- Startpagina tijdschrift
- Volume 26 (2022)
- Special issue : 150 years of CRA-W
- Re-authorization of gelatin and collagen of ruminant origin in non-ruminant feed: a new analytical challenge for the control of the feed ban
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Re-authorization of gelatin and collagen of ruminant origin in non-ruminant feed: a new analytical challenge for the control of the feed ban

Documenten bij dit artikel
Version PDF originaleRésumé
Ré-autorisation de la gélatine et du collagène de ruminant dans les aliments pour non-ruminants : un nouveau défi analytique pour le contrôle des restrictions dans les aliments pour bétail
Description du sujet. La révision récente des dispositions relatives à l’interdiction des protéines animales dans l’alimentation des animaux de rente autorise l’utilisation de collagène et de gélatine de ruminant dans les aliments à destination de non-ruminant. Cette levée favorisera l’utilisation de denrées alimentaires recyclées (Former Foodstuffs ou FFS) dans l’alimentation des porcs et des volailles.
Objectifs. L’étude visait à déterminer comment ces ingrédients pourraient avoir une incidence sur la capacité de détecter les protéines animales transformées (PAT) provenant de ruminants dans les aliments pour animaux en utilisant la méthode PCR officielle et d’évaluer la valeur ajoutée d’un protocole de spectrométrie de masse en développement.
Méthode. La présence d’ADN de ruminants dans des échantillons d’hydrolysat de collagène, de gélatine et de FFS prélevés dans l’industrie a été évaluée à l’aide de la méthode officielle d’extraction d’ADN et de PCR. Cela a permis d’évaluer les propriétés d’inhibition de PCR par la gélatine et le collagène. Les échantillons collectés ont également été soumis à une analyse protéomique par spectrométrie de masse (UHPLC-MS/MS) ciblant les protéines de ruminants, y compris le collagène, afin de distinguer les sous-produits de ruminants (non autorisés ou autorisés).
Résultats. Les résultats montrent la complémentarité des approches PCR et UHPLC-MS/MS dans le contexte de l’utilisation de denrées alimentaires recyclées dans l’alimentation animale. Leur combinaison a permis de prouver que la présence d’ADN de ruminants dans les échantillons contenant des FFS était plus liée à la présence de lait qu’à la présence de gélatine. Par contre, certains échantillons ont montré une augmentation de la valeur de cycle seuil (Ct) qui pourrait correspondre à un effet inhibiteur dû à l’ajout de gélatine.
Conclusions. Dans le contexte de l’économie circulaire, les FFS sont une source intéressante de nutriments pour l’alimentation animale. Toutefois, en raison de la présence de produits laitiers, il faut s’attendre à une interférence avec les méthodes officielles et de fausses suspicions de matières interdites. De plus, un effet de masquage de PAT de ruminant par les FFS est aussi possible.
Abstract
Description of the subject. A recent revision of the feed ban provisions authorizes the use of ruminant collagen and gelatin in feed for non-ruminant farmed animals. This authorization will promote the use of former foodstuffs (FFS) in poultry and pig feed.
Objectives. The study aimed to investigate how these ruminant materials could impact the capacity to detect processed animal proteins (PAP) of ruminant origin in feed using the official PCR method and to evaluate the added value of a mass spectrometry protocol in development.
Method. Presence of ruminant DNA in samples of collagen hydrolysate, gelatin and FFS collected from the industry was assessed using the official DNA extraction and PCR method. This allowed to evaluate the PCR inhibition properties of gelatin and collagen. The same samples were also submitted to a mass spectrometry-based proteomics (UHPLC-MS/MS) protocol targeting ruminant proteins, including collagen, to distinguish between ruminant by-products (unauthorized or authorized).
Results. The results show the complementarity of PCR and UHPLC-MS/MS approaches in the context of the use of former foodstuffs in animal feed. Their combination has allowed to evidence that the presence of ruminant DNA in samples containing FFS was more linked to the presence of milk than to the presence of gelatin. On contrary, some samples have shown an increase of the cycle threshold value (Ct) that could correspond to an inhibitory effect due to gelatin addition.
Conclusions. In the context of the circular economy, FFS is an interesting source of nutriment for animal feed. However, due to the presence of dairy ingredients, interference with official methods giving false suspicion of prohibited materials is to be expected. Furthermore, a masking effect of the presence of PAP due to a PCR inhibitory effect by FFS is also possible.
Inhoudstafel
Received 7 December 2021, accepted 7 December 2022, available online 20 December 2022
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1About 88 million tons of food are wasted every year in the EU accounting not only for economic damage but also climate impact (Council of the EU, 2016). It is therefore the ambitious goal of the European Commission (2021) to reduce food waste by 50% by 2030 in the frame of their recent ‘Farm to Fork’ strategy. According to the EU regulation (European Commission, 2017), former foodstuffs (FFS) are foodstuffs, other than catering reflux, which were manufactured for human consumption, but which are no longer intended for its consumption. These products of high nutritional value can be produced with biscuits, bread, chocolate, etc. Their reuse as feed may be a brick paving the way to better sustainability by recycling food waste in proteins of high nutritional value. In 2020, the European Food Safety Authority (EFSA) gave a green light to the use of ruminant collagen and gelatin in feed for non-ruminant farmed animals (EFSA Panel on Biological Hazards [BIOHAZ], 2020). Based on this text, the EU Commission has adopted a revision of the feed ban provisions authorizing the use of these ingredients (European Commission, 2021). This lifting would allow the use of former foodstuffs containing collagen hydrolysate and gelatin in feed.
2The subject of this study was to evaluate the possible implications of the recent modification of the legislation on the capacity to detect processed animal proteins (PAP) of ruminant origin in feed. Collagen hydrolysates, gelatins and FFS were collected with the support of the industry and ruminant DNA content was evaluated using the official DNA extraction and PCR method. Because gelatin and collagen are reputed to be powerful PCR inhibitors (Kim et al., 2000; Schrader et al., 2012; Liu et al., 2015), it was relevant to assess the possibility that traces of ruminant PAP in co-presence of collagenous material might not be detected using the same PCR protocol. In addition, the same samples were also submitted to a mass spectrometry (MS) protocol (Lecrenier et al., 2018) targeting ruminant proteins, including collagen, to distinguish between (unauthorized or authorized) ruminant by-products.
2. Materials and methods
2.1. Samples
3Nine gelatin (Gela-01 to 09) and seven collagen hydrolysate (Colla-01 to 07) samples of ruminant origin were collected from European industries. Twelve feed mix and FFS (Feed-01 to 12) with different gelatin contents were supplied by EFPPA (European Former Foodstuffs Processors Association). All samples were kept at 4 °C, and subsamples were ground at 2 mm with a rotor mill (ZM200, Retsch, Haan, Germany). For PCR analysis, subsamples were submitted to a second grinding with a 0.5 mm sieve when the samples contained a level of fat allowing such fine grinding.
2.2. Polymerase chain reaction (PCR)
4The ruminant DNA content of the samples was characterized by real time-PCR according to the EURL-AP Standard Operating Procedures (European Union Reference Laboratory for Animal Proteins in feedingstuffs, 2013a and 2013b). A cut-off value (in cycles) allowing the distinction between significant signals from non-significant ones was set using the ERM-AD482 RUMINANT pDNA CALIBRANT from the Joint Research Centre (JRC).
2.3. Mass spectrometry (UHPLC-MS/MS)
5The UHPLC-MS/MS approach was used for the simultaneous detection of targeted ruminant collagen, hemoglobin and milk proteins. Sample preparation and MS analysis were based on previously published protocols (Lecrenier et al., 2018) with minor changes. The peptides used as markers for the detection of ruminant hemoglobin (VGGHAAEYGAEALER, AAVTAFWGK, EFTPVLQADFQK and VVAGVANALAHR), casein (HQGLPQEVLNENLLR and NAVPITPTLNR), beta-lactoglobulin (LSFNPTQLEEQCHI and VLVLDTDYK) and ruminant collagen (GEPGPAGAVGPAGAVGPR, GSTGEIGPAGPpGPpGLR and GPpGESGAAGPTGPIGSR) were selected according to previous studies (Buckley, 2016; Lecrenier et al., 2018; Lecrenier et al., 2021).
3. Results
6PCR results are summarized in tables 1 and 2. The overall analysis of the results shows that ruminant DNA has been detected in 75% (12/16) of gelatin and collagen hydrolysate samples. The majority of the samples (8/12) gave early signals with a mean Cycle Threshold (Ct) value of 25.3 cycles. One sample of collagen hydrolysate (Colla-07) gave a very early signal of 15.8 cycles. Three of the four samples giving a negative result for the detection of ruminant DNA were produced from bovine bones. The tissue origin of the remaining one (Colla-06) is unknown. No significant difference linked to the feed material nature (gelatin or collagen hydrolysate) was observed.
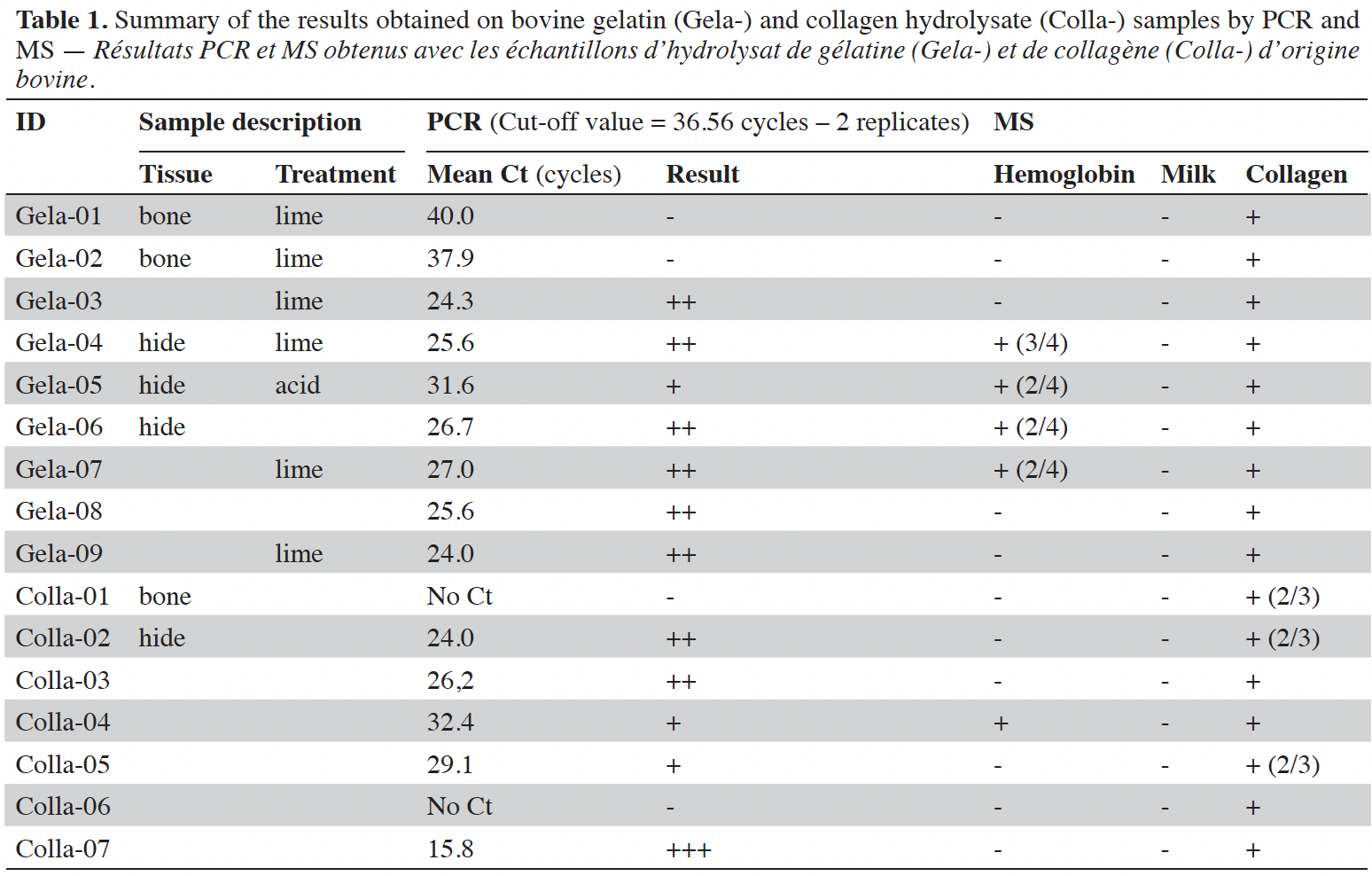
7By MS, collagen peptides were detected in all gelatin and collagen hydrolysate samples. In 81% of the cases (13 out of 16 samples), all collagen peptides (3/3) were identified. In three last samples, two of the three collagen peptides (2/3) were detected. This is only observed in collagen hydrolysate samples (Colla-01, 02 and 05) and concerns almost half of this sample type (3 out of 7 samples). Hemoglobin peptides were identified in 44% of the gelatin samples (Gela-04, 05, 06 and 07) and in only one collagen hydrolysate sample (Colla-04). As expected, no milk protein was detected in gelatin and collagen hydrolysate samples.
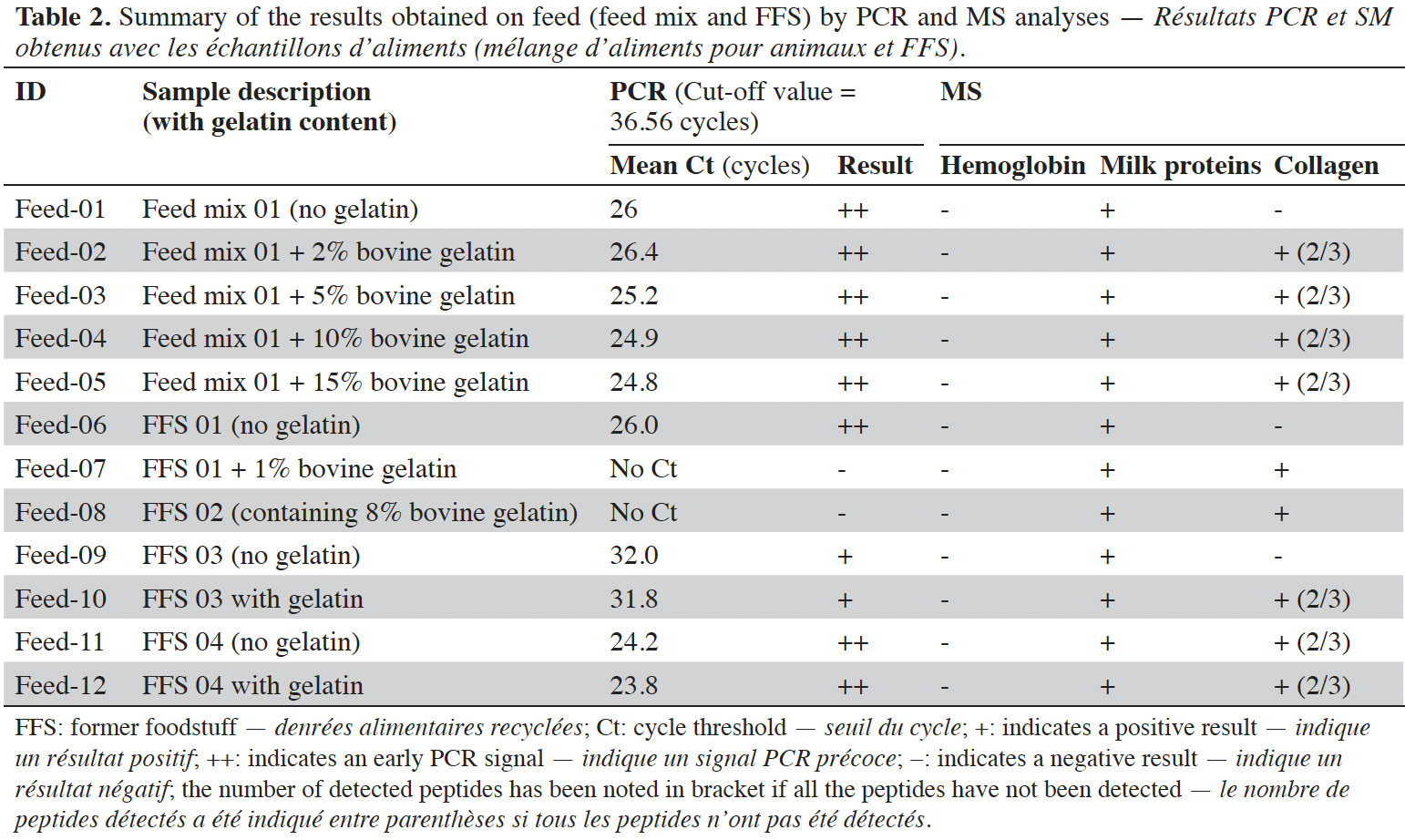
8Feed analyses show that the majority of the samples (10 out of 12) contained ruminant DNA and gave early signals with a mean Ct value of 26.5 cycles. It has to be noticed that increased inclusion of bovine gelatin added in Feed mix 01 (Feed-02 to 05) improves the PCR signal (from 26.4 cycles to 24.8 cycles respectively). Ruminant DNA was not detected in Feed-07 and Feed-08. Feed-07 was produced based on FFS 01 (Feed-06) containing detectable ruminant DNA with addition of 1% gelatin. The negative result obtained with Feed-08 is also puzzling as it contains a high amount (8%) of bovine gelatin. By considering MS results, the presence of ruminant DNA seems to be linked to the use of dairy products in the feed. Indeed, milk proteins were detected in all feed samples, but also in Feed-07 and Feed-08. Collagen peptides were identified in all feed declared to contain gelatin. Only one sample (Feed-11) described to be not adulterated with gelatin has given a positive result for gelatin. No hemoglobin was detected in all feed.
4. Discussion
9Amplifiable DNA was extractable in both gelatin and collagen hydrolysate, produced by enzymatic hydrolysis of collagen. DNA amounts seem to be highly influenced by the tissue origin and the processing, but the remaining DNA is detectable in most of the samples. PCR signals are mostly moderate with collagen and gelatin samples. However, strong PCR signals are observed with FFS and feed containing such FFS. These results let us assume that the use of FFS in a feed may yield a PCR response giving rise to false suspicions of prohibited materials. MS analyses performed on the same set of samples show that collagen markers are detected in all samples containing gelatin or collagen whatever the treatment (acid or alkaline). The use of milk markers shows that the presence of ruminant DNA in samples containing FFS will be more linked to the presence of milk than to the presence of gelatin.
10Concerning the potential inhibitory effect of gelatin for PCR, the analyses performed have revealed a suspicion of such effect in Feed-07 and Feed-08. The addition of gelatin in Feed-06 to produce Feed-07 made it impossible to amplify the ruminant DNA present in the feed whereas Feed-06 gave a strong PCR amplification. The same hypothesis can be made for Feed-08 that was confirmed to contain milk protein by MS, even if the feed matrix used to prepare Feed-08 was not available to confirm it. However, this inhibitory effect was observed in a limited number of samples and will be investigated more deeply.
5. Conclusions
11These results show the high complementarity of PCR and MS approaches in the context of the use of former foodstuffs in animal feed and the analytical conclusion in terms of prohibited versus authorized material content from ruminant origin.
Bibliographie
Buckley M., 2016. Species identification of bovine, ovine and porcine type 1 collagen; comparing peptide mass fingerprinting and LC-based proteomics methods. Int. J. Mol. Sci., 17, 445-445, doi.org/10.3390/ijms17040445
Council of the European Union, 2016. Conclusion, Brussels, 28 June 2016, 10730/16, https://ec.europa.eu/food/system/files/2017-08/fw_lib_council_food-losses-food-waste_2016.pdf, (20/12/2022).
EFSA Panel on Biological Hazards (BIOHAZ), 2020. Potential BSE risk posed by the use of ruminant collagen and gelatine in feed for non-ruminant farmed animals. EFSA J., 18, 1-68, doi.org/10.2903/j.efsa.2020.6267
European Commission, 2017. Commission Regulation (EU) 2017/1017 of 15 June 2017 amending Regulation (EU) No 68/2013 on the Catalogue of feed materials. Off. J. Eur. Union, L159, 48-119.
European Commission, 2021. Commission Regulation (EU) 2021/1372 of 17 August 2021 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as regards the prohibition to feed non-ruminant farmed animals, other than fur animals, with protein derived from animals. Off. J. Eur. Union, L295, 1-17.
European Union Reference Laboratory for Animal Proteins in feedingstuffs, 2013a. EURL-AP Standard Operating Procedure - DNA extraction using the "Wizard® Magnetic DNA purification system for Food" kit, https://www.eurl.craw.eu/wp-content/uploads/2021/01/EURL-AP-SOP-DNA-extraction-V1.1.pdf, (03/11/2021).
European Union Reference Laboratory for Animal Proteins in feedingstuffs, 2013b. EURL-AP Standard Operating Procedure - Detection of ruminant DNA in feed using real-time PCR, https://www.eurl.craw.eu/wp-content/uploads/2021/05/EURL-AP-SOP-Ruminant-PCR-V1.3.pdf, (03/11/2021).
Kim S., Labbe R.G. & Ryu S., 2000. Inhibitory effects of collagen on the PCR for detection of Clostridium perfringens. Appl. Environ. Microbiol., 66, 1213-1215, doi.org/10.1128/AEM.66.3.1213-1215.2000
Lecrenier M.C. et al., 2018. A mass spectrometry method for sensitive, specific and simultaneous detection of bovine blood meal, blood products and milk products in compound feed. Food Chem., 245, 981-988, doi.org/10.1016/j.foodchem.2017.11.074
Lecrenier M.-C. et al., 2021. Inter-laboratory study on the detection of bovine processed animal protein in feed by LC-MS/MS-based proteomics. Food Control, 125, 107944, doi.org/10.1016/j.foodcont.2021.107944
Liu D. et al., 2015. Collagen and gelatin. Annu. Rev. Food Sci. Technol., 6, 527-557, doi.org/10.1146/annurev-food-031414-111800
Schrader C., Schielke A., Ellerbroek L. & Johne R., 2012. PCR inhibitors - occurrence, properties and removal. J. Appl. Microbiol., 113, 1014-1026, doi.org/10.1111/j.1365-2672.2012.05384.x






