. Chemosphere, 181, 208-215, doi.org/10.1016/j.chemosphere.2017.04.065
Taran N. et al., 2017. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett., 12, 60, doi.org/10.1186/s11671-017-1839-9
Tardieu F., Draye X. & Javaux M., 2017. Root water uptake and ideotypes of the root system: whole‐plant controls matter. Vadose Zone J., 16, 1-10, doi.org/10.2136/vzj2017.05.0107
Trujillo-González J.M. et al., 2016. Heavy metal accumulation related to population density in road dust samples taken from urban sites under different land uses. Sci. Total Environ., 553, 636-642, doi.org/10.1016/j.scitotenv.2016.02.101
Vezza M.E. et al., 2018. Arsenic stress effects on root water absorption in soybean plants: physiological and morphological aspects. Plant Physiol. Biochem., 123, 8-17, doi.org/10.1016/j.plaphy.2017.11.020
Vieira E.A. et al., 2017. Mechanisms of desiccation tolerance in the bromeliad Pitcairnia burchellii Mez: biochemical adjustments and structural changes. Plant Physiol. Biochem., 121, 21-30, doi.org/10.1016/j.plaphy.2017.10.002
Zaouali W. et al., 2020. Copper-induced changes in growth, photosynthesis, antioxidative system activities and lipid metabolism of cilantro (Coriandrum sativum L.). Biologia, 75, 367-380, doi.org/10.2478/s11756-020-00419-9
Zhang H., Li X.Y., Chen L. & Huang Z.R., 2020. The photosynthetic performance of two Citrus species under long-term aluminum treatment. Photosynthetica, 58, 228-235, doi.org/10.32615/ps.2019.145






