- Accueil
- Volume 26 (2022)
- Numéro 3
- Detarium microcarpum Guill. & Perr. fruit properties, processing and food uses. A review
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Detarium microcarpum Guill. & Perr. fruit properties, processing and food uses. A review

Document(s) associé(s)
Version PDF originaleRésumé
Propriétés, transformation et utilisations alimentaires des fruits de Detarium microcarpum Guill. & Perr. (synthèse bibliographique)
Introduction. Detarium microcarpum est une plante fruitière ligneuse, largement présente dans les forêts de l'Afrique subsaharienne. Cette revue présente les propriétés nutritionnelles et fonctionnelles des fruits de D. microcarpum et leurs utilisations dans différents produits alimentaires.
Littérature. Les deux parties comestibles du fruit de D. microcarpum sont la pulpe et la graine. Ces pulpes et graines ont un taux de glucides presque identique (40,2-66,1 %). La pulpe contient des protéines (2,9-6,1 %), des lipides (0,7-2,2 %), des minéraux : magnésium (51-84 mg·100 g-1), potassium (1 017 mg·100 g-1) et des vitamines (vitamine C : 4,6-4,7 mg·100 g-1). La graine aussi contient des protéines (34,5-37,1 %), des lipides (23 %), des minéraux et des vitamines (vitamine C : 9,3-28,1 mg·100 g-1). La poudre des graines présente des propriétés fonctionnelles : masse volumique apparente (0,5-0,9 g·cm-3), capacité d'absorption d'eau (3,5-11,7 ml·g-1), capacité d'absorption d'huile (0,7-3,1 ml·g-1), solubilité (2,0-3,0 %), capacité d'émulsion (4,3-4,4 %), stabilité de l'émulsion (3,4-3,5 %), pouvoir moussant (8,7-8,8 cm3) et stabilité de la mousse (7,0-9,3 cm3) ; elle améliore également la viscosité de la pâte. La pulpe de D. microcarpum est utilisée dans les jus et confitures, tandis que la graine est utilisée comme stabilisant ou épaississant dans le jus, la confiture, la sauce tomate, la sauce traditionnelle, le pain blanc, le hamburger et la crème glacée.
Conclusions. La plupart des travaux étudiés sur la graine de D. microcarpum n’ont pas exploré au-delà des propriétés de sa gomme. De même, ceux sur l’utilisation de la pulpe n’ont abordé que le jus et la confiture. Il se présente dès lors une large possibilité de recherche dans le contexte de la formulation des aliments à base des fruits de D. microcarpum.
Abstract
Introduction. Detarium microcarpum is a woody fruit plant, widely found in the dense dry and clear forests of sub-Saharan Africa. This review presents the nutritional and functional properties of D. microcarpum fruits pulp and seed, as well as their uses in food products.
Literature. The two edible parts of the fruit of D. microcarpum are pulp and seed. These pulp and seed have an almost identical carbohydrate content (40.2-66.1%). Pulp contains proteins (2.9-6.1%), lipids (0.7-2.2%), and minerals: magnesium (51-84 mg·100g-1), potassium (1,017 mg·100g-1) and vitamins (vitamin C: 4.6-4.7 mg·100g-1). Seed also contains proteins (34.5-37.1%), lipids (23%), minerals and vitamins (vitamin C: 9.3-28.1 mg·100g-1). Seed powder exhibits functional properties such as bulk density (0.5-0.9 g·cm-3), water absorption capacity (3.5-11.7 ml·g-1), oil absorption capacity (0.7-3.1 ml·g-1), solubility (2-3%), emulsion capacity (4.3-4.4%), emulsion stability (3.4-3.5%), foaming capacity (8.7-8.8 cm3) and foam stability (7.0-9.3 cm3); it also improves the viscosity of dough. Pulp is used in juice and jam, while seeds are used as a stabilizer or as a thickener in juice, jam, tomato sauce, traditional soup, white bread, raw beef burger, and ice cream.
Conclusions. Most studied works on the seed of D. microcarpum did not explore beyond the properties of its gum. Likewise, those on the pulp only approached its use in juice and jam. Therefore, it presents a large possibility for research in the context of the formulation of foods containing D. microcarpum fruits.
Table des matières
Received 6 June 2021, accepted 27 June 2022, available online 3 October 2022
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1The World Health Organization reports that “wild” plants may support the health and nutritional needs of 80% of people living in developing countries (Van Andel, 2006). Non-timber forest products (NTFPs) would therefore represent a considerable food and economic stake for local populations. In addition, it is recognized that rational exploitation of local resources, through improved processing technologies, would make a valid contribution to the sustainable development of the continent (Issaoui‐Mansouri, 2010). Detarium spp. were identified among the NTFPs of major importance in Africa. The genus Detarium spp. belongs to the class of Magnoliopsida, subclass of Rosidae, order of Fabales, family of Fabaceae, subfamily of Caesalpinioideae, tribe of Detarieae (Kouyate, 2005). Detarium has several species including Detarium microcarpum Guill. & Perr. (Akah et al., 2012). Detarium microcarpum inhabits dry lands, wooded savannas, and open forests of the Sudano-Guinean and Sudano-Sahelian zones of the African continent (Arbonnier, 2002). Its range extends from Senegal to Sudan, in particular in Benin, Burkina Faso, Cameroon, Côte d’Ivoire, Gambia, Ghana, Guinea, Guinea Bissau, Mali, Niger, Nigeria, Central African Republic, Senegal, Sudan and Chad (Kouyate, 2005). It is known as: “sweet dattock” or “tallow tree” or “sweet Detar” in English and “Détar sucré” or “petit Détar” in French. It is a fruit tree of great socio-economic importance in West Africa (Agbo et al., 2017). The pulp of this fruit is known to be rich in sugar, vitamin C and certain minerals (Makalao et al., 2016). The seed contained in the stone of the fruit is also rich in nutritional and functional properties and has the particularity of being rich in hydrocolloids (59.8 g·100 g-1) which gives it binding and thickening properties (Uzomah & Odusanya, 2011). Indeed, D. microcarpum seeds powder revealed several functional and pasting properties such as: water and oil absorption, foaming ability, emulsion capacity, swelling power and very good aptness for use as stabilizer or thickening agent (Onweluzo et al., 1999a; Onweluzo et al., 1999b; Akpata & Miachi, 2001; Amandikwa et al., 2017; Peace & Adekunle, 2018). It is also proven that the processing method of D. microcarpum pulp or seed has a considerable effect on these different properties (Amandikwa et al., 2017; Peace & Adekunle, 2018; Michael et al., 2019). Consequently, this review aims to present an updated report of the nutritional and functional properties of pulp and seed of D. microcarpum fruit, to highlight the effect of processing methods on these properties and finally to make an inventory of their use in food products. To achieve these objectives, data collection has been done in scientific databases such as: Web of science, Scopus/Science Direct, Springer, Wiley, MDPI, and Google Scholar. The following expressions and keywords were used: Detarium microcarpum, fruit of Detarium microcarpum, seed of Detarium microcarpum, nutritional and functional properties of fruits of Detarium microcarpum, use of fruits of Detarium microcarpum. More than 120 original articles and 2 review articles were obtained. The selection according to the objectives made it possible to retain 43 original articles and 2 review articles. In the article analysis, priority was given to the most recent articles, especially those less than 10 years old. These selected articles were arranged by categories, which at the same time correspond to the structure of the development of this review, including:
2– generalities;
3– nutritional and functional properties;
4– effects of processes on the properties of D. microcarpum seed;
5– use of the pulp and seed of the fruits of D. microcarpum in food products.
2. Literature
2.1. Morphological characterization and conservation of D. microcarpum
6Detarium microcarpum is a shrub or small tree with an irregular crown, 5-10 m tall (Figure 1a). The tree is recognizable by its leaves, 15 cm long, paripinnate or imparipinnate (Arbonnier, 2002; Akah et al., 2012), with translucent ones. They contain four to twelve alternate or sub-opposite leaflets 11 cm long and 3 to 5 cm wide (Arbonnier, 2002). The bark is reddish brown, cracked on woody twigs, clear, smooth, and greenish yellow on young shoots; its flowers are grouped in axillary panicles 15 to 25 cm long and 6 to 10 cm wide (Kouyate, 2005; Akah et al., 2012). The fruit of D. microcarpum (Figure 1b) is a globular or sub globular, flattened drupe 3 to 8 cm in diameter. According to Kouyate (2005), it is made up of three main parts: the epicarp (dark green, hard for immature fruits, light green tending to brown and brittle for ripe fruits), the greenish mesocarp or pulp (Figure 1c), intermingled with fibers inserted on the stone corresponding to the edible portion of the fruit, and the stone (Figure 1d), woody, covered with fibrous meshes containing a single ovoid and flattened seed of dark brown color. The fruits develop between early January and May, while ripening happens between February and April in arid sub-Saharan Africa from Senegal east to Sudan (Kouyate & Lamien, 2011). In Mali, the average fruit production of a population of D. microcarpum possessing 268 trees·ha-1 reaches 1.6 ton·ha-1 of fruits (Kouyate et al., 2016). Detarium microcarpum is among threatened species (Agbo et al., 2017). The main threats facing Detarium species are the massive harvesting of leaves, roots and stems and especially logging for wood. Excessive logging, mainly for fuel, the expansion and intensification of agriculture, and uncontrolled fires prevent regeneration in D. microcarpum. Today, trees with trunks larger than 30 cm in diameter are rarely found within a 10 km radius of villages (Kouyate & Lamien, 2011). Perhaps the best conservation strategy for D. microcarpum is to encourage the use of its fruits in food and also through a well-planned domestication process (Kouyate & Lamien, 2011). Identifying areas of abundance that can serve as in situ conservation areas and setting up seed banks would contribute to the conservation of D. microcarpum (Agbo et al., 2017). Detarium microcarpum can be propagated either by seed, or by stump rejection, or by sucker (Kouyate, 2005). The seeds are orthodox. Propagation by seed requires pretreatment with boiling water or sulfuric acid, then soaking in lukewarm water for 24 h or cutting the hard seed coat with a sharp object. Germination in situ is favored by the intervention of phytophagous termites which bury the fruit of Detarium microcarpum by their constructions and thus maintain a certain humidity around it, even on skeletal soils. By vegetative propagation, D. microcarpum has a great capacity for vegetative multiplication by regeneration in coppice and suckering of stumps or roots. Also lateral root segments, measuring 20 cm in length and 15 to 60 mm in diameter and from mature cultivated trees, can be used for cuttings of the species in the nursery (Kouyate, 2005).
 Figure 1. Detarium microcarpum: tree (a), fruits (b), pulp (c) and seeds (d) — Detarium microcarpum: arbre (a), fruits (b), pulpe (c) et graines (d).
Figure 1. Detarium microcarpum: tree (a), fruits (b), pulp (c) and seeds (d) — Detarium microcarpum: arbre (a), fruits (b), pulpe (c) et graines (d).
Source : D. Tchatcha.
2.2. Nutritional and functional properties of D. microcarpum fruit pulp and seed
7Pulp. The fruit pulp of D. microcarpum contains a relatively low moisture (Table 1). The values are between 7.1 and 12.2% (Kini et al., 2010; Oibiokpa et al., 2014; Makalao et al., 2016). The carbohydrate content is the highest among the main constituents, it varies from 40.1 to 65.4% (Mariod et al., 2009; Oibiokpa et al., 2014; Makalao et al., 2016) and represents more than 81.2% of the dry matter of dried pulp (Kini et al., 2010). Proteins vary from 2.9 to 6.12% (Kouyate et al., 2009; Kini et al., 2010; Oibiokpa et al., 2014; Makalao et al., 2016). However, Mariod et al. (2009) found a protein level of up to 30.0% ± 0.4 on the pulp of D. microcarpum fruits collected in Sudan. The lipid content of the pulp of D. microcarpum is between 0.7 and 2.2% (Mariod et al., 2009; Kini et al., 2010; Oibiokpa et al., 2014; Makalao et al., 2016). The vitamin C content varies from 4.6 to 4.7 mg·100 g-1 (Kouyate et al., 2009; Makalao et al., 2016), but Oibiokpa et al. (2014) found 55.1 mg·100 g-1. Magnesium (51-84 mg·100 g-1), a fundamental trace element for the cell, is also present at interesting levels in the pulp (Kini et al., 2010; Makalao et al., 2016). The pulp of D. microcarpum fruit is also very rich in potassium (1.017 mg·100 g-1) (Kini et al., 2010). The potassium content is comparable to those of almond fruits (600 mg·100 g-1) and potatoes (500 mg·100 g-1) classified as foods rich in potassium (Alais & Linden, 1991). According to Oibiokpa et al. (2014) and Umaru et al. (2007), the fruit of D. microcarpum, like most wild fruits, contains small amount of anti-nutritional compounds such as: saponin (1.2-2.7 mg·100 g-1), oxalate (1.1-1.3 mg·100 g-1), phytate (0.2-0.4 mg·100 g-1), tannins (0.2-0.4 mg·100 g-1) and cyanides (0.1 mg·100 g-1).
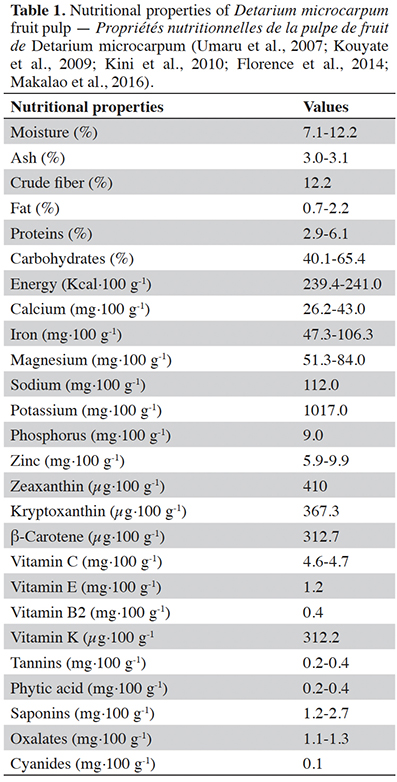
8With regard to the nutritional contribution of the pulp of D. microcarpum fruit, the contents of vitamin C, calcium, magnesium and protein are lower than the daily allowances recommended by FAO/WHO (vitamin C, 45 mg·day-1; calcium, 1,000 mg·day-1; magnesium, 220-260 mg·day-1; protein, 13.5-15.5 g·day-1. However, the iron content (47.3-106.3 mg·100 g-1) can meet the daily intake recommended by FAO/WHO (2004), 27.4-58.8 mg·day-1. In addition, the zinc content (5.9-9.9 mg·100 g-1) is lower than the daily intake recommended by FAO/WHO for girls aged 10-18 (14.4 mg·day-1) and men aged 10-65 years and over (14-17.1 mg·day-1), but may satisfy women aged 19 to 65 and over (9.8 mg·day-1). This is the reason why Makalao et al. (2016) and Oibiokpa et al. (2014) concluded that the integration of D. microcarpum fruit pulp in combination with other foodstuffs in the daily diet could overcome the challenges associated with the lack of carbohydrates in terms of energy, mineral and vitamin C intakes.
9Seed. Several studies show that D. microcarpum seeds contain (Table 2) a relatively low moisture, values ranging from 2.2 to 16.7% (Akpata & Miachi, 2001; Uhegbu et al., 2009; Amandikwa et al., 2017; Peace & Adekunle, 2018; Michael et al., 2019). Low values (2.2-9.9%) were found by Akpata & Miachi (2001), Peace & Adekunle (2018), Michael et al. (2019) and the highest values (11.7-16.7%) were found by Uhegbu et al. (2009) and Amandikwa et al. (2017). The sugar content is the highest among the main constituents, ranging from 39.0 to 66.1% dry matter (Akpata & Miachi, 2001; Anhwange et al., 2004; Uhegbu et al., 2009; Amandikwa et al., 2017; Peace & Adekunle, 2018). For proteins, the highest levels have been evaluated between 34.5 and 37.1% by Akpata & Miachi (2001), Anhwange et al. (2004); the lowest values (8.8-17.7%) were found by Uhegbu et al. (2009), Amandikwa et al. (2017), Peace & Adekunle (2018), Michael et al. (2019). For fats, they range from 1.01% (Peace & Adekunle, 2018) to 23% (Amandikwa et al., 2017). Other authors found intermediate values (Akpata & Miachi, 2001; Anhwange et al., 2004; Uhegbu et al., 2009; Michael et al., 2019). The fat content of D. microcarpum seeds is low (about 7%). Apart from oleic acid (29.1%), behenic acid (25.4%), palmitic acid (12.5%) and lignoceric acid (11%), fat from the seeds of D. microcarpum contains beta-carotene, plant sterols, phospholipids and glycolipids (Sowemimo et al., 2011). Several toxicological studies show the absence of gossypol and detectable mycotoxins. The works of Anhwange et al. (2004) showed that the fat of the seeds of D. microcarpum does not oxidize quickly and remain a long time under liquid form. They also showed that this fat can be kept during a long period. These works allowed them to deduct that the fat of the seeds of D. microcarpum can be used in the human and animal food (Anhwange et al., 2004). Fairly similar values for fibers and ash were found, ranging between 1 and 7% (Akpata & Miachi, 2001; Uhegbu et al., 2009; Amandikwa et al., 2017; Peace & Adekunle, 2018; Michael et al., 2019). Most authors have used standard conventional methods for analyzing these key constituents. Detarium microcarpum seeds contain calcium, magnesium, iron, sodium, phosphorus and potassium with values ranging respectively from 0.185; 0.022; 0.01; 0.0125 and 0.15 g·100 g-1 to 0.35; 0.24; 0.031; 0.275 and 1.836 g·100 g-1 (Anhwange et al., 2004; Uhegbu et al., 2009; Amandikwa et al., 2017; Michael et al., 2019). The magnesium content of D. microcarpum seeds (0.24 g·100 g-1) is significantly higher than that of corn or oats (0.125 g·100 g-1) (Diop et al., 2010). Similarly, the calcium content (0.35 g·100 g-1) is higher than that of milk (0.125 g·100 g-1) (Diop et al., 2010). The iron content is of importance, 0.031 g·100 g-1, considering the minimum nutritional intakes recommended by FAO/WHO which are 0.009 g·day-1 for men to 0.027 g·day-1 for women (Alais & Linden, 1991). With regard to vitamins in D. microcarpum seeds, little work has been done. Nevertheless, Michael et al. (2019), reported vitamin A (986.3 to 5364.1 IU·100 g-1), vitamin B1 (0.18-0.36 mg·100 g-1), vitamin B3 (0.09-0.29 mg·100 g-1, vitamin B6 (0.04-0.21 mg·100 g-1), vitamin C (9.32-28.05 mg·100 g-1) and vitamin D (0.25-0.42 mg·100 g-1).
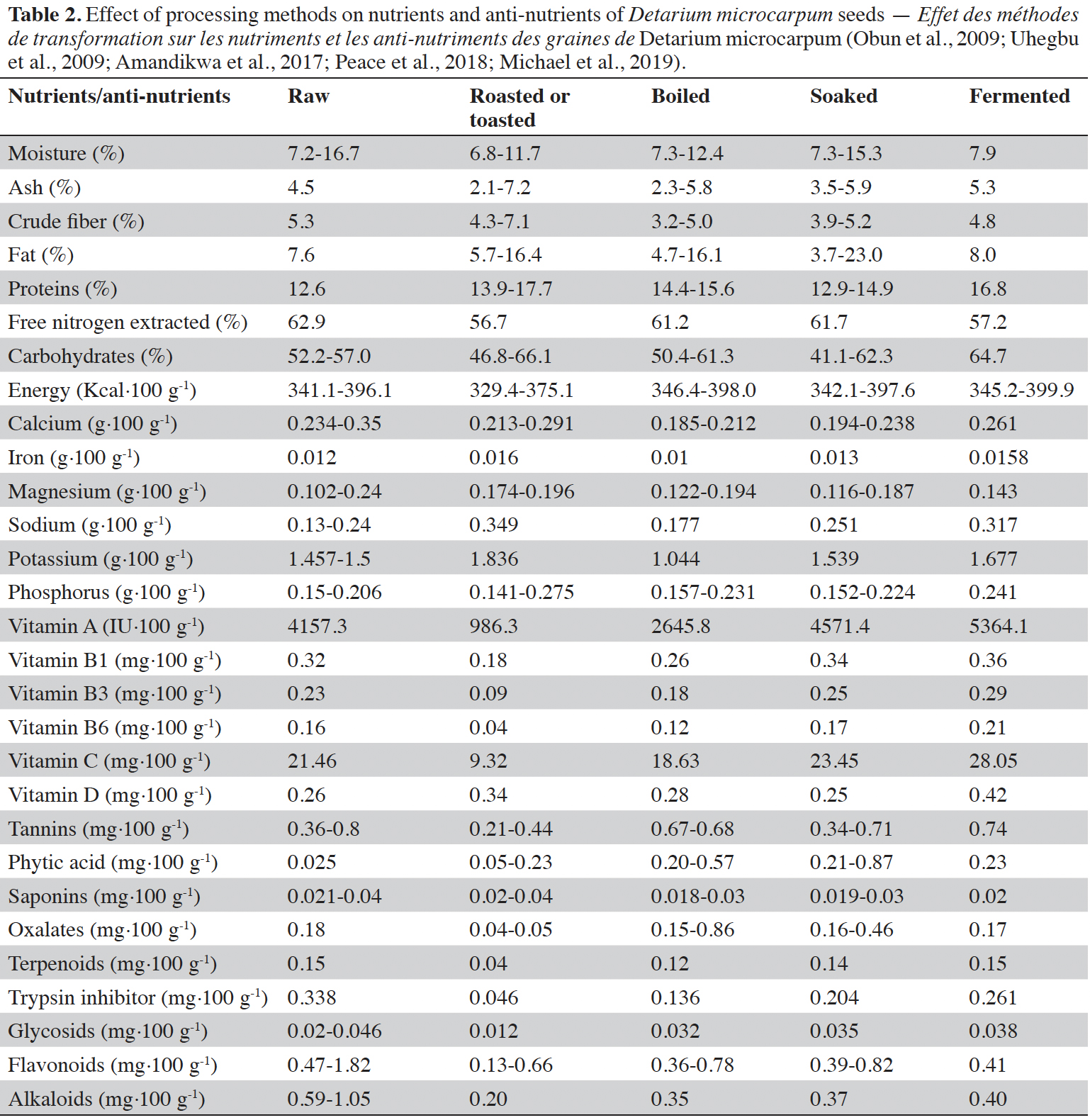
10Detarium microcarpum seeds contain some anti-nutrients that can be removed or inactivated by different processing methods. Anhwange et al. (2004), Uhegbu et al. (2009), Amandikwa et al. (2017), Peace & Adekunle (2018) and Michael et al. (2019) investigated the anti-nutrients in D. microcarpum seeds and found that they contain a trypsin inhibitor (0.338-0.46 mg·100 g-1), phytic acid (0.025-0.87 mg·100 g-1), tannins (0.67-0.74 mg·100 g-1), saponins (0.04-0.4 mg·100 g-1), oxalates (0.04-0.86 mg·100 g-1), terpenoids (0.04-0.15 mg·100 g-1), glycosids (0.12-0.25 mg·100 g-1), flavonoids (0.13-1.82 mg·100 g-1) and alkaloids (0.2-1.05 mg·100 g-1). Amandikwa et al. (2017), Peace et al. (2018) and Michael et al. (2019) investigated the effects of some processing methods on the anti-nutrients composition of D. microcarpum seeds and found that roasting, soaking and boiling treatments reduced the anti-nutrients concentration in D. microcarpum kernels significantly. However, dehulling did not lead to significant decreases. Moreover, fermentation reduced the anti-nutrients contents of D. microcarpum seeds (Michael et al., 2019). Akpata & Miachi (2001), Owuamanam et al. (2016), Amandikwa et al. (2017) and Peace & Adekunle (2018) have found the following functional and pasting properties: bulk density (0.51-0.92 g·cm-3), water absorption capacity (3.47-11.73 ml·g-1), oil absorption capacity (0.73-3.13 ml·g-1), solubility (2.02-3.01%), emulsion activity (4.25-4.40%), emulsion stability (3.44-3.54%), foaming capacity (8.70-8.75 cm3) and foaming stability (7.0-9.30 cm3). According to Uzomah & Odusanya (2011), these functional and pasting properties of D. microcarpum seeds are due to their richness in hydrocolloids (water-soluble non-starch polysaccharides and proteins), mainly xyloglucans (59.8 g·100 g-1). Thanks to these hydrocolloids, the flour obtained from the seeds of D. microcarpum shows promise as thickening and gelling agent.
2.3. Effect of processing methods on D. microcarpum seeds nutritional and functional properties
11The effects of different processing methods of seeds of D. microcarpum such as: roasting, boiling, soaking and fermentation on chemical and nutritional characteristics of the flours obtained were reported, a summarizing table being included (Table 2). According to Michael et al. (2019), the minimum values of water content (6.8%) are obtained with roasting of the seeds, on the other hand the highest values are obtained with soaking (15.3%). In terms of proteins and carbohydrates, the variations are not very noticeable between the different processing methods, but there is a difference with raw seeds (Michael et al., 2019). Regarding lipids, the values of raw seeds and fermented seeds are similar, on the other hand those of roasted, boiled and soaked seeds are different. The maximum values are between 7.6-8.0% in the first case and 16-23% in the second case. An increase in vitamins is observed in soaked and fermented seeds compared to raw seeds. Roasting the seeds dramatically reduces anti-nutrient levels compared to all other processing methods. For seeds of D. microcarpum, roasting would be the best method of removing anti-nutritional compounds (Amandikwa et al., 2017; Michael et al., 2019).
12Peace & Adekunle (2018) and Michael et al. (2019) found that seed processing methods improve their functional and pasting properties. Indeed, among all the functional and pasting properties, it is only at the level of the apparent density that the raw seed recorded a higher value than the transformed seeds: 0.92 g·cm-3 for the raw seed against 0.78 g·cm-3 for those fermented (Akpata & Miachi, 2001; Uzomah & Odusanya, 2011; Peace & Adekunle, 2018; Michael et al., 2019). For water absorption, oil absorption and foaming capacities, roasted seeds have the highest values (Table 3).
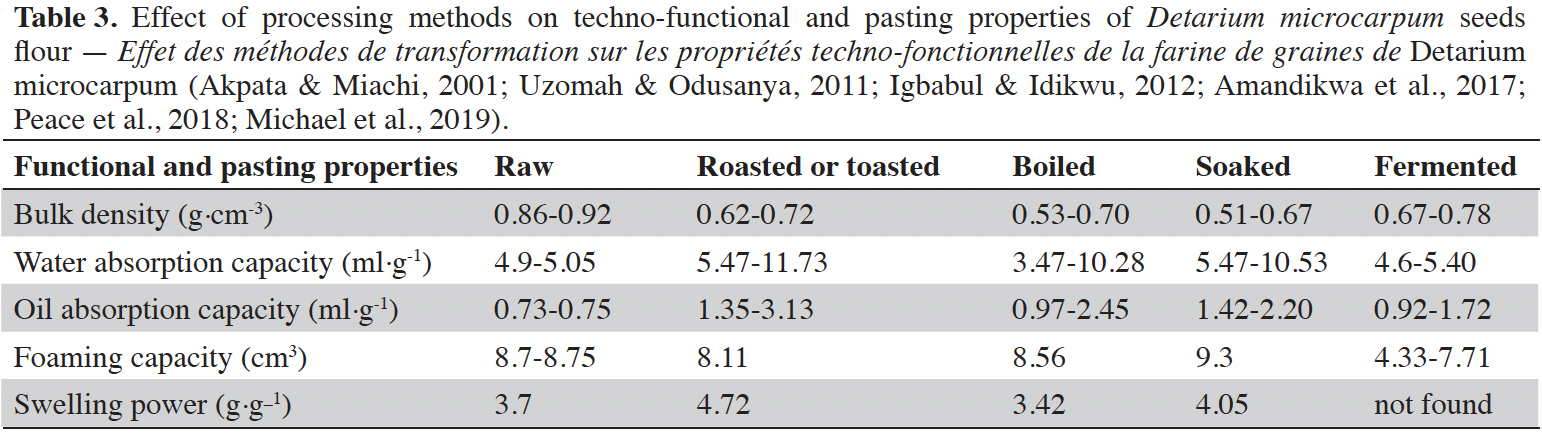
2.4. Utilization of Detarium microcarpum pulp and seed in food products
13Beverages. The first products one lean towards when it comes to fruit are drinks. The pulp and seed of D. microcarpum make no exception to this rule. Indeed, among the works studied, several used the pulp or the seed in the production of drinks. Mariod et al. (2009) used the pulp to produce juice and jams in Sudan. To produce juice, they took the powder from the pulp of D. microcarpum and added water, sugar and citric acid (Table 4), obtaining a juice concentrated at 56% of total soluble solid. They also produced jam from the pulp of D. microcarpum, by adding water, sucrose, and pectin. The sensory properties of the jam and juice obtained were evaluated by panelists, the two products being accepted and appreciated for their color, smell, taste and texture. These products based on D. microcarpum pulp were preferred over control samples which are products already found on the market in Sudan. However, apart from the work of Mariod et al. (2009), who used the pulp of D. microcarpum to produce juice and jam, we did not find any other works that used the pulp of D. microcarpum for the production of other food products. Nevertheless, the seed of D. microcarpum has been used in the production of several food products, mainly as a stabilizer. Detarium microcarpum seed powder has been used as a stabilizer in pineapple jam, mango and orange juices (Onweluzo et al., 1999). These different juices and jam were stabilized for two months at room temperature (26 °C ± 2) with the gum of the seed of D. microcarpum and pectin, the products being highly appreciated by the evaluators.
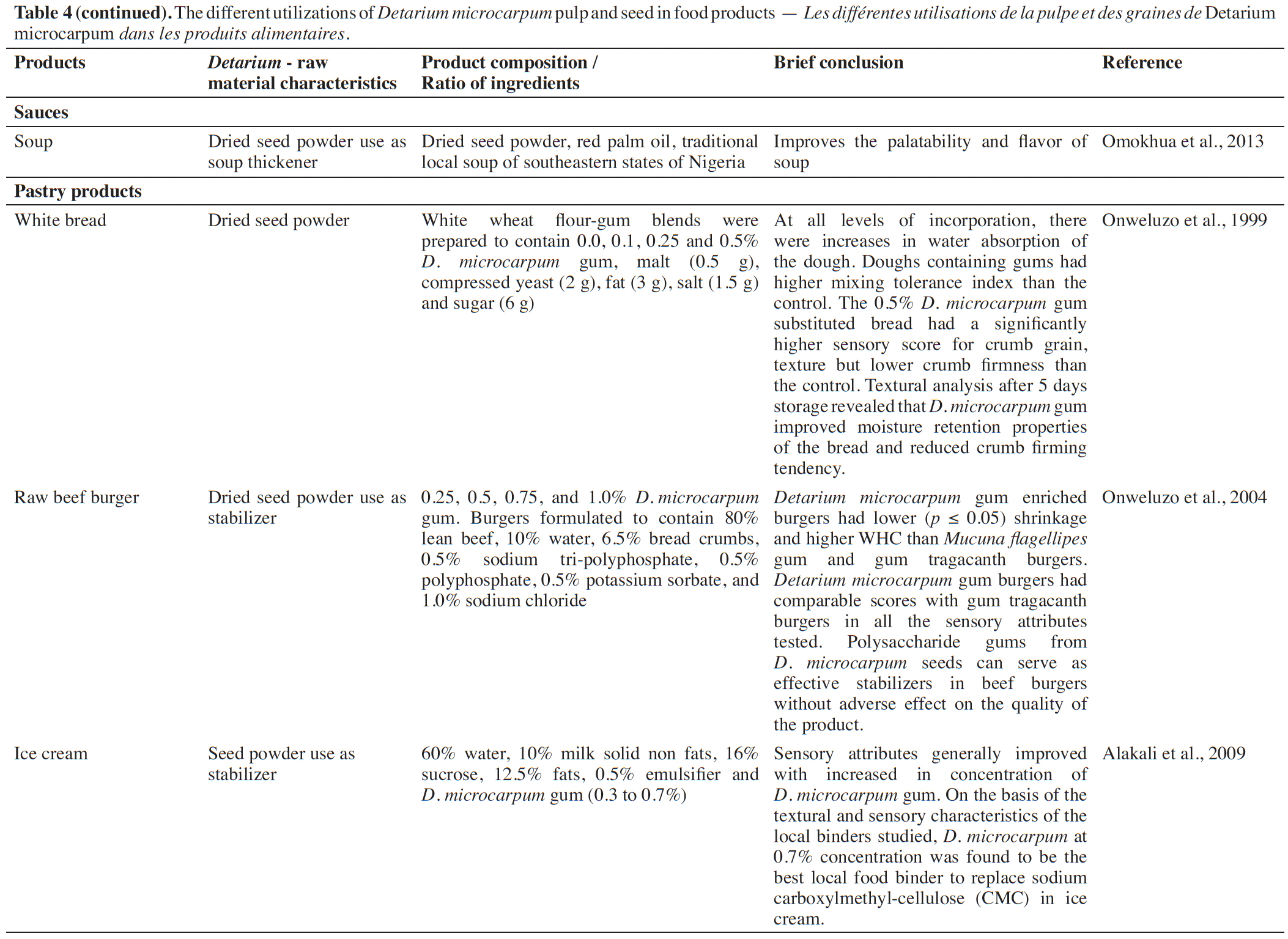
14Sauces. Powder from the seed of D. microcarpum has been used either as a stabilizer in tomato sauce (Onweluzo et al., 1999) or as a thickener in traditional sauces in southeastern Nigeria (Omokhua et al., 2013). Onweluzo et al. (1999) extracted the gum from the seed of D. microcarpum, adding 10 g·l-1 on tomato puree for stabilizing purposes (Table 4). This sauce was much appreciated even after two months of conservation in glass bottle maintained at room temperature (26 °C ± 2). For soup (traditional sauce from South-East Nigeria), Omokhua et al. (2013) observed an improvement in palatability and flavor compared to traditional sauces without the addition of powder from the seed of D. microcarpum.
15Pastry products. The gum extracted from the seed of D. microcarpum was already used as stabilizer in white bread, raw beef burger and ice cream. Onweluzo et al. (1999) used 0.5% D. microcarpum gum in the white bread preparation and obtained a product very appreciated for the crumb and the texture; however, the firmness values of the crumb were lower than that of control bread. After five days of storage, the bread with 0.5% D. microcarpum gum, Onweluzo et al. (1999) observed an improvement in the water retention of the bread which even tended to firm up. To use D. microcarpum gum as a stabilizer in raw beef burger, Onweluzo et al. (2004) experimented four formulations (0.25; 0.5; 0.75 and 1% of D. microcarpum gum). All used ratios improved the quality and stability of the product; however, it was concluded to use D. microcarpum gum at a maximum of 0.75% in the production of the raw beef burger, for better quality and good economic return. Alakali et al. (2009) used 0.3 to 0.7% D. microcarpum gum as a stabilizer in ice cream, observing that the higher the gum concentration of D. microcarpum, the better the quality attributes of the ice cream. Alakali et al. (2009) concluded that the use at 0.7% concentration of D. microcarpum gum was the best alternative to replace sodium carboxylmethyl-cellulose (CMC) in ice cream.
3. Conclusions
16This review shows that the reported values of macronutrients, minerals, vitamins, anti-nutrients, functional and pasting properties of D. microcarpum pulp and seeds vary considerably. These variations may be due to the quality of the samples (mixing of samples, or samples obtained on the markets or samples from different trees), origin of samples, age of samples and treatment before analysis, analytical methods used, storage conditions, treatment method, genetic variation, soil structure and chemical composition. Food composition can be greatly influenced by environment such as type of soil, soil fertility, water or intensity of sunlight. Indeed, Diop et al. (2010) characterized Detarium fruits from six different zones of Senegal and observed variations in the results obtained caused by the variability of the raw material, including habitat, maturity and storage conditions of samples. Furthermore, the investigated literature shows that the analyzed samples were selected and handled differently. For instance, some researchers purchased their D. microcarpum fruits or seeds from local markets, e.g. Akpata & Miachi (2001), Uhegbu et al. (2009) and Owuamanam et al. (2016). Authors who bought D. microcarpum seeds from the market mostly found the highest values. For example, the highest values of proteins of seeds have been evaluated between 34.48 and 37.1% by Akpata & Miachi (2001) and Anhwange et al. (2004), who bought the seeds at the market; lowest values (8.8-17.70%) were found by Amandikwa et al. (2017), Peace & Adekunle (2018) and Michael et al., (2019), who collected the seeds in the field. It was also observed that researches published in the last ten years reported the lowest values intervals for the main chemical constituents of Detarium fruits. This could be explained by the evolution of technology over the last ten years; more efficient devices have been developed and analysis methods have become more precise. In all the reviewed literature, no studies on the effect of processing methods on the pulp properties of D. microcarpum have been encountered.
17Overall the current work bring together up-to-date knowledge for understanding that the fruit of D. microcarpum contains essential nutrients (carbohydrates, potassium, and iron, and vitamins, seed gum) which justifies the technological and nutritional interest of this wild plant. The work carried out has deeply investigated these properties and explored several areas for valuing both the pulp and the seed in the formulation of food products. Several studies have confirmed that seed processing methods influence nutritional and functional properties. These effects are mostly advantageous because they reduce the level of anti-nutritional compounds and improve certain functional properties. Consequently, boiled and soaked seeds are recommended for use as a thickener for sauces, while roasted seeds are recommended for use in pastry products. The specificity observed through the various studies on the use of the fruit of D. microcarpum in food products is that they have mostly extracted the gum from the seed for use. Almost all of the works studied on the use of the seed has always extracted the gum and it is this gum alone that is exploited. This work has not explored the other properties of the seed, so it is limited to the gum. Likewise, the work studied on the use of pulp in food products has also been limited to juices and jam. Similarly, few works have been encountered on the quantities of D. microcarpum fruit available, the current quantitative use and the quantities available for a future development in countries where the plant is present. Therefore, a wide range of research possibilities in the context of the formulation of foods containing the pulp or the whole seed of D. microcarpum fruit and statistical data on the quantities of the fruit of D. microcarpum might be open.
Acknowledgements
18This work received financial support from the University of Abomey-Calavi through the Competitive Research Fund Program at the University of Abomey-Calavi (PFCR/UAC, 3rd phase) 2019-2021 and from the Olga Triballat Institute through the Research Projects Award Program, which we sincerely thank.
19We also thank the Francophone University Agency (AUF) and the Faculty of Food Science and Technology of the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca for their support through the “Eugen Ionescu” Mobility Scholarship.
20This work was supported by a grant of the Romanian Ministry of Education and Research, CCCDI - UEFISCDI, project number PN-III-P2-2.1-PED-2019-5346, within PNCDI III.
Bibliographie
Agbo I.R. et al., 2017. Impacts des usages traditionnels sur la vulnérabilité de Detarium microcarpum Guill. & Perr. (Caesalpiniaceae) dans le district phytogéographique Zou au Bénin (en Afrique de l’Ouest). Int. J. Biol. Chem. Sci., 11(2), 730, doi.org/10.4314/ijbcs.v11i2.16
Akah P. et al., 2012. Genus Detarium: ethnomedicinal, phytochemical and pharmacological profile. Phytopharmacology, 2(3), 367-375, http://inforesights.com/phytopharmacology/archive.html, (10/03/2021).
Akpata M.I. & Miachi O.E., 2001. Proximate composition and selected functional properties of Detarium microcarpum. Plant Foods Hum. Nutr., 56(4), 297-302, doi.org/10.1023/A:1011836332105
Alais C. & Linden G., 1991. Food biochemistry. 1st ed. Springer.
Alakali J.S., Okonkwo T.M. & Uwu L.U., 2009. Effect of food binders on the textual and sensory characteristics of ice cream. Afr. J. Biotechnol., 8(12), 2853-2856.
Amandikwa C., Bede E.N. & Eluchie C.N., 2017. Effects of processing methods on proximate composition, mineral content and functional properties of “Ofor” (Detarium microcarpum) seed flour. Int. J. Sci. Res., 6(5), 966-970, doi: 10.21275/ART20172977.
Anhwange B.A., Ajibola V.O. & Oniye S.J., 2004. Chemical studies of the seeds of Moringa oleifera (Lam) and Detarium microcarpum (Guill and Sperr). J. Biol. Sci., 4(6), 711-715, doi.org/10.3923/jbs.2004.711.715
Arbonnier M.A., 2002. Arbres, arbustes et lianes des zones sèches d’Afrique de l’Ouest. Montpellier, France : CIRAD.
Diop N. et al., 2010. Le ditax (Detarium senegalense J.F. Gmel.) : principales caractéristiques et utilisations au Sénégal. Fruits, 65(5), 293-306, doi.org/10.1051/fruits/2010025
FAO/WHO, 2004. Vitamin and mineral requirements in human nutrition. 2nd ed. Roma: FAO; Geneva, Switzerland: World Health Organization.
Igbabul B.D. & Idikwu H.O., 2012. Effect of fermentation on some functional properties of Mucuna sloanei and Detarium microcarpum. J. Food Technol., 10(3), 83-86, doi.org/10.3923/jftech.2012.83.86
Issaoui‐Mansouri K., 2010. Souveraineté alimentaire : un concept en émergence, http://redtac.org/possibles/files/2010/10/S1-C1-IssaouiB.pdf, (24/08/2022).
Kini F., Ouédraogo S. & Guissou P.I., 2010. Propriétés nutritionnelles et thérapeutiques du fruit de Detarium microcarpum Guill. & Perr. Fruit Veg. Cereal Sci. Biotechnol, 4(special issue), 26-30.
Kouyate A.M., 2005. Aspects ethnobotaniques et étude de la variabilité morphologique, biochimique et phénologique de Detarium microcarpum Guill. & Perr. au Mali. Thèse de doctorat : Ghent University (Belgique).
Kouyate A.M., Van Damme P., De Meulenaer B. & Diawara H., 2009. Contribution des produits de cueillette dans l’alimentation humaine. Cas de Detarium microcarpum. Afrika focus, 22(1), 77-88, doi.org/10.1163/2031356X-02201007
Kouyate A.M. & Lamien N., 2011. Conservation and sustainable use of genetic resource of priority food tree species in sub-saharan Africa Detarium microcarpum, https://citarea.cita-aragon.es/citarea/bitstream/10532/1687/1/2011_343EN.pdf (10/3/2021).
Kouyate A.M., Nacoulma B.M.I., Lykke A.M. & Thiombiano A., 2016. Estimation de la production fruitière des espèces ligneuses alimentaires en Afrique sub-saharienne. Ann. Sci. Agron., 20, 69-78.
Makalao M.M., Savadogo A., Zongo C. & Traore A.S., 2016. Composition nutritionnelle de 10 fruits sauvages consommés dans trois départements du Tchad. Int. J. Biol. Chem. Sci., 9(5), 2385-2400, doi.org/10.4314/ijbcs.v9i5.11
Mariod A.A., Mirghani M.E.S., Abdul A.B. & Abdelwahab S.I., 2009. Detarium microcarpum Guill and Perr fruit proximate chemical analysis and sensory characteristics of concentrated juice and jam. Afr. J. Biotechnol., 8(17), 4217-4221.
Michael K., Sogbesan O.A., Onyia L.U. & Kefas M., 2019. Effect of processing methods on the nutritional and anti-nutritional value of Detarium microcarpum (Guill and Sperr) seed meals. Int. J. Appl. Res., 5(5), 68-72, allresearchjournal.com/archives/2019/vol5issue5/PartB/5-4-3-364.pdf
Obun C.O., Yahaya S. & Lekene B., 2009. Evaluation of nutritive value of processed and unprocessed Detarium microcarpum (Guill and Sperr) seed meal fed to broiler chicks. J. Agric. For. Social Sci. (JOAFSS), 7(1), 214-222.
Oibiokpa F.I., Adoga G.I., Saidu A.N. & Shittu K.O., 2014. Nutritional composition of Detarium microcarpum fruit. Afr. J. Food Sci., 8(6), 342-350, doi.org/10.5897/AJFS2014.1161
Omokhua G.E., Abbey W.M. & Olaleye S.M., 2013. Use of indigenous technology in processing and utilization of non-timber forest product in south-eastern Nigeria. J. Agric. Soc. Res., 13(2), 68-75.
Onweluzo J.C., Vijayalakshmi M.R., Vijayanand P. & Eipeson W.E., 1999a. Detarium microcarpum polysaccharide as a stabilizer in processed fruit products. LWT Food Sci. Technol., 32(8), 521-526, doi.org/10.1006/fstl.1999.0592
Onweluzo J.C., Leelavathi K. & Haridas R.P., 1999b. Effect of Detarium microcarpum (Dm) and Mucuna flagellipes (Mf) gums on the quality of white bread. Plant Foods Hum. Nutr., 54(2), 173-182, doi.org/10.1023/a:1008111610481
Onweluzo J.C., Obanu Z.A. & Okwandu M.C., 2004. Potentials of gum from Detarium microcarpum (DM) and Mucuna flagellipes (MF) seeds as raw beef burger stabilizers. Plant Foods Hum. Nutr., 59(4), 137-141, doi.org/10.1007/s11130-004-0027-0
Owuamanam C.I. et al., 2016. Functional properties of seed flours of Detarium microcarpum and Mucuna sloanei as affected by sodium chloride and palm oil : a response surface methodology approach. Futo J. Ser., 2(2), 361-378.
Peace P.D. & Adekunle O.A., 2018. Effects of some processing methods on antinutritional, functional and pasting characteristics of Detarium microcarpum seed flours. Ann. Food Sci. Technol., 19(1), 69-78.
Sowemimo A.A. et al., 2011. Chemical composition, antimicrobial activity, proximate analysis and mineral content of the seed of Detarium senegalense JF Gmelin. Afr. J. Biotechnol., 10(48), 9875-9879, doi: 10.5897/ajb11.1416
Uhegbu O.F., Onwuchekwa C.C., Iweala E.E.J. & Kanu I., 2009. Effect of processing methods on nutritive and antinutritive properties of seeds of Brachystegia eurycoma and Detarium microcarpum from Nigeria. Pak. J. Nutr., 8(4), 316-320, doi.org/10.3923/pjn.2009.316.320
Umaru H.A., Adamu R., Dahiru D. & Nadro M.S, 2007. Levels of antinutritional factors in some wild edible fruits of Northern Nigeria. Afr. J. Biotechnol., 6(16), 1935-1938.
Uzomah A. & Odusanya O.S., 2011. Mucuna sloanei, Detarium microcarpum and Brachystegia eurycoma seeds: a preliminary study of their starch-hydrocolloids system. Afr. J. Food Sci., 5(13), 733-740, doi.org/10.5897/AJFS11.088
Van Andel T., 2006. Les produits forestiers autres que le bois d’œuvre : la valeur des plantes sauvages. Wageningen, Pays-Bas: Fondation Agromisa & CTA, https://publications.cta.int/fr/publications/publication/1338/index.html, (10 March 2021).






