- Home
- Volume 26 (2022)
- Numéro 2
- Physiological and anatomical responses of Crambe abyssinica to repeated exposure to water deficit
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Physiological and anatomical responses of Crambe abyssinica to repeated exposure to water deficit

Attached document(s)
original pdf fileRésumé
Réponses physiologiques et anatomiques de Crambe abyssinica à une exposition répétée au déficit hydrique
Description du sujet. Les réponses des plantes à un seul épisode de sècheresse sont assez courantes. Cependant, dans la nature, les plantes sont exposées à des cycles répétés de sècheresse et de réhydratation, et les conséquences de ces épisodes de sècheresse répétitifs sont moins bien comprises.
Objectifs. L’objectif de cette étude était de comprendre comment la performance du crambe dans des conditions limitantes en eau est affectée par une exposition antérieure au déficit hydrique.
Méthode. Les plantes de crambe ont été cultivées dans des pots de 5,5 l et exposées à un (1DH) ou trois (3DH) cycles de déficit hydrique. À titre de référence, les plantes ont été cultivées avec une irrigation quotidienne. À la fin de trois cycles de déficit hydrique, l'anatomie des feuilles, la teneur en eau relative des feuilles (RWC), la conductance stomatique, l'extravasation des électrolytes et la fluorescence de la chlorophylle ont été analysées.
Résultats. En cas de déficit hydrique, la RWC a considérablement diminué dans les plantes à 1DH. Cependant, dans les plantes de FMS CR 1307, l'application de trois cycles de déficit hydrique n'a pas changé le RWC et a diminué la conductance stomatique et l'extravasation des électrolytes par rapport aux plantes de référence. De même, la différence cinétique dans la fluorescence de la chlorophylle (bande K et bande L) a indiqué une stabilité et une efficacité améliorées dans l'utilisation de l'énergie.
Conclusions. Nos résultats montrent que le stress de sècheresse imposé sur trois cycles de déficit hydrique induit des ajustements dans la physiologie et l'anatomie des plantes de crambe. La lignée FMS CR 1307 était mieux en mesure de stocker les informations des évènements stressants précédents, améliorant ainsi ses performances en cas de déficit hydrique.
Abstract
Description of the subject. It is common for cultivated plants to face a single water deficit event, but in the wild plants are exposed to repeated cycles of drought and rehydration and the consequences of such repetitive events are less well understood.
Objectives. The objective of this study was to understand how crambe’s performance under water limiting conditions is affected by previous exposure to water deficit.
Method. Crambe plants were grown in 5.5 l pots and exposed to one (1WD) or three (3WD) water deficit cycles. As reference, plants were grown with daily irrigation. At the end of three water deficit cycles, the leaf anatomy, leaf relative water content (RWC), stomatal conductance, extravasation of electrolytes and chlorophyll fluorescence were analyzed.
Results. Under water deficit, RWC significantly decreased in 1WD plants. However, in plants of the FMS CR 1307 lineage, the application of three water deficit cycles did not change the RWC and decreased the stomatal conductance and extravasation of electrolytes compared to the reference plants. Likewise, the kinetic difference in chlorophyll fluorescence (K-band and L-band) indicated improved stability and efficiency in utilizing energy.
Conclusions. Our results show that the drought stress imposed three times induced adjustments in the physiology and anatomy of crambe plants. The FMS CR 1307 lineage was better able to store information from previous stressful events than the FMS Brilhante and FMS CR 1326 lineages, showing better performance under water deficit.
Table of content
Received 20 January 2021, accepted 28 March 2022, available online 20 April 2022
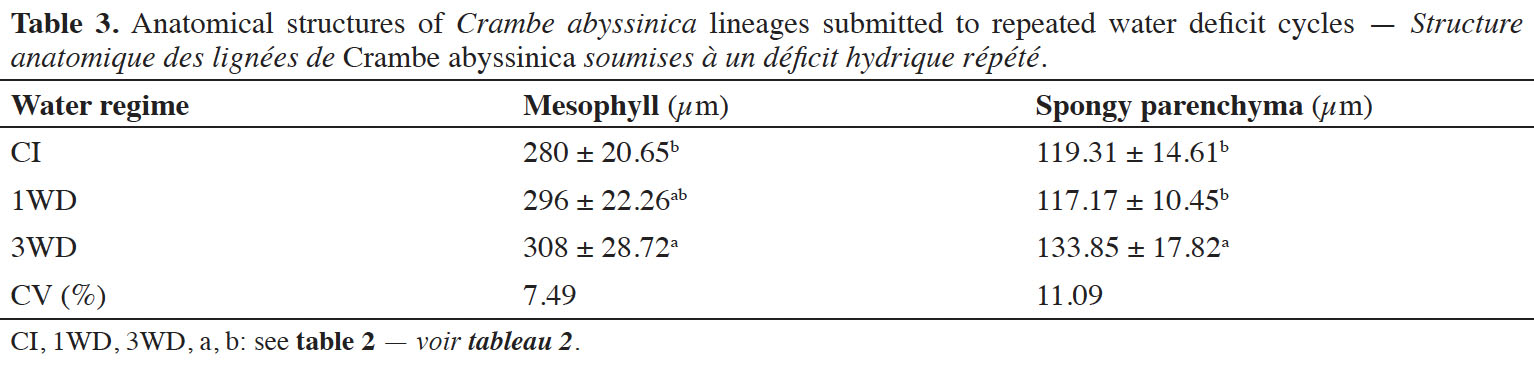
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1Among the environmental conditions affecting plants, drought is the main stress factor affecting the growth, development and yield of crops (Zhang et al., 2018a). Drought can affect plants in several ways. One of the first effects of water deficit in plants is stomatal closure, which is regulated by abscisic acid (ABA). The stomatal closure to avoid water loss through transpiration can lead to a reduction in the photosystem II (PSII) efficiency. In addition, this causes limitations in CO2 absorption in the mesophyll, which consequently reduces its fixation (Mehta et al., 2010; Flexas et al., 2012; Marcos et al., 2018). In scientific studies, plants’ responses to drought are generally analyzed with respect to a single water deficit event.
2In nature, plants are exposed to recurrent cycles of water deficit and rehydration, and the responses to these repeated events are still not well understood (Fleta-Soriano & Munné-Bosch, 2016; Menezes-Silva et al., 2017). According to Mickelbart et al. (2015), plants generally exhibit stress tolerance or avoid stress through mechanisms of acclimation and adaptation that evolved through natural selection. Thus, after stress recognition, plants regulate their responses to reestablish cellular homeostasis, reducing the effects of immediate stress. These changes in the acclimation period tend to allow plants to respond faster and more efficiently to future environmental stresses (Fleta-Soriano & Munné-Bosch, 2016; Marcos et al., 2018), enabling them to live in a great diversity of habitats (Fleta-Soriano & Munné-Bosch, 2016).
3Studies of previous exposure to one or repeated cycles of various abiotic stresses (water deficit, salinity, high temperature) have demonstrated this improvement in plant resistance to future exposure, a reaction known as “stress memory” (Hu et al., 2015; Hu et al., 2016; Zhang et al., 2018a; Marcos et al., 2018). When potato plants (Solanum tuberosum L. cv Atlantic) were exposed to water deficit, they showed increases in the leaf relative water content (RWC) and leaf thickness, increasing water storage capacity, preventing excessive transpiration and ensuring more efficient water use (Zhang et al., 2018a). Increases in root biomass of sugarcane plants (Saccharum spp. variety IACSP94-2094) submitted to three water deficit cycles caused improved water uptake (Marcos et al., 2018). Reductions in electrolyte extravasation were observed in plants of Lolium perenne L. (cv. 'Quickstart II') exposed to salinity (Hu et al., 2016), while improved recovery of PSII by decreased inhibition of photosynthetic electron transport fluxes was noted in Festuca arundinacea Schreb. plants exposed to high temperatures (Hu et al., 2015).
4The majority of studies reporting stress memory have used analysis of transcriptome, epigenome, proteome and metabolome (Hu et al., 2015; Hu et al., 2016; Fleta-Soriano & Munné-Bosch, 2016; Menezes-Silva et al., 2017). However, these techniques are expensive and time-consuming. In this study, we investigated stress memory utilizing analyses of simple morphophysiological aspects, such as chlorophyll a fluorescence. This method is highly sensitive in detecting plant responses and has been widely used to assess the tolerance of a species/genotype to abiotic stresses (Mehta et al., 2010).
5Crambe abyssinica Hochst. is an annual plant native to the coastal region of Ethiopia, but now cultivated in many tropical and subtropical regions. The crop has high potential to produce biodiesel (Batista et al., 2018). Its cultivation has many agronomic advantages, such as low nutritional requirements and high morphological plasticity (Zanetti et al., 2016), allowing crop rotation and providing a source of clean energy, helping to mitigate global warming. The crambe FMS Brilhante lineage is considered tolerant to water deficit (Lara-Fioreze et al., 2013; Martins et al., 2017), but the responses of the FMS CR 1307 and 1326 lineages to water deficit are still unknown. The physiological responses of these two lineages may be related to the natural selection process, since they came from natural cross-breeding between different FMS Brilhante genotypes.
6In order to understand how crambe’s performance under drought conditions is affected by previous exposure to water deficits, we evaluated the photochemical adjustments of plants through chlorophyll a fluorescence analyses; changes in relative water content (RWC) and anatomic characteristics of the diffusive system through stomatal conductance measurements (gs); and cell membrane stability through analysis of extravasation of electrolytes (EE). We hypothesized that crambe plants subjected to previous droughts would exhibit improved performance, achieved through changes in morphophysiology under water deficit.
2. Materials and methods
2.1. Plant growth and experimental design
7The experiment was conducted in a greenhouse (18°43’S longitude, 39°51’W latitude, altitude of 39 m) from June to September. Seeds of crambe (FMS Brilhante, FMS CR 1307 and 1326 lineages) were obtained from the Mato Grosso do Sul Foundation (MS Foundation), in Maracaju, MS. Seeds were previously disinfested with 70% ethanol for 2 min, 1% sodium hypochlorite (v/v) for 20 min and Ridomil® fungicide for 10 min, followed by triple lavage with autoclaved distilled water. Then the seeds were placed to germinate in 5.5 l pots (15 seeds per pot) filled with soil (75.5% sand, 17.2% clay and 5.2% silt). The soil was submitted to previous chemical analysis to correct nutrient and pH levels. Thirty grams of single super phosphate was added per pot three times during the experiment. In addition, applications of insecticide (Evidence®) and fungicide (Ridomil®) were performed according to culture manual. After 20 days, the seedlings were thinned to one per pot.
8The plants were irrigated daily for 36 days to maintain the soil moisture around 80% of field capacity (≅ 0.20 m3·m-3), after which they were divided into three groups:
9– plants irrigated daily (continuously irrigated - CI);
10– plants submitted to one water deficit cycle (1WD);
11– plants submitted to three water deficit cycles with periods of water deficit recovery (3WD) (Menezes-Silva et al., 2017). Soil water levels (m3·m-3) were monitored using a soil water sensor (ProCheck, version 4, Decagon Devices).
12Each water deficit cycle was imposed by suspending the irrigation until the soil moisture content reached approximately 30% (≅ 0.06 m3·m-3) and the stomatal conductance of plants reached values below 10 mmol m2·s-1, which occurred after seven days. The recovery phase occurred after four days; the time required for the stomatal conductance (gs) of plants submitted to water deficit to reach the values observed in the continuously irrigated (CI) plants. After the full recovery from the water deficit cycle (seven days of water deficit and four days of recovery), two additional cycles of water deficit were applied to plants, totaling three water deficit cycles (3WD), as described above. These cycles were similar in intensity and duration, and all treatments (CI, 1WD and 3WD) were evaluated at the same time at the end of the experiment. Therefore, the measurements were performed at the end of the dehydration and rehydration, phase of the first and third dry cycle (at 4 days after the soil moisture reached the values observed in the plants irrigated continuously [CI]). Thus, the plants were same age at the end of the experiment. All measurements were made between 5 a.m. and 10 a.m. in fully expanded leaves (third or fourth leaf from the apex).
2.2. Leaf relative water content (RWC), stomatal conductance (gs) and extravasation of electrolytes (EE)
13Leaf relative water content (RWC), stomatal conductance (gs) and extravasation of electrolytes (EE) were measured in three (n = 3), nine (n = 9) and three (n = 3) plants per treatment, respectively, at the end of the experiment. RWC was determined following the methods proposed by Barrs & Weatherley (1962). The stomatal conductance was measured on the abaxial leaf surface using a leaf porometer (SC-1, Decagon Devices). The degree of membrane integrity was determined by measuring extravasation of electrolytes (EE) as described by Bajji et al. (2002) with modifications. For such analysis, fresh leaves (5 mm diameter leaf discs) were randomly harvested and washed with distilled water. Leaf discs were placed in test tubes containing 20 ml of distilled and deionized water at room temperature on a rotary shaker for 6 h. Subsequently, the initial electrical conductivity of the medium (EC1) was evaluated. Afterward, the tubes with the discs were placed for 2 h at 90 ºC in a water bath (EC2). Test tubes containing 20 ml of distilled and deionized water were used as control, which were also exposed to the same conditions (at room temperature [C1] and at 90 ºC for 2 h in a water bath [C2]). The EE was expressed as a percentage using the formula: (EC1 - C1) / (EC2 - C2) x 100.
2.3. Chlorophyll a fluorescence analysis
14The chlorophyll a fluorescence transients were measured in nine plants per treatment (n = 9) at the end of the experiment using a fluorometer (Handy-PEA, Hansatech, King’s Lynn, Norfolk, UK). All measurement procedures were based on Braga et al. (2020). The fluorescence kinetics and the JIP test parameters (Table 1) were analyzed according to Strasser et al. (2004), Stirbet & Govindjee (2011), and Wang et al. (2016).
2.4. Leaf anatomy analysis
15At the end of the experiment for each treatment, the thickness of the spongy and palisade parenchyma, number of xylem vessels were determined in seven leaves from different plants (n = 7). The collected samples were fixed in FAA (formaldehyde, acetic acid, and 50% ethanol, 0.5:/0.5:/9, v/v) and stored in 50% ethanol (Johansen, 1940). Cross-sections were made in the middle region of leaves (third fully expanded leaf from the apex). The sections were visualized and the images captured with a Leica DM 1,000 light microscope coupled to a Leica ICC50 HD digital camera (Wetzlar, Germany). The UTHSCSA-Imagetool® software was used to measure the anatomical characteristics as revealed by the photomicrographs.
2.5. Analysis of growth traits
16The growth analysis consisted of measuring the leaf area, root and shoot dry weight. Nine plants (n = 9) were evaluated in each treatment at the end of the experiment. The leaf area was determined using a LI-COR leaf area meter (Model LI-3100). Root and shoot dry weight were determined in grams (g) after the plants were placed in paper bags and dried for 72 h at 60 ºC.
2.6. Statistical analysis
17The experimental design was completely randomized in a factorial scheme consisting of different cycles of water deficit (plants continuously irrigated [CI]; plants exposed to one water deficit cycle [1WD]; and plants exposed to three water deficit cycles [3WD]) and three crambe lineages (FMS Brilhante, FMS CR 1307 and CR 1326), with nine replicates (nine pots/one plant per pot) per treatment. The data were submitted to analysis of variance (ANOVA) and the means were compared using the Tukey test (p < 0.05 or p < 0.001) using the statistical program Sisvar® (Ferreira, 2014). The results were presented as mean ± standard deviation (SD). In addition, the relative standard deviation was presented as the percent coefficient of variation (CV%).
3. Results
3.1. Leaf relative water content (RWC), stomatal conductance (gs) and extravasation of electrolytes (EE)
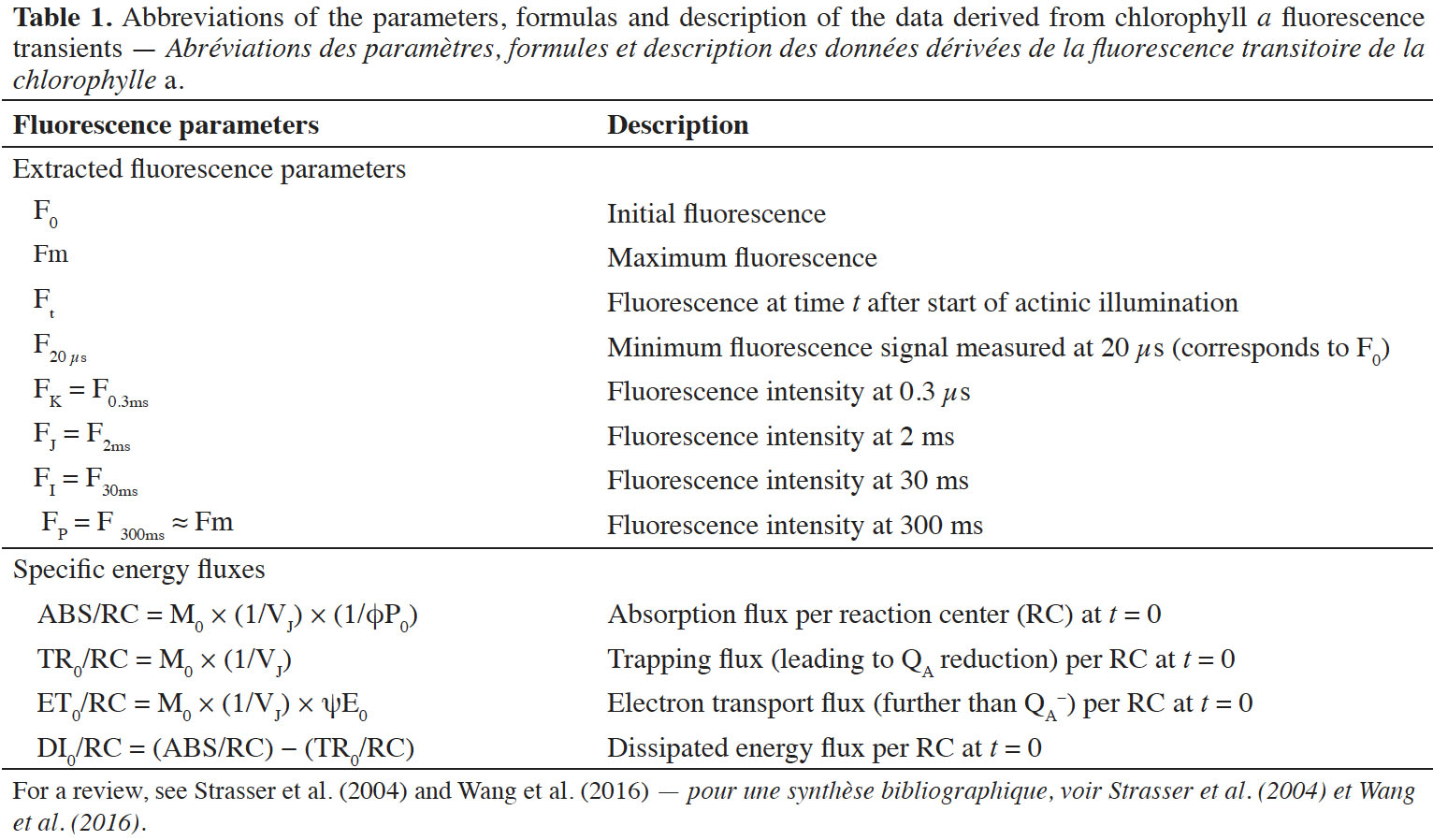
18Significant differences (p ≤ 0.001) in leaf relative water content (RWC) related to water deficit cycles and crambe lineages (FMS Brilhante, FMS CR 1307 and 1326) were observed (Figure 1A). At the end of the first (1WD) and the third cycle (3WD), visual symptoms of leaf wilt were evident in all plants. Plants exposed to one water deficit cycle (1WD) showed lower leaf relative water content than the continuously irrigated (CI) plants (Figure 1A). After three water deficit cycles (3WD), all crambe lineages showed some increase of RWC compared to the plants exposed to 1WD cycle. However, only FMS CR 1307 showed RWC values statistically similar to the continuously irrigated plants (CI) at 3WD (84.5% and 85.5%, respectively).
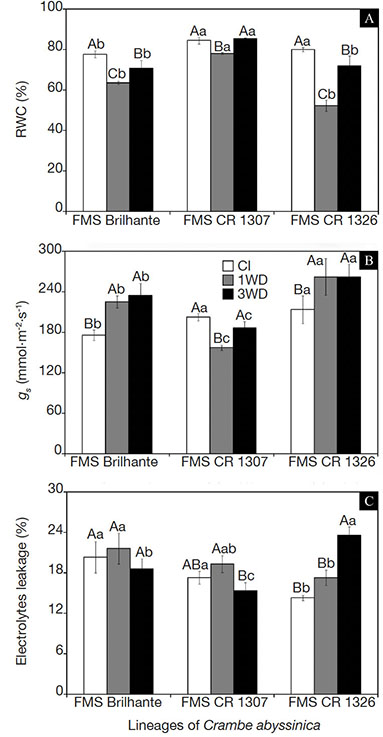
Figure 1. Leaf relative water content (RWC) (A), stomatal flow rate gs (B) and extravasation of electrolytes (EE) (C) of three Crambe abyssinica lineages under continuous irrigation (CI) or subject to one (1WD) or three drought events (3WD) and subsequent recovery — Teneur en eau relative des feuilles (TER) (A), débit stomatique (ds) (B) et fuite d’électrolyte (FE) (C) de trois lignées de Crambe abyssinica sous irrigation continue (IC) ou soumises à un cycle de déficit hydrique (1DH) ou trois cycles de déficit hydrique (3DH) et récupération ultérieure.
Columns followed by the same letter, uppercase for water regime and lowercase for lineage, do not differ significantly from each other according to the Tukey test, p ≤ 0.001 (± SD) — Les colonnes suivies de la même lettre, majuscules pour le régime hydrique et minuscules pour le génotype, ne diffèrent pas significativement les unes des autres selon le test de Tukey, p ≤ 0,001 (± ET).
19The stomatal conductance values in plants that experienced 1WD cycle decreased only in FMS CR 1307 compared to the CI plants and was higher in the 3WD plants (p ≤ 0.001) (Figure 1B). When the crambe lineages were compared under water deficit, FMS CR 1307 showed the lowest gs values after one and three cycles, while similar gs values were observed for FMS Brilhante and FMS CR 1326 (Figure 1B).
20Regarding the degree of membrane integrity, the EE increased in FMS CR 1326 plants exposed to one and three water deficit cycles but decreased in FMS CR 1307 submitted to three water deficit cycles, with no change observed for one water deficit cycle (Figure 1C). Comparing all lineages at 3WD, FMS CR 1326 showed increases of about 26.8 and 54.2% compared to FMS Brilhante and FMS CR 1307, respectively, and about 65% in comparison to CI plants.
3.2. Chlorophyll a fluorescence transients
21Chlorophyll a fluorescence transient OJIP reflects the dynamics of oxirreduction processes involved in the electron transference between plant photosystems. As shown in figure 2A, 1WD and 3WD plants showed a typical polyphasic OJIP shape (Stirbet & Govindjee, 2011), from a basal level (F0) to a maximum level (Fm), and the J and I steps were well defined. The variable relative fluorescence between the O and J steps [obtained at 0.02 and 2 ms, respectively and presented as VOJ = (Ft -F0) / (FJ–F0)] and between O and K steps [0.02 and 30 ms, respectively, presented as VOK = (Ft–F0)/(FK– F0)] were normalized and showed kinetic differences of ΔVOJ = VOJ (treatment) - VOJ (control) and ΔVOK = VOK (treatment) - VOK (control), respectively (Figure 2B and C). The kinetic difference ΔVOK and ΔVOJ made the L- and K-bands visible, with peaks around 0.26 and 0.16 ms, respectively.
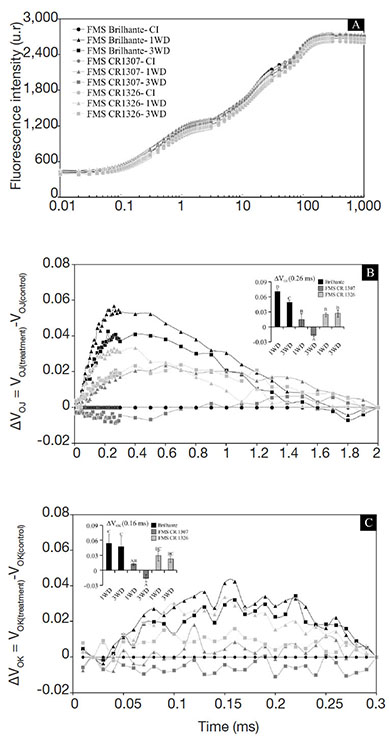
Figure 2. Fluorescence intensity (A), VOJ (B), and VOK (C) obtained through the double normalization of O-J (ΔVOJ = VOJ(treatment) - V OJ(control)) and O-K (ΔVOK = VOK(treatment)-VOK(control)) respectively, of three Crambe abyssinica lineages under continuous irrigation (CI) or subject to one (1WD) or three (3WD) drought events and subsequent recovery — Intensité de fluorescence (A), VOJ (B) et VOK (C) obtenue par la double normalisation de O-J (ΔVOJ = VOJ(traitement) -VOJ(contrôle)) et O-K (ΔVOK = VOK (traitement) -VOK (contrôle)) respectivement de trois lignées de Crambe abyssinica sous irrigation continue ou soumises à un ou trois épisodes de sécheresse et à une récupération ultérieure.
Columns followed by the same letter do not differ significantly from each other according to the Tukey test, p ≤ 0.001 (± SD) — Les colonnes suivies de la même lettre ne diffèrent pas significativement les unes des autres selon le test de Tukey, p ≤ 0,001 (± ET).
22Overall, L- and K-bands with positive amplitudes were observed for all crambe lineages analyzed. In both FMS Brilhante and FMS CR 1326, the stability of the oxygen-evolving complex (OEC) (K-band) decreased (p ≤ 0.001) after 3WD cycles. In FMS CR 1307, negative and significant values of K-band were observed after 3WD (see insert in figure 2B). Furthermore, the energetic connectivity (L-band) values were significantly lower (p ≤ 0.001) in FMS CR 1307 treated with 3WD cycles (see insert in figure 2C). However, these alterations were not significant for FMS Brilhante and FMS CR 1326 (Figure 2C). Among the lineages, the lowest K- and L-band values were observed for FMS CR 1307 submitted to water deficit.
23The parameters of specific energy fluxes varied significantly (p ≤ 0.05) as a function of repeated exposures to water deficit, except for the FMS Brilhante lineage. However, no difference was observed between lineages (Table 2). For FMS CR 1326, plants 1WD and 3WD showed increased TR0/RC and ET0/RC. For FMS CR 1307, increases in the ABS/RC, TR0/RC and ET0/RC were noted at 1WD and 3WD. There were significant decreases in DI0/RC and RC/ABS, and the lowest values were found in 3WD plants, which was similar to that obtained in the control plants (Table 2).
3.3. Leaf anatomy
24Both mesophyll and spongy parenchyma thickness were influenced by the water regimes (p ≤ 0.001), but this influence was independent of lineage. Significant increases of mesophyll and spongy parenchyma thickness values were observed in plants submitted to 3WD (9.0% and 10.8%, respectively) compared to CI plants (Table 3).
25The palisade parenchyma thickness and number of xylem vessels differed (p ≤ 0.05) in function of water deficit cycles and crambe lineages. The exposure of FMS Brilhante and FMS CR 1307 to 1WD increased the palisade parenchyma thickness compared to the CI plants. All lineages submitted to 3WD showed similar palisade parenchyma thickness values to that found in CI, evidencing full recovery (Figure 3). For FMS CR 1326, no change in the palisade parenchyma thickness was observed after the first water deficit cycle, but after 3WD, reduction of 41.7% in thickness was observed. Comparing all lineages after 3WD, FMS CR 1326 showed decreases in the palisade parenchyma thickness of about 54.3 and 43.9% compared to FMS Brilhante and FMS CR 1307, respectively (Figure 3A). Reductions in the number of xylem vessels were observed in FMS Brilhante after 1WD and 3WD (24.7% and 52.4%, respectively) compared with CI plants (Figures 3B and 4). No change in vessel number was observed for FMS CR 1307 and 1326 (Figure 3B).
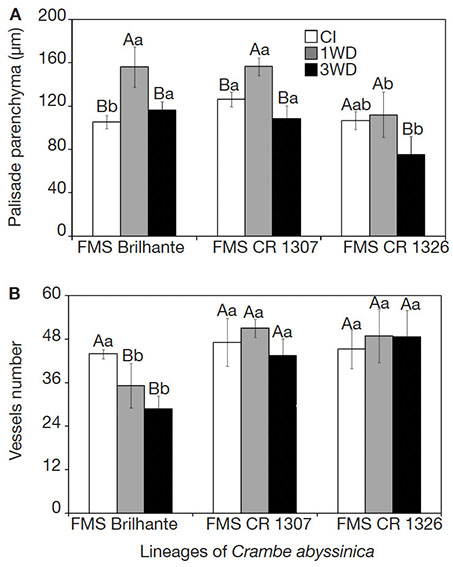 Figure 3. Palisade parenchyma (A) and number of xylem vessels (B) of three Crambe abyssinica lineages under continuous irrigation (CI) or subject to one (1WD) or three drought events (3WD) and subsequent recovery — Parenchyme palissade (A) et nombre de vaisseaux xylème (B) de trois lignées de Crambe abyssinica sous irrigation continue (IC) ou soumis à un cycle de déficit hydrique (1DH) ou à trois cycles de déficit hydrique (3DH) et récupération ultérieure.
Figure 3. Palisade parenchyma (A) and number of xylem vessels (B) of three Crambe abyssinica lineages under continuous irrigation (CI) or subject to one (1WD) or three drought events (3WD) and subsequent recovery — Parenchyme palissade (A) et nombre de vaisseaux xylème (B) de trois lignées de Crambe abyssinica sous irrigation continue (IC) ou soumis à un cycle de déficit hydrique (1DH) ou à trois cycles de déficit hydrique (3DH) et récupération ultérieure.
Columns followed by the same letter, uppercase for water regime and lowercase for lineage, do not differ significantly from each other according to the Tukey test, p ≤ 0.001 (± SD) — Les colonnes suivies de la même lettre, majuscules pour le régime hydrique et minuscules pour le génotype, ne diffèrent pas significativement les unes des autres selon le test de Tukey, p ≤ 0,001 (± ET).
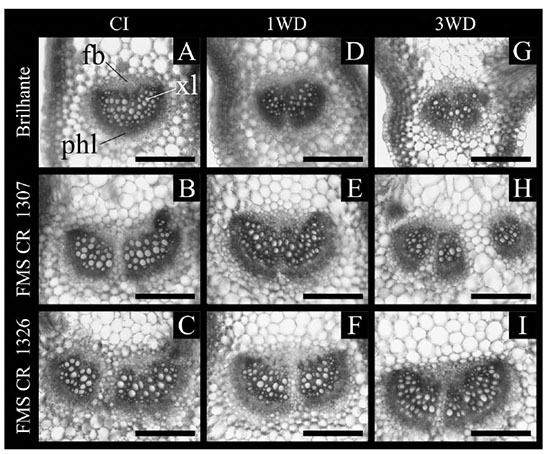
Figure 4. Cross-sections of Crambe abyssinica lineages submitted to continuous irrigation (CI) or subject to one (1WD) or three (3WD) drought events and subsequent recovery — Coupes transversales de lignées de Crambe abyssinica soumises à une irrigation continue (IC) ou soumises à un (1DH) ou trois (3DH) épisodes de sècheresse et à un rétablissement ultérieur.
Bar — barre = 50 µm; xl: xylem — xylème; phl: phloem — phloème; fb: fibers — fibres.
3.4. Analysis of growth
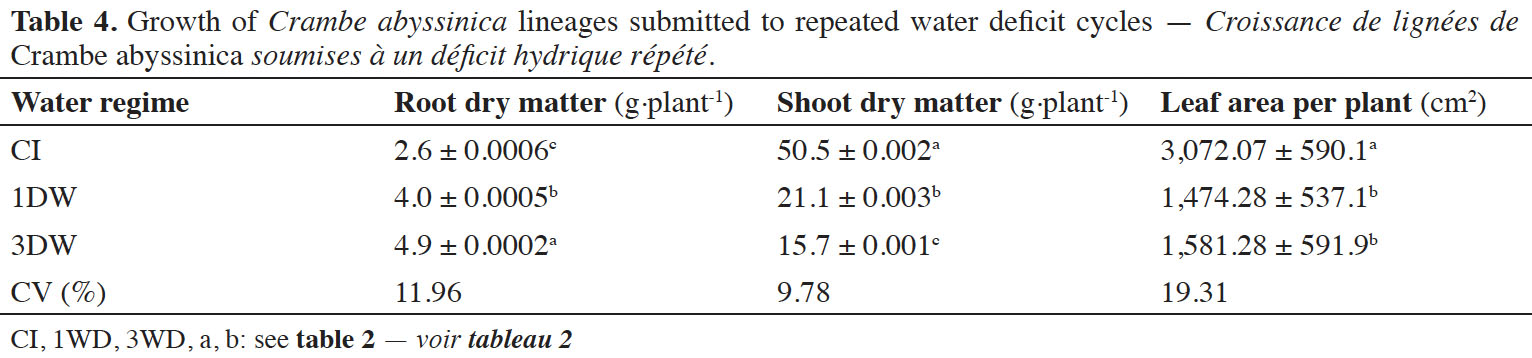
26The root dry matter, shoot dry matter and leaf area values of crambe plants varied significantly (p ≤ 0.001) independently of lineage (Table 4). Increases in root dry matter of plants exposed to 1WD and 3WD were observed (≅ 35.0% and 46.9%, compared to CI plants). In addition, decreased shoot dry matter and leaf area values were obtained when the plants were exposed to 1WD and 3WD cycles (approximately 139.3% and 226.6%, respectively for shoot dry matter and 108.3% and 94.3%, respectively, for leaf area). However, the alterations observed in leaf area between 1WD and 3WD were not significant (Table 4).
4. Discussion
27Under drought stress, the RWC tends to decline, which is directly related to soil water content. This parameter is an important indicator of water stress level in plants (Kumar et al., 2018). In our study, the increase of RWC in 3WD plants compared to 1WD plants suggests increased capacity of crambe plants to retain water and more efficient water use with each water deficit cycle. However, only the FMS CR 1307 lineage showed full recovery of RWC values after 3WD compared with continuously irrigated plants (CI). These higher RWC values of 3WD plants were associated with the decreases observed in gs, which likely reduced the loss of water through leaf transpiration (Gao et al., 2018), improving water maintenance in leaf tissue. The higher RWC values observed in lineage FMS CR 1307 under water deficit is associated with its greater ability to maintain high water content in the leaf tissue under water deficit conditions, compared to the FMS Brilhante and FMS CR 1326 plants.
28The maintenance of RWC is also evidenced by osmoregulatory mechanisms (Marcos et al., 2018). Osmoregulation ensures the preservation of protein structures and functions under low water availability (Verlues et al., 2006; Li et al., 2020). By comparing the lineages after 3WD cycles, the reductions observed for EE in FMS CR 1307 served as an indication of less damage to the cell membranes, suggesting the presence of osmoregulation. According to Bajji et al. (2002), the cell membrane is one of the first structures affected by stresses, while according to Trabelsi et al. (2019), it is generally accepted that their integrity and stability under water deficit conditions can be correlated with plant tolerance. Thus, the maintenance of RWC and decrease of gs and EE observed in the FMS CR 1307 lineage compared to CI plants suggest that FMS CR 1307 plants exposed to 3WD cycles can develop a differential acclimation through morphophysiological adjustment.
29The water regimes clearly influenced the photosynthetic apparatus performance. Crambe lineages exposed to 3WD presented different responses to the water deficit, affecting the photochemical parameters. In this study, all lineages showed O-J-I-P curves with typical polyphasic behavior, indicating that all plants were photosynthetically active after treatments. The occurrence of positive K- and L-bands in FMS Brilhante and FMS CR 1326 after exposure to 1WD and 3WD cycles and for FMS CR 1307 after 1WD cycle, respectively, reflects inhibition of electron donation to YZ (Tyr161 of protein D1) and disaggregation of PSII, respectively. Probably the water deficit resulted in damage to the OEC, and consequently degradation of D1 protein (Strasser, 1997) and instability of the subunits associated with PSII (Lin et al., 2009). Thus, for the FMS Brilhante and FMS CR 1326 lineages, each water deficit cycle increased the damage due to lack of electron donation from the OEC to oxidized PSII (Takahashi & Badger, 2011), which may have resulted in photoinhibition (Shin et al., 2020). In a previous study, plants in the vegetative phase of the lineage FMS CR 1326 were exposed to a single cycle of water deficit and showed a better ability to use excitation energy and greater stability of the photosynthetic system (Braga et al., 2020). However, in the present study, FMS CR 1326 plants exposed to 3WD cycles and resumption showed lower energy use and stability of the photosynthetic apparatus. This means that the FMS CR 1326 lineage is not able to maintain performance after consecutive periods of water stress. This result may indicate that some defense mechanisms, such as the accumulation of glycine, betaine, proline, and sugars (De Ronde et al., 2004), can decrease with each new water deficit cycle, reducing the stability of PSII. FMS Brilhante presented the highest positive amplitudes for both K- and L-bands in 1WD plants. A higher amplitude of the L-band denotes that the water deficit caused dissociation of the PSII antenna complex (CP43 and CP47) (Chen et al., 2013).
30The FMS CR 1307 lineage exposed to 1WD showed increased ABS/RC and reduced stability of RC and their connection with the light-harvesting antenna complexes (RC/ABS) (Chen et al., 2014). These results suggest inactivation of the reaction centers and/or increase of the light-harvesting antenna size (Meng et al., 2016). This is an indication of susceptibility to photoinhibition due to flaws in the regulation mechanisms of the excess light absorbed (Franić et al., 2017). The FMS CR 1307 lineage exposed to one water deficit cycle also showed an increase in TR0/RC. Increased TR0/RC values along with proportional increases in ABS/RC indicate suppressed repair of damage to the OEC (Takahashi & Murata, 2008; Kalaji et al., 2014). In this study, the impairment caused to OEC was verified by the positive K-band in 1WD plants. However, part of the absorbed energy was dissipated as heat (DI0/RC). The improvement of energy dissipation protects the leaves against photooxidative damage (Franić et al., 2017). The results obtained in this study also suggest that repeated cycles of water deficit did not reduce the efficiency of photosynthesis by reaction centers (RCs) in the FMS Brillante lineage. However, after the exposure to 1WD and 3WD cycles, the FMS CR 1326 lineage presented increased TR0/RC and ET0/RC values.
31The exposure of plants to 3WD resulted in negative K- and L-bands only for FMS CR 1307. This result may indicate a photoprotective mechanism of the reaction centers associated with PSII. Photoprotection works by increasing electron transport from OEC to protein D1 and protecting the grouping of PSII units. Thus, the results denote better use of excitation energy and greater system stability acquired after each new water deficit cycle. According to De Ronde et al. (2004), osmoregulation is an efficient mechanism to protect the OEC under environmental stress. In our study, as previously mentioned, reductions of EE showed lower degree of cell membrane damage in FMS CR 1307, suggesting a possible osmoregulatory mechanism, which increased the stability of the OEC. Moreover, this lineage, when submitted to 3WD, presented higher ET0/RC and reduced DI0/RC. This may indicate that after the recovery from water deficit cycles, the FMS CR 1307 lineage had a more efficient photosynthetic apparatus, with increased electron transport from the OEC to protein D1 (negative L-band) and direction of electrons to reduce pheophytin, QA and the other electron acceptors of the carrier chain. This probably occurred in order to convert the excitation energy into redox energy, which leads to CO2 fixation (Strasser et al., 2000). According to Hu et al. (2015), stress memory can help delay the impairment of CO2 fixation induced by abiotic stress, inhibiting the energy flow through of active RCs. These responses indicate photochemical memory of stress. Our data indicate that tolerance was improved under water deficit conditions and that the FMS CR 1307 crambe lineage is better at coping with drought stress than FMS Brilhante and FMS CR 1326.
32Besides the changes in leaf physiology, alterations in the leaf anatomy are also important for plants to adapt to drought conditions (Wu et al., 2018). The increases of palisade parenchyma thickness in 1WD plants suggested improvement of water relations and protection of leaf tissues to allow plant growth under water deficit conditions (Bacelar et al., 2004). In order to reduce the water loss of leaves, plants may develop increased palisade parenchyma thickness, thus improving the mechanical resistance of the parenchyma (Bacelar et al., 2004; Oliveira et al., 2018). However, previous exposure to water deficit in the 3WD plants resulted in a compact arrangement of the palisade parenchyma layers, reducing the thickness with each new cycle of water deficit. This result indicates the need to increase light uptake and to facilitate the penetration of light into the deeper cell layers, to improve the photosynthetic performance of the plants (Gotoh et al., 2018), since the palisade parenchyma is closely linked to the photosynthetic process by the number of chloroplasts in palisade cells, as reported by Liu et al. (2010). In addition, smaller mesophyll cells are an essential response to increased water stress because they can resist turgor pressure, thus contributing to turgor maintenance more effectively under water deficit (Boughalleb et al., 2015). In contrast to the palisade, spongy parenchyma thickness increased. After a period of water restriction, plants may increase the spongy tissue rather than the palisade tissue (Toscano et al., 2019). This modification is an important anatomical strategy, since it can improve the diffusion toward the fixation sites in order to increase the concentration gradient between inter-cellular spaces and the atmosphere, in turn increasing the competition among cells for CO2 and light (Ennajeh et al., 2010; Fang & Xiong, 2015).
33After 3WD cycles, no change in the number of xylem vessels was observed in lineages FMS CR 1307 and 1326, which also might have increased the leaves’ hydration, mainly in FMS CR 1307, in which higher RWC maintenance was observed. This result indicates that the exposure of these crambe lineages, during the non-reproductive stage, submitted to 3WD cycles did not result in changes in the number of xylem vessels. Although a reduction in vessels could decrease the hydraulic conductivity, it could also lead to loss of vessels due to greater predisposition to embolism (Guha et al., 2018; Zhang et al., 2018b).
34An increase in root dry weight of plants submitted to 1WD and 3WD was observed. Sugarcane plants (Saccharum spp.) variety IACSP94-2094 exposed to repeated water deficit cycles also showed increases in root dry matter (Marcos et al., 2018). According to the authors, this increase is related to large and controlled H2O2 concentrations in the roots, not causing oxidative damage but increasing root growth. In this sense, plants under water deficit invest in root growth to increase water uptake from the soil. However, by increasing root growth, the shoot dry matter tends to decrease. In our study, after 3WD cycles, reductions in shoot dry matter and leaf area were observed. This reduction may be related to the need to increase light capture, since a moderate light can reduce biomass production (Gotoh et al., 2018). These reductions of leaf area resulted in increased mesophyll thickness, which increased the water storage capacity in 3WD plants, facilitating the CO2 absorption and maintaining the photosynthetic activity under water deficit conditions (Chartzoulakis et al., 2000). The higher water storage capacity of 3WD plants was associated with increases in RWC, compared to 1WD plants. The results obtained in this study are the first demonstration that C. abyssinica plants exposed to repeated water deficit cycles are better able to cope with water shortage than their counterparts that experienced one water deficit exposure. According to Ding et al. (2013), a memory of stress can facilitate plants' future responses in unfavorable situations.
35In summary, the results obtained in this study showed that previous exposure of lineage FMS CR 1307 to 3WD resulted in increased electron transport or energy exchange between independent photosystem II (PSII) units, showing activated stress memory, which was regulated by the PSII recovery process, in addition to greater energy transport flow. This result indicates that the transient chlorophyll a fluorescence is a good stress/tolerance indication tool for plants (Meng et al., 2016; Falqueto et al., 2017; Gupta, 2019) and an indicator of plant stress memory. Finally, from a practical perspective, our data indicate that tolerance of C. abyssinica to water deficit can be improved while saving water and energy through less frequent irrigation in regions characterized by low precipitation.
5. Conclusions
36In conclusion, our results showed that exposure of crambe plants to three water deficit cycles (3WD) induced stress memory, involving physiological and anatomical changes. The stress memory resulting from previous exposure to water deficit was associated with the maintenance of the RWC, reductions in gs, maintenance of the stability of cell membranes and utilization of excitation energy and greater stability of the system, besides modifications in the thickness of the mesophyll tissues. The FMS CR 1307 lineage showed better photochemical performance as well as anatomical adjustments that were synchronized with the maintenance of cell membrane integrity and stability. These results confirm the existence of water deficit memory in the PSII of crambe plants, besides more efficient water use.
Acknowledgements
37We are grateful to the Espírito Santo Research and Innovation Foundation (FAPES) for the financial support and scholarship. We are also thankful to the MS Foundation for providing the plant material and technical support during the study. The authors would also like to thank Luiz Carlos de Almeida Rodrigues, Thayna dos Santos Silva, Railda Martins Borges, João Vitor G. Silva, Myllena Lorenn Ferreira de Souza, Patrícia dos Santos Oliveira, Lucas de Almeida Leite and Marcel Merlo Mendes for their technical assistance.
Bibliographie
Bacelar E. et al., 2004. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol., 24, 233-239, doi.org/10.1093/treephys/24.2.233
Bajji M., Kinet J.M. & Lutts S., 2002. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul., 36, 61-70, doi.org/10.1023/A:1014732714549
Barrs H.D. & Weatherley P.E., 1962. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci., 15, 413-428, doi.org/10.1071/BI9620413
Batista P.F. et al., 2018. Nitric oxide mitigates the effect of water deficit in Crambe abyssinica. Plant Physiol. Biochem., 129, 310-322, doi.org/10.1016/j.plaphy.2018.06.012
Boughalleb F., Abdellaoui R., Hadded Z. & Neffati M., 2015. Anatomical adaptations of the desert species Stipa lagascae against drought stress. Biologia, 70, 1042-1052, doi.org/10.1515/biolog-2015-0125
Braga P.C.S. et al., 2020. Differential response of photosystem II and I photochemistry in leaves of two Crambe abyssinica Hochst. lineages submitted to water deficit. Photosynthetica, 58, 1122-1129, doi.org/10.32615/ps.2020.065
Chartzoulakis K. et al., 2000. Effects of water stress on water relations, gas exchange and leaf structure of olive tree. Acta Hortic., 537, 241-247, doi.org/10.17660/actahortic.2000.537.25
Chen K., Chen L., Fan J. & Fu J., 2013. Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. Photosynth. Res., 116, 21-31, doi.org/10.1007/s11120-013-9883-5
Chen S.G., Strasser R.J. & Qiang S., 2014. In vivo assessment of effect of phytotoxin tenuazonic acid on PSII reaction centers. Plant Physiol. Biochem., 84, 10-21, doi.org/10.1016/j.plaphy.2014.09.004
De Ronde J.A. et al., 2004. Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J. Plant Physiol., 161, 1211-1224, doi.org/10.1016/j.jplph.2004.01.014
Ding Y. et al., 2013. Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol., 13, 229, doi.org/10.1186/1471-2229-13-229
Ennajeh M., Vadel A.M., Cochard H. & Khemira H., 2010. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol., 85, 289-294, doi.org/10.1080/14620316.2010.11512670
Falqueto A.R. et al., 2017. Effects of drought stress on chlorophyll a fluorescence in two rubber tree clones. BMC Plant Biol., 224, 238-243, doi.org/10.1016/j.scienta.2017.06.019
Fang Y. & Xiong L., 2015. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci., 72, 673-689, doi.org/10.1007/s00018-014-1767-0
Ferreira D.F., 2014. Sisvar: a guide for its bootstrap procedures in multiple comparisons. Ciênc. Agrotecnol., 38, 109-112, doi.org/10.1590/S1413-70542014000200001
Fleta-Soriano E. & Munné-Bosch S., 2016. Stress memory and the inevitable effects of drought: a physiological perspective. Front. Plant Sci., 7, 143, doi.org/10.3389/fpls.2016.00143
Flexas J. et al., 2012. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci., 193-194, 70-84, doi.org/10.1016/j.plantsci.2012.05.009
Franić M., Galić V., Mazur M. & Šimić D., 2017. Effects of excess cadmium in soil on JIP-test parameters, hydrogen peroxide content and antioxidant activity in two maize inbreds and their hybrid. Photosynthetica, 55, 1-10, doi.org/10.1007/s11099-017-0710-7
Gao R. et al., 2018. Physiological responses to drought in three provenances of Discorea nipponica Makino. J. Appl. Bot. Food Qual., 91, 261-270, doi.org/10.5073/JABFQ.2018.091.034
Gotoh E. et al., 2018. Chloroplast accumulation response enhances leaf photosynthesis and plant biomass production. Plant Physiol., 178, 1358-1369, doi.org/10.1104/pp.18.00484
Guha A. et al., 2018. Hydraulic anatomy affects genotypic variation in plant water use and shows differential organ specific plasticity to drought in Sorghum bicolor. Environ. Exp. Bot., 156, 25-37, doi.org/10.1016/j.envexpbot.2018.08.025
Gupta R., 2019. Tissue specific disruption of photosynthetic electron transport rate in pigeonpea (Cajanus cajan L.) under elevated temperature. Plant Signaling Behav., 14, 6, doi.org/10.1080/15592324.2019.1601952
Hu T., Liu S.Q., Amombo E. & J. Fu M., 2015. Stress memory induced rearrangements of HSP transcription, photosystem II photochemistry and metabolism of tall fescue (Festuca arundinacea Schreb.) in response to high-temperature stress. Front. Plant Sci., 6, 403, doi.org/10.3389/fpls.2015.00403
Hu T. et al., 2016. Stress memory induced transcriptional and metabolic changes of perennial ryegrass (Lolium perenne) in response to salt stress. Physiol. Plant., 156, 54-69, doi.org/10.1111/ppl.12342
Johansen D.A., 1940. Plant microtechnique. 4th ed. New York, NY, USA: Mc Graw-Hill.
Kalaji H.M. et al., 2014. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem., 81, 16-25, doi.org/10.1016/j.plaphy.2014.03.029
Kumar M.S., Mawlong I., Ali K. & Tyagi A., 2018. Regulation of phytosterol biosynthetic pathway during drought stress in rice. Plant Physiol. Biochem., 129, 11-20, doi.org/10.1016/j.plaphy.2018.05.019
Lara-Fioreze A.C.C. et al., 2013. Genetic diversity among progenies of Crambe abyssinica Hochst. for seed traits. Ind. Crop Prod., 50, 771-775, doi.org/10.1016/j.indcrop.2013.07.039
Li T., Wang R., Zhao D. & Tao J., 2020. Effects of drought stress on physiological responses and gene expression changes in herbaceous peony (Paeonia lactiflora Pall.). Plant Signaling Behav., 15, 5, doi.org/10.1080/15592324.2020.1746034
Lin Z.-H. et al., 2009. CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol., 9, 43, doi.org/10.1186/1471-2229-9-43
Liu C. et al., 2010. Influence of drought intensity on the response of six woody species subjected to successive cycles of drought and rewatering. Physiol. Plant., 139, 39-54, doi.org/10.1111/j.1399-3054.2009.01341.x
Marcos F.C.C. et al., 2018. Drought tolerance of sugarcane is improved by previous exposure to water deficit. J. Plant Physiol., 223, 9-18, doi.org/10.1016/j.jplph.2018.02.001
Martins R.F.A., Souza A.F.C., Pitol C. & Falqueto A.R., 2017. Physiological responses to intense water deficit in two genotypes of crambe (Crambe abyssinica Hochst.). Aust. J. Crop Sci., 11, 821-827, doi.org/10.3316/informit.091182519373093
Mehta P., Jajoo A., Mathur S. & Bharti S., 2010. Chlorophyll a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol. Biochem., 48, 16-20, doi.org/10.1016/j.plaphy.2009.10.006
Menezes-Silva P.E. et al., 2017. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot., 68, 4309-4322, doi.org/10.1093/jxb/erx211
Meng L.L. et al., 2016. Effects of drought stress on fluorescence characteristics of photosystem II in leaves of Plectranthus scutellarioides. Photosynthetica, 54, 414-421, doi.org/10.1007/s11099-016-0191-0
Mickelbart M.V., Hasegawa P.M. & Bailey-Serres J., 2015. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet., 16, 237-251, doi.org/10.1038/nrg3901
Oliveira I., Meyerb A., Afonso S. & Gonçalves B., 2018. Compared leaf anatomy and water relations of commercial and traditional Prunus dulcis (Mill.) cultivars under rain-fed conditions. Sci. Hortic., 229, 226-232, doi.org/10.1016/j.scienta.2017.11.015
Shin Y.K., Bhandari S.R., Cho M.C. & Lee J.G., 2020. Evaluation of chlorophyll fluorescence parameters and proline content in tomato seedlings grown under different salt stress conditions. Hortic. Environ. Biotechnol., 61, 433-443, doi.org/10.1007/s13580-020-00231-z
Stirbet A. & Govindjee V., 2011. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol., B, 104, 236-257, doi.org/10.1016/j.jphotobiol.2010.12.010
Strasser B.J., 1997. Donor side capacity of photosystem II probed by chlorophyll a fluorescence transients. Photosynth. Res., 52, 147-155, doi.org/10.1023/A:1005896029778
Strasser R., Srivastava A. & Tsimilli-Michael M., 2000. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M., Pathre U. & Mohanty P., eds. Probing photosynthesis: mechanism, regulation and adaptation. Bristol: Taylor & Francis, 445-483, doi.org/10.1007/978-1-4020-3218-9_12
Strasser R.J., Michael M.T. & Srivastava A., 2004. Analysis of the chlorophyll a fluorescence transient. In: George C., Govindje V. & Papageorgiou G.C., eds. Chlorophyll a fluorescence: a signature of photosynthesis. Springer: The Netherlands, 321-362.
Takahashi S. & Murata N., 2008. How do environmental stresses accelerate photoinhibition? Trends Plant Sci., 13, 178-182, doi.org/10.1016/j.tplants.2008.01.005
Takahashi S. & Badger M.R., 2011. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci., 16, 1360-1385, doi.org/10.1016/j.tplants.2010.10.001
Toscano S., Ferrante A. & Romano D., 2019. Response of Mediterranean ornamental plants to drought stress. Horticulturae, 5, 6, doi.org/10.3390/horticulturae5010006
Trabelsi L. et al., 2019. Impact of drought and salinity on olive water status and physiological performance in an arid climate. Agric. Water Manage., 213, 749-759, doi.org/10.1016/j.agwat.2018.11.025
Verlues P.E. et al., 2006. Methods and concepts in quantifying resistance to drought salt and freezing, abiotic stress that affect plant water status. Plant J., 45, 523-539, doi.org/10.1111/j.1365-313X.2005.02593.x
Wang Y.W. et al., 2016. Chlorophyll a fluorescence analysis of high-yield rice (Oryza sativa L.) LYPJ during leaf senescence. Photosynthetica, 54, 422-429, doi.org/10.1007/s11099-016-0185-y
Wu Y., Hong W. & Chen Y., 2018. Leaf physiological and anatomical characteristics of two indicator species in the limestone region of southern China under drought stress. Pak. J. Bot., 50, 1335-1342.
Zanetti F. et al., 2016. Crambe abyssinica a non-food crop with potential for the Mediterranean climate: insights on productive performances and root growth. Ind. Crop Prod., 90, 152-160, doi.org/10.1016/j.indcrop.2016.06.023
Zhang S. et al., 2018a. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric., 17, 336-347, doi.org/10.1016/S2095-3119(17)61758-1
Zhang J. et al., 2018b. Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol., 176, 2082-2094, doi.org/10.1104/pp.17.01432






