- Home
- Volume 27 (2023)
- Numéro 1
- Effect of year of cultivation on the oil content and fatty acid composition of chia seeds (Salvia hispanica L.) grown in France
View(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Effect of year of cultivation on the oil content and fatty acid composition of chia seeds (Salvia hispanica L.) grown in France

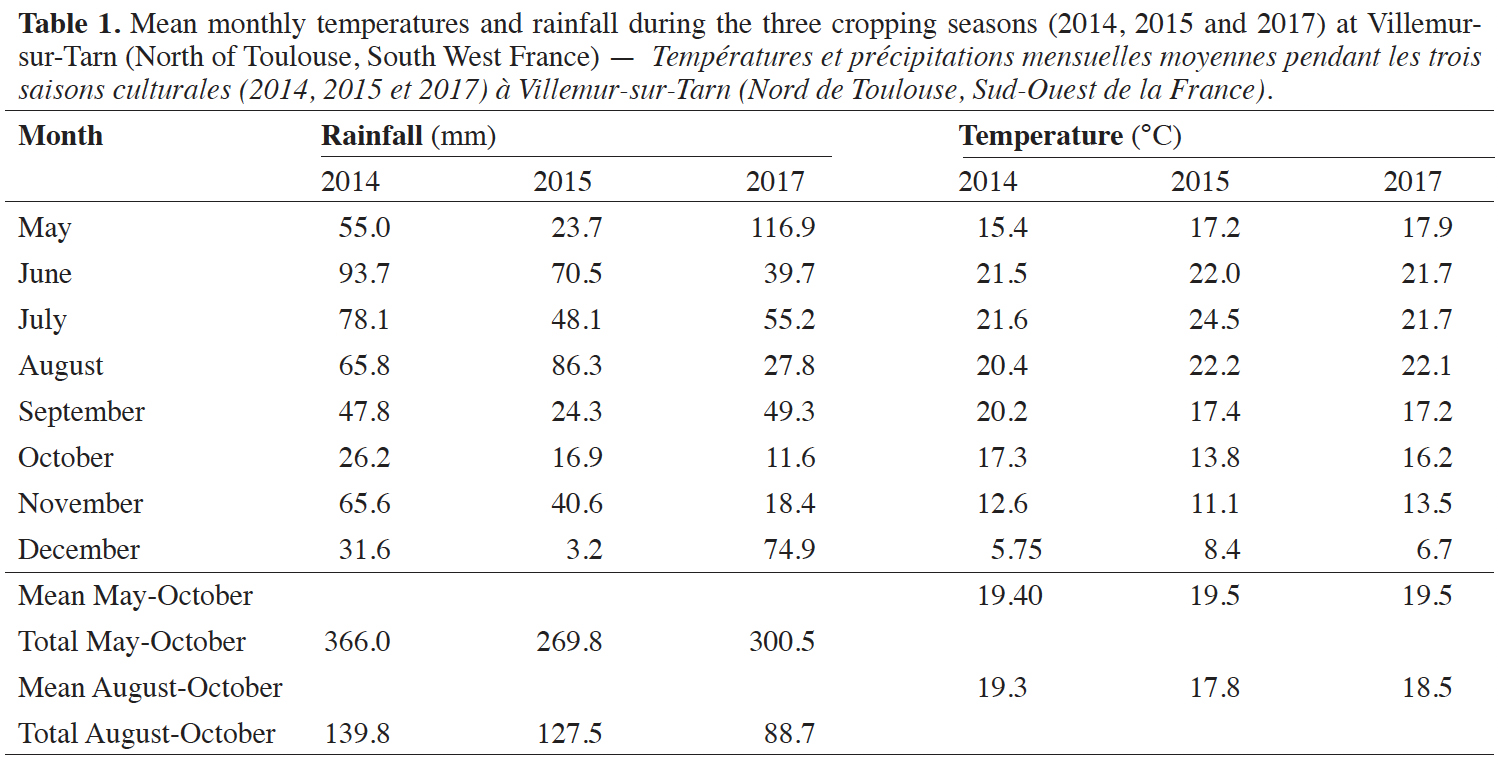
Attached document(s)
original pdf fileRésumé
Effet de l'année de culture sur la teneur en huile et la composition en acides gras des graines de chia (Salvia hispanica L.) en France
Description du sujet. La sauge espagnole ou chia (Salvia hispanica) a suscité, en raison de la composition de ses graines, un intérêt croissant au cours de la dernière décennie. Elle est maintenant cultivée dans plusieurs pays européens.
Objectifs. Les buts de cette étude étaient d'examiner la teneur en huile et la composition des graines de chia cultivées durant trois années dans le sud-ouest de la France. Les teneurs en huile, en cendres et en protéines ont été évaluées, la composition en acides gras, stérols et tocophérols a été déterminée dans les graines.
Methode. Le cultivar Oruro (Panam, France) a été utilisé et cultivé en 2014, 2015 et 2017 à Villemur-sur-Tarn (sud-ouest de la France).
Resultats. L'année de culture a affecté les teneurs en huile, en protéines (Kjeldahl) et en cendres, ainsi que la teneur et la composition en acides gras (GC-FID), en stérols (GC) et en tocophérols (HPLC). Les teneurs en huile (comprises entre 30,6 et 34,7 %) et en cendres (4,8-5,2 %) étaient plus élevées en 2014 et 2015 (pluviométrie plus importante qu'en 2017). La teneur en protéines était plus élevée en 2017 (21,7 %) que les deux autres années (17,5 et 19,9 %). Cette tendance était attendue, étant donné la corrélation négative entre les teneurs en huile et en protéines. Les acides gras polyinsaturés ont prédominé et leurs teneurs étaient les plus élevées en 2015 et les plus faibles en 2017. Les teneurs en acides gras saturés et monoinsaturés ont suivi le schéma inverse (les plus faibles en 2015 et les plus élevées en 2017). Les stérols et les tocophérols se sont accumulés à des niveaux plus élevés dans les graines en 2014 qu'en 2017.
Conclusions. Ainsi, tous les traits de qualité des graines de chia cultivées en France ont été affectés par les conditions climatiques de l'année de culture.
Abstract
Description of the subject. Spanish sage or chia (Salvia hispanica) has attracted increasing interest over the last decade due to the composition of its seeds. It is now cultivated in several European countries.
Objectives. The aims of this study were to investigate the oil content and composition of seeds from chia cultivated over a three-year period in South-West France.
Method. The cultivar Oruro (Panam, France) was used for this study. It was cultivated in 2014, 2015 and 2017 at Villemur-sur-Tarn (South-West France). Oil, ash and protein contents (Kjeldahl) was assessed, fatty acids (GC-FID), sterols (GC), tocopherols (HPLC) composition was determined on seeds.
Results. Cultivation year affected oil, protein and ash contents, and the content and composition of fatty acid, sterols and tocopherols. Oil (range of 30.6-34.7%) and ash (4.8-5.2%) contents were higher in 2014 and 2015 (higher rainfall than 2017). Protein content was higher in 2017 (21.7%) than in the other two years (17.5-19.9%). This trend was expected, given the negative correlation between oil and protein contents. Polyunsaturated fatty acids predominated, and their levels were highest in 2015 and lowest in 2017. Saturated and monounsaturated fatty acid levels followed the opposite pattern (lowest in 2015 and highest in 2017). Sterols and tocopherols accumulated to higher levels in the seeds in 2014 than in 2017.
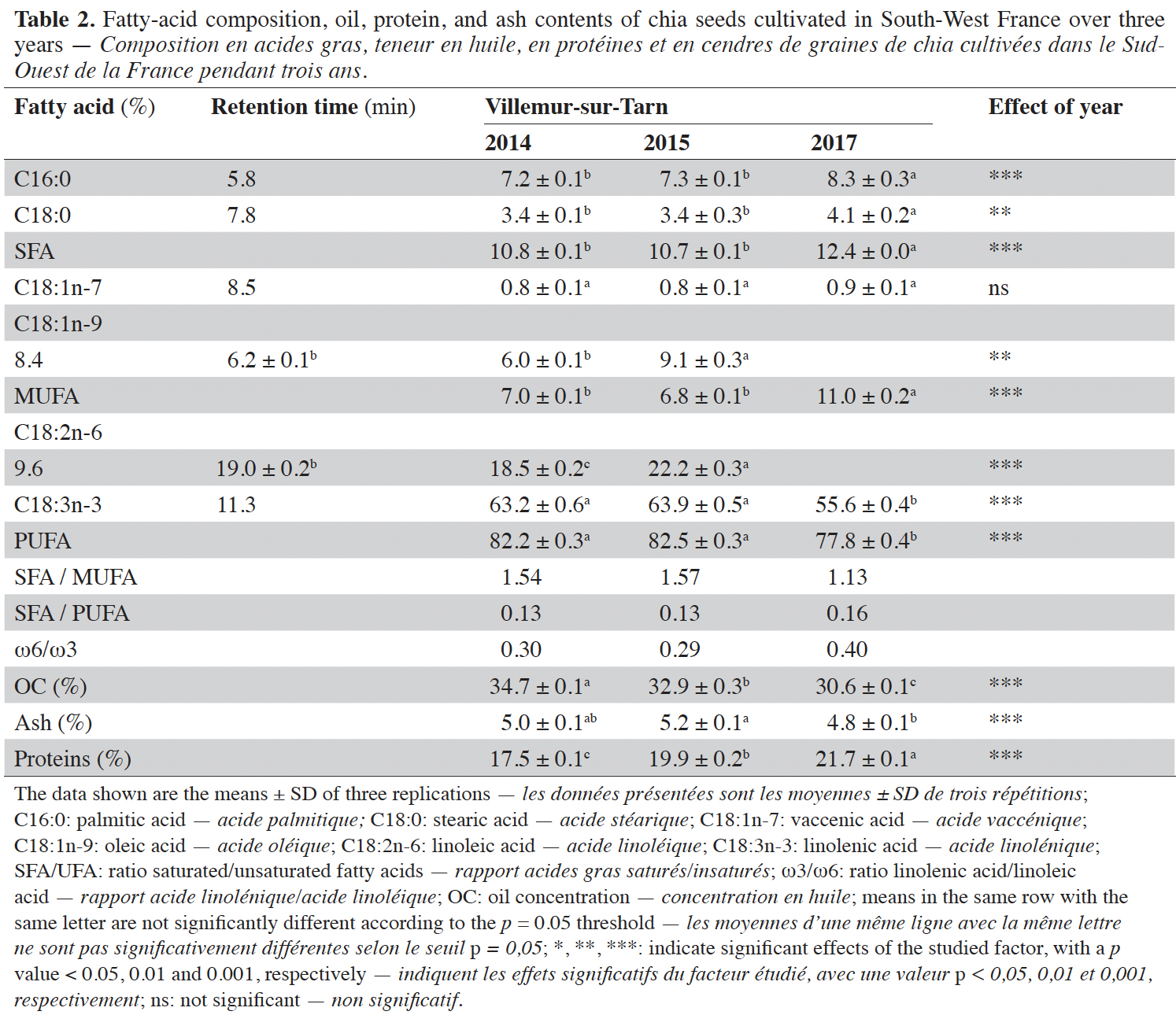
Conclusions. All the seed quality traits of chia cultivated in France were affected by the climatic conditions of the year of cultivation.
Table of content
Received 7 December 2021, accepted 16 January 2023, available online 31 January 2023
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1The Lamiaceae family is known for its aromatic species, used in both culinary dishes and for medicinal purposes. Salvia hispanica L. (known as chia or Spanish sage), an ancient Central American crop (Capitani et al., 2012), is a member of this family that has received considerable attention due to its oil content and enrichment in omega-3 and omega-6 (Ciftci et al., 2012; Mohd-Ali et al., 2012; Zettel & Hitzmann, 2018). Chia (Figure 1) is an ancestral crop in Central and South America (Zettel & Hitzmann, 2018), with seeds rich in mono- and polyunsaturated fatty acids (almost 90% of the total fatty acids) (Zettel & Hitzmann, 2018; Gravé et al., 2019). The seeds contain large amounts of fiber non-allergenic, highly digestible and contain all the essential amino acids for human nutrition (de Falco et al., 2017; Grancieri et al., 2019; Kulczyński et al., 2019). Moreover, chia has a few industrial uses in functional foods, baked goods, dairy products, fillets of Tilapia, and in pharmaceuticals and cosmetics (de Falco et al., 2017; Zettel & Hitzmann, 2018; Muños-González et al., 2019; Câmara et al., 2020; Mas et al., 2020). This multipurpose plant also has several benefits for human health (Mohd-Ali et al., 2012; de Falco et al., 2017).
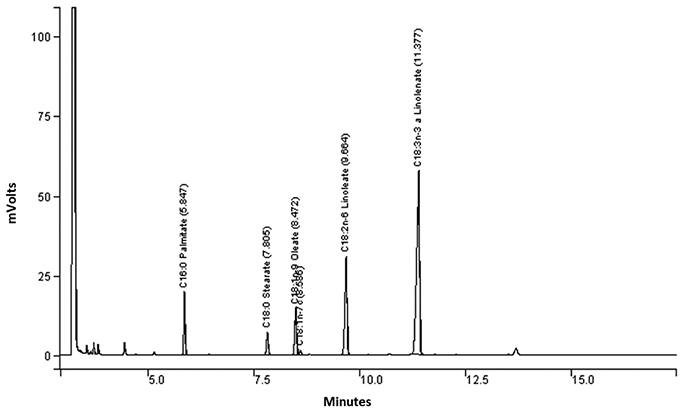 Figure 1. Chromatogram of fatty acid composition of chia seeds cultivated in South-West France over three years — Chromatogramme de la composition en acides gras de graines de chia cultivées dans le Sud-Ouest de la France pendant trois ans.
Figure 1. Chromatogram of fatty acid composition of chia seeds cultivated in South-West France over three years — Chromatogramme de la composition en acides gras de graines de chia cultivées dans le Sud-Ouest de la France pendant trois ans.
2The addition of chia to rat feed rich in sucrose has been shown to decrease free triacylglycerol concentration and hyperglycemia (Creus et al., 2016; Ferreira et al., 2020), and to have anti-inflammatory osteoarthritis-preventing effects in albino rats (Arpitha et al., 2019). Antioxidant, anticholesterolemic, antihypertensive and hypoglycemic effects have been reported (Grancieri et al., 2019). Chia seeds improve the absorption and oxidation of glucose and restore tissue sensitivity to insulin, thereby having a major effect on health (Enes et al., 2020). Chañi et al. (2018) showed that dietary supplementation with chia increased body weight gain, bone mineral density and bone mineral content.
3Chia seeds contain large amounts of oil enriched in polyunsaturated fatty acids, phytosterols and tocopherols (Zettel & Hitzmann, 2018; Gravé et al., 2019; Grimes et al., 2019). Studies, mostly performed in South American countries, have shown that oil levels depend on climatic conditions, cultivar, location, soil composition and stage of ripening (Ayerza, 1995; Ayerza & Coates, 2011; Gravé et al., 2019). Interest in chia seed for human nutrition and health has increased over the last decade in Europe (Gravé et al., 2019; Grimes et al., 2019), principally due to the acceptance of chia as a novel food ingredient (European Commission, 2009; 2013). Several studies testing South American cultivars in Europe have been performed with a view to introducing chia cultivation into Italy (Bochicchio et al., 2015), Greece (Bilalis et al., 2016; Karkanis et al., 2018) and Germany (Grimes et al., 2019). Panam (Villemur-sur-Tarn, South-West France) has released a chia cultivar (Oruro, registration number 20332) adapted to European conditions, which has been cultivated in the South of France since 2013. Grimes et al. (2019) showed that year of cultivation and environmental conditions influenced the yield and biochemical composition of the seeds, whereas these traits were unaffected by sowing density and the rate of fertilizer application. No information has been obtained concerning the effect of cultivation year on the biochemical traits of chia in France since its introduction. In this study, we therefore investigated the effect of year (climatic conditions) on the lipid composition, oil, protein and ashes contents of seeds of chia cultivar Oruro cultivated in South-West France.
2. Materials and methods
2.1. Plant material and environmental conditions
4Field-based participatory experiments were conducted over three years (2014, 2015 and 2017) at Villemur-sur-Tarn (40 km north of Toulouse, South-West France, 43°51′59″N, 1°30′21″E). We used the Oruro cultivar (Panam, France) for these experiments. This variety developed in France is adapted to long days with a cycle of 150 days. It presents cream-brown seeds. Each year three plots of 20 m² were delimited randomly in the field and harvested separately from the farmer’s production. For each plot three technical samples were performed.
5Seeds were sown at a density of 4 kg·ha-1 and a depth of 1 cm. Sowings were carried out 12th, 6th and 20th May in 2014, 2015 and 2017, respectively. Each year, plants received 60 units·ha-1 ammonium nitrate. Weeds were managed by supply of 2 l·ha-1 Basamais and 1 l·ha-1 Fusilade.
6The crops grew entirely in rainfed conditions, with no additional water supplied. Flowering occurred at 27th, 18th and 30th of August in 2014, 2015 and 2017, respectively. The seeds were ripe at the end 21th, 11th and 31th of October in 2014, 2015 and 2017, respectively. These allow to a cycle duration of 158, 155 and 153 days in 2014, 2015 and 2017, respectively.
7Table 1 presents rainfall and temperatures during the plant cycle. At Villemur-sur-Tarn, rainfall during the cropping season was lower in 2015 and 2017 than in 2014 (Table 1). Differences were observed between the three cropping seasons during the August-September period, corresponding to the seed filling stage. Indeed, 2014 had the warmest and wettest of the growing seasons, whereas 2015 and 2017 presented similar, lower temperatures. Moreover, rainfall levels in 2017 were almost half those in the other two years. The soil on this farm is clay-loam with high water-holding capacity and an organic matter content of 4.9% and a pH of 6.4. At maturity, four samples of one square meter, randomly chosen, were harvested (end of October) and seeds collected. The seed yield was the average value of seeds weight harvested by the farmer.
8All reagents were purchased from VWR (Fontenay-sous-Bois, France).
2.2. Extraction and determination of oil concentration in chia seeds
9Chia seed powder was obtained by grinding dried seeds (IKA Werke MF 10 basic, Staufen, Germany). From this powder, three samples (20 g), as technical replicates, were used for oil extraction (5 h) at atmospheric pressure in a Soxhlet-type apparatus (Fischer, Illkirch, France). The solvent was cyclohexane (VWR, Fontenay-sous-Bois, France). Subsequently, a rotary evaporator (Fischer, Illkirch, France) at low pressure (around 150 mbars thanks to its connection to a laboratory filter pump) and 35 °C was used to remove the solvent. The extracted oil was dried at 35 °C for at least 12 h and the yield calculated from the oil masses obtained on dry mass basis.
10Moisture content was obtained by heating samples of seeds at 80 °C during 48 h.
2.3. Fatty acids composition
11Fatty acids were determined after transesterification (norm ISO 5509: 2000). The seeds (1 g) were crushed with 10 ml cyclohexane and the mixture was then centrifuged at 10,000 x g for 15 min at room temperature. Three extractions were performed, and the organic phases were pooled, filtered on anhydrous Na2SO4 and dried under nitrogen. The fatty acid profile of the triacylglycerols was determined after trans-methylation with TMSH (0.2 M trimethylsulfonium hydroxide in methanol) according to AFNOR Method NF EN ISO 12966-3. Fatty acid methyl esters (FAME) were analyzed by gas chromatography (Roche et al., 2006).
12We used a CP-select CB column (50 m long, 0.32 mm i.d., 0.25 µm film thickness), with helium as the carrier gas, at a flow rate of 1.2 ml·min-1; the split injector (1:100) and FID were maintained at 250 °C; the initial oven temperature was set at 185 °C for 40 min, then increased to 250 °C at a rate of 15 °C·min-1, before maintenance at this temperature for 10 min.
2.4. Sterol content
13Sterol content was also determined by gas chromatography (Roche et al., 2006). We introduced 50 µl of a 2 mg·ml-1 solution of cholestanol (Sigma-Aldrich, Saint-Louis, United States) in chloroform into a 15 ml screw-top tube. Chloroform was then eliminated in the nitrogen flow, and 100 mg of oil followed by 2 ml 1 M KOH in ethanol were added. The tube was vortexed and heated at 75 °C (water bath) for 30 min. The mixture was cooled to room temperature, 1 ml of distilled water was added and the mixture was vortexed again. The unsaponifiable material was then extracted in 6 ml cyclohexane, and 160 µl of the cyclohexane phase was silylated with 40 µl BSTFA/TMCS (99/1 mixture). The sample was briefly heated in an oven at 103 °C and was then analyzed by gas chromatography.
14A sample volume of 1 µl was injected into the Perkin Elmer device equipped with an Agilent VF-5ms column (30 m long, internal diameter of 0.25 mm, film thickness of 0.25 µm) coupled to a flame ionization detector operating at 355 °C. The carrier gas was helium, at a column head pressure of 100 kPa. The thermal program for the injector was 55 °C for 0.5 min, increasing to 340 °C at a rate of 200 °C·min-1, 340 °C for 30 min. The thermal program for the oven was 160 °C for 0.5 min, increasing to 260 °C at a rate of 20 °C·min-1, then at to 300 °C at a rate of 2 °C·min-1 and to 350 °C at a rate of 45 °C·min-1. Commercial standards or reference values were used to identify the various sterols, and the internal standard (cholestanol) was used for their quantification.
2.5. Tocopherol content
15As previously described (Fabre et al., 2015), tocopherol content was assessed by high-performance liquid chromatography. Oil (20 mg) was dissolved in 1 ml cyclohexane and 20 µl of the resulting solution was injected into a Dionex liquid chromatography equipment fitted with a Kromasil 100 SIL 5 µm column (250 mm long, with an internal diameter of 4 mm). The mobile phase consisted of 99.5% isooctane and 0.5% isopropanol (v/v) and the flow rate was 1.1 ml·min-1. A fluorimeter (Dionex) was used for detection, at an excitation wavelength of 290 nm and an emission wavelength of 317 nm. Reference standards were used to identify the tocopherols and quantification was obtained through external calibration.
2.6. Ash and protein contents determination
16Calcination at 450 °C for 6 h was performed on chia seeds in order to evaluate the ash contents according to the ISO 749:1977 standard.
17Protein contents were evaluated by using an indirect method based on the determination of the percent total nitrogen (% N) by the Kjeldahl method, in accordance with the ISO 5983‐1:2005 standard. Kjeldahl’s method involves mineralization of the total organic nitrogen of the sample to generate ammoniacal nitrogen, which is then determined by titration against acid. A mass of 0.8 g of dried kernels delipidated by the Soxhlet method was placed in a glass tube for mineralization, to which 12.5 ml of 95% sulfuric acid was added. The mixture was placed in a fume hood for 16 h. It was then mineralized by heating at 400 °C for 1 h. Finally, the analysis was performed with a KjeltecTM 8400 analyzer.
18The conversion factor between total N and protein (NPCF) was 5.3, calculated as described by Mosse (1990) and confirmed by Gosukonda (2020).
2.7. Statistical data analysis
19Statistical analyses were performed for all the traits measured. Analyses of variance and Duncan tests were performed to determine the significance of differences between years. These analyses were performed with a statistical package (Sigmastat Ver. 2.0, USA).
3. Results
3.1. Oil, protein and ash contents
20The mean fatty-acid composition, oil, protein and ash contents of seeds harvested for three years (2014, 2015 and 2017) at Villemur -sur-Tarn are presented in table 2. All quality traits were affected by year of cultivation. Indeed, seed oil content in 2017 was 2% lower than that in 2015 and 4% lower than that in 2014. Protein content was highest in 2017 and lowest in 2014, the years during which the seed filling period was driest and wettest, respectively (Table 2). The seed yield was the average value of seeds weight harvested by the farmer. Yield values were 415, 298 and 322 kg·ha-1 in 2014, 2015 and 2017, respectively.
3.2. Fatty-acid composition
21The fatty-acid profiles obtained during the three years of the study are displayed in table 2. Figure 1 presents the chromatogram of fatty acids composition. As expected, the proportion of polyunsaturated fatty acids (PUFA) was high, with 55.6-63.9% -linolenic (C18:3n-3) and 77.8-82.5% PUFA.
22Fatty-acid composition was influenced by year. Indeed, similarly to oil content, the observed fatty-acid composition in 2017 contrasted with those observed in 2014 and 2015 (Table 2). Indeed, the seeds harvested in 2017 were characterized by 2% and 5% higher levels of saturated (SFA) and monounsaturated (MUFA) fatty acids than those harvested in 2014 and 2015. Conversely, PUFA levels were lower in 2017 than in other years (Table 2), and this affected the SFA-to-PUFA ratio (Table 2).
3.3. Phytosterol and tocopherol content and composition
23As for fatty acids, the content and composition of sterols was affected by year of cultivation (Table 3). Total sterol content was highly variable (182.7-215.2 mg·100 g-1 seed). Total sterol content was lowest in 2017. As expected, desmethylsterols constituted the main group in chia, accounting for at least 87.1% (in 2014) of total sterols.
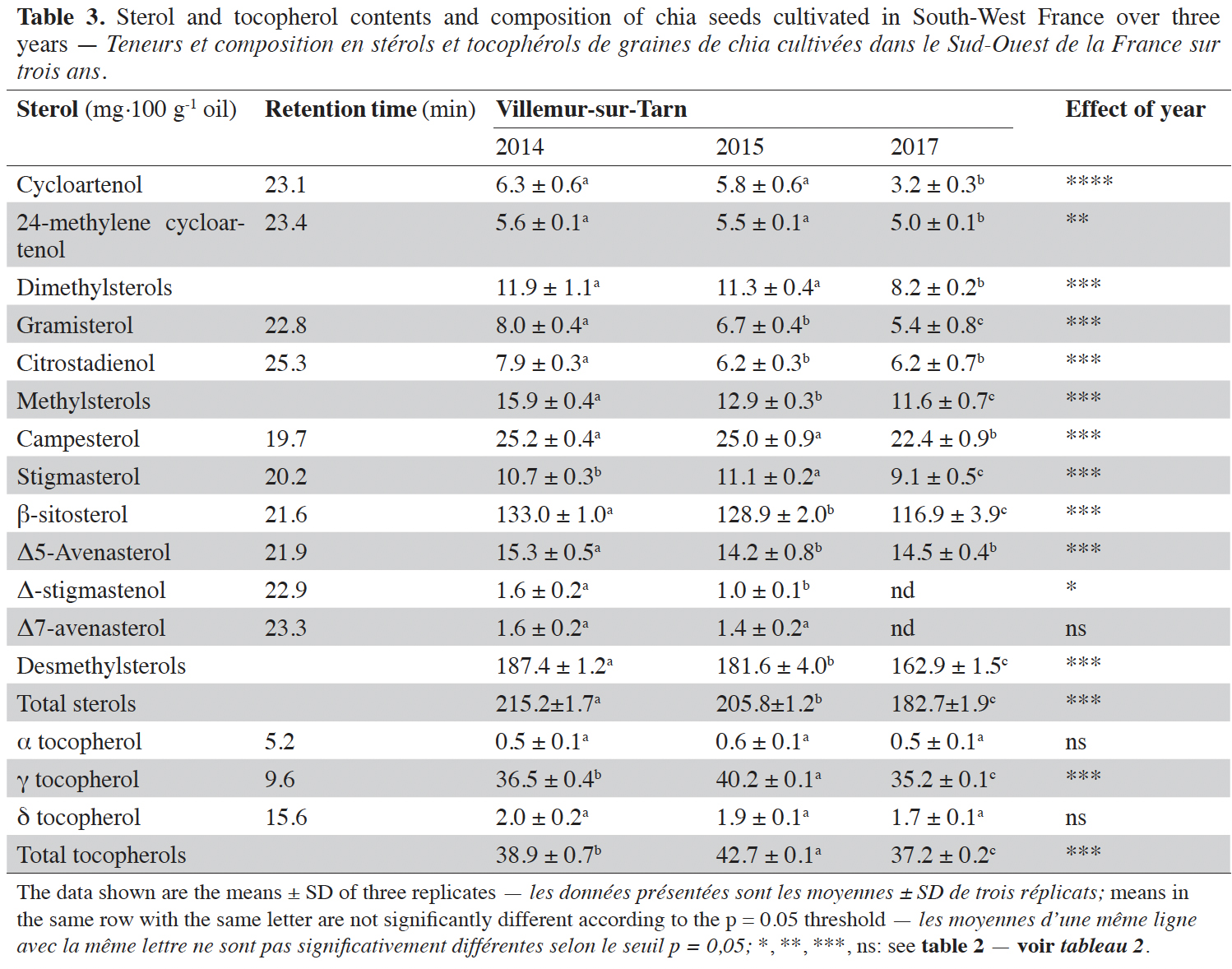
24Tocopherol contents and compositions are shown in table 3. Figure 2 presents the chromatograms of sterol (A) and tocopherol (B) compositions. As for other lipids, tocopherol levels were lower in 2017 (50% lower than in 2015; Table 3). The predominant isomer of tocopherol was tocopherol, which accounted for more than 94% of total tocopherol content (Table 3).
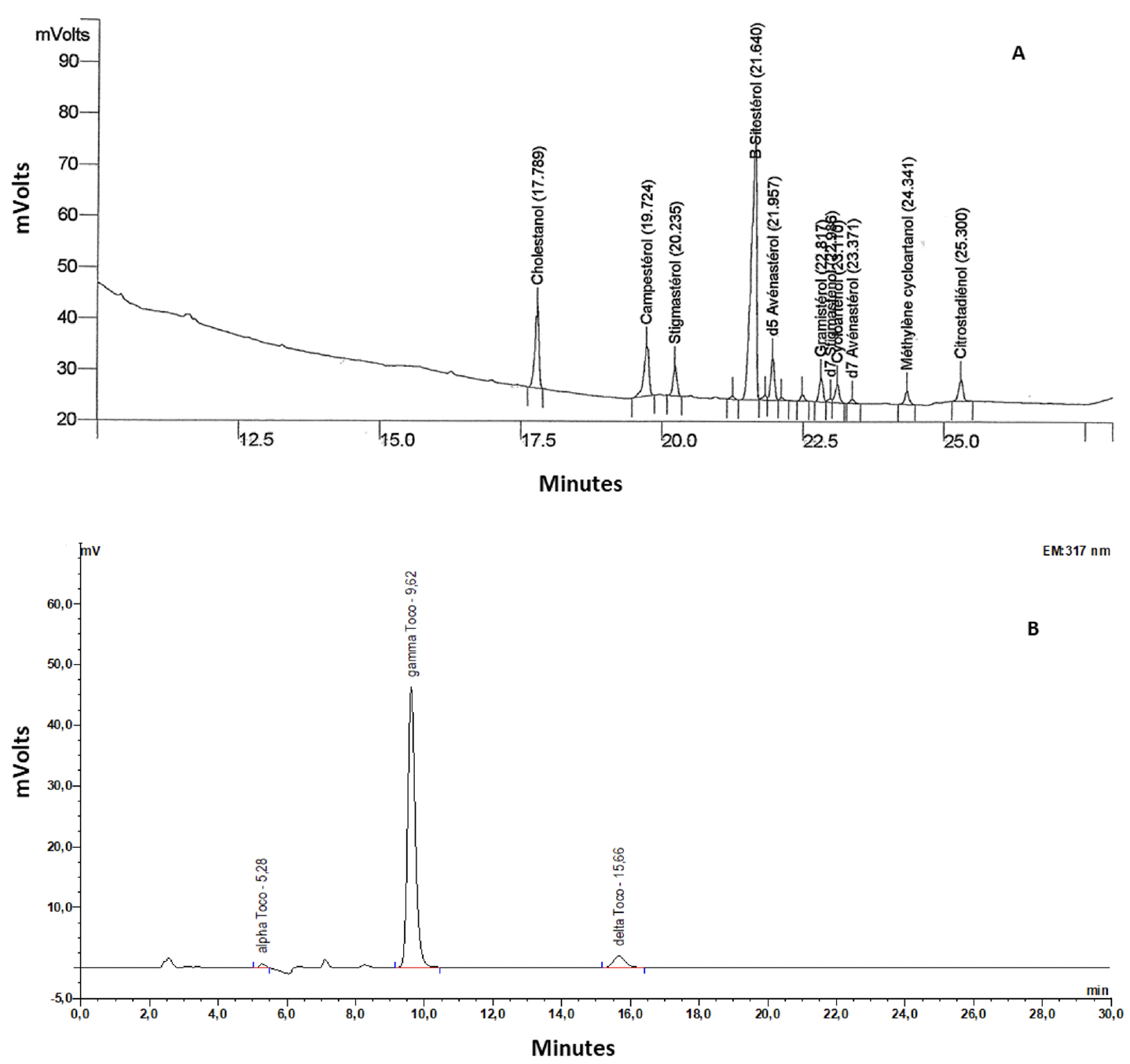 Figure 2. Chromatograms of sterol (A) and tocopherol (B) compositions of chia seeds cultivated in South-West France over three years — Chromatogrammes de la composition en stérols (A) et en tocophérols (B) de graines de chia cultivées dans le Sud-Ouest de la France pendant trois ans.
Figure 2. Chromatograms of sterol (A) and tocopherol (B) compositions of chia seeds cultivated in South-West France over three years — Chromatogrammes de la composition en stérols (A) et en tocophérols (B) de graines de chia cultivées dans le Sud-Ouest de la France pendant trois ans.
4. Discussion
4.1. Oil, protein and ash contents and seed yield
25Oil concentration varied by 4% among years (Table 2), and was within the range of values reported in other countries: 20.3 to 40.1% oil, depending on genotype and selection, location, elevation, seed ripening stage, climatic conditions, irrigation and solvent or extraction methods (Ayerza, 1995; Ayerza & Coates, 2011; Ciftci et al., 2012; Ixtaina et al., 2012; Amato et al., 2015; Dąbrowski et al., 2017; de Falco et al., 2018; Sayed-Ahmad, 2018; Gravé et al., 2019; Grimes et al., 2019). The range observed with Oruro under our experimental conditions was wider than that for the charcoal-seeded genotype G8 grown under different conditions of row spacing, sowing density and nitrogen fertilizer rates in Germany (Grimes et al., 2019) and lower than G8 cultivated under different irrigation treatments in Italy (de Falco et al., 2018). These differences may be due to genotype, climatic conditions, cropping practices or a combination of these factors. A year effect was observed for this trait. Indeed, the seeds harvested in 2017, season characterized by the lowest levels of rainfall during seed filling, had the highest protein concentration (Table 2). These results are within the range (12.3-26.0%) reported in previous studies, which varied with location, genotype, climatic conditions and agricultural practices (Ayerza & Coates, 2004; Sandoval-Oliveros & Paredes-López, 2013; Segura-Campos et al., 2014; de Falco et al., 2018; Sayed-Ahmad, 2018; Grimes et al., 2019). Moreover, a conversion factor between N content and protein level of 6.25 is generally used. This results in higher values for crude protein content and may introduce differences between studies. We used a factor of 5.3 in this study because the study of Gosukonda (2020), in accordance with the work of Mosse (1990), showed that the use of amide nitrogen is the most appropriate method in the determination of nitrogen to protein conversion factors (NPCFs), giving for chia a value of 5.3. So crude protein content should probably be considered to be slightly higher than that reported here when comparing with the apparent values obtained in studies in which 6.25 was used as the universal conversion factor.
26The opposite temporal pattern was observed for lipid accumulation (Table 2). The opposite trends observed for oil and protein contents may be explained by the impact of the climatic conditions encountered during seed formation and filling. Indeed, in 2017, there was less rainfall during seed development and filling than in 2014 and 2015 (Table 1). These conditions affected the proportions of lipids and proteins in the seeds, leading to an asynchrony of accumulation. In sunflower, proteins have been reported to accumulate earliest in akenes, before lipids (Blanchet, 1987; Roche et al., 2006). Moreover, the oil content of oilseed species depends on the activity of fatty acid synthetases, and these enzymes are differentially activated during fruit development (Roche et al., 2019; Nguyen et al., 2020). Water deficits probably have a stronger negative effect on the FA synthetase system than on protein accumulation. This hypothesis is supported by the lower ash content in 2017 than in both 2014 and 2015 (Table 2). Indeed, ash (minerals) accumulates in wheat grains through active transport in the phloem rather than passively via the transpiration stream (Merah, 2001), mirroring the maintenance of photosynthesis during seed filling. Protein content was lower in 2014 than in 2015 and 2017. Climatic conditions during the seed filling period may account for these differences. Indeed, 2014 was rainier than 2015 and 2017, and the more favorable conditions for oil production (Table 1) resulted in higher oil concentrations rather than an accumulation of protein (Table 2).
27The yield, based on seeds weight harvested by the farmer, ranged from 298 kg·ha-1 (2015) and 415 kg·ha-1 (2014). These results can be explained by the differences in water availability during plant cycle (Table 1). Nevertheless, chia yielded more in 2017 than in 2015. This discrepancy could be explained by the fact that rainfall was less limited during seed formation and filling in 2015 than in 2017 (Table 1). Our results agree with those observed in Italy under rainfed conditions (de Falco et al., 2018). Unfortunately, these results cannot be compared with those reported by Grimes et al. (2019) in Germany who have measured seed yield by plant and not by area unit.
4.2. Fatty-acid composition
28The profiles of fatty-acid profiles observed for three cropping seasons varied largely (Table 2). These ranges are wider than those reported in Germany (Grimes et al., 2019), Canada (Abad & Shahidi, 2020), Poland (Dąbrowski et al., 2017), Argentina, Bolivia and Ecuador (Ayerza, 1995). Our results are consistent with those reported for different methods of extraction with solvent (Ixtaina et al., 2012; Sayed-Ahmad, 2018), at different stages of seed ripening (Gravé et al., 2019) and at different sites (Ayerza & Coates, 2004), but are lower than those reported in Mexico (Segura-Campos et al., 2014).
29Fatty acid composition presented large variation according to the year of cultivation (Table 2). These results may be explained by genotype effects (Ayerza, 1995; Ayerza & Coates, 2004) or the impact of site or environmental parameters (Roche et al., 2006; 2016; 2019; Nguyen et al., 2020). In this study, the same cultivar (Oruro) was used at the same site over a three-year period. We can therefore assume that the differences in fatty-acid composition observed were due to differences in the prevailing climatic conditions at the site among years. Temperature and rainfall are the climatic parameters with the greatest influence on fatty-acid composition in several oilseed species (Merah et al., 2012; Roche et al., 2016; Roche et al., 2019; Nguyen et al., 2020). Saturated fatty acids are the first to be synthesized. Desaturation involves several enzymes and transforms stearic acid into oleic, linoleic and then linolenic acids (Petit-Pigeard, 2002). The observed increase in PUFA levels was mainly due to increases in SFA + MUFA levels (Figure 3). Indeed, and as expected, a negative correlation was observed between PUFA and SFA + MUFA levels, across years and cropping systems. This result mirrors the effect of climatic conditions on desaturase activities, resulting in a lower PUFA in 2017. Indeed, the lower PUFA concentration resulted solely from a decrease in linolenic acid levels, probably due to lower levels of 15 desaturase activity. This hypothesis is also supported by the higher levels of linoleic acid accumulation in 2017 than in the other years (Table 2), and the higher levels of SFA and MUFA in 2017.
30In our study, the ω-6/ω-3 fatty-acid ratio ranged from 0.29-0.40, consistent with previous reports (Amato et al., 2015; Sayed-Ahmad, 2018). As already reported and confirmed in our study, chia can be considered a healthy source of balanced fatty acids for dietary consumption in Europe (Amato et al., 2015; Sayed-Ahmad, 2018; Gravé et al., 2019).
4.3. Phytosterol and tocopherol content and composition
31The sterol content and composition were found to be affected by year of cultivation (Table 3). Surprisingly, only a few studies have reported sterol content and composition in chia seeds (Ciftci et al., 2012; Dąbrowski et al., 2017; Sayed-Ahmad, 2018). The values reported here are lower than those reported in previous studies. These differences may be due to differences in genotype, climatic conditions, seed filling stage, extraction and measurement methods (Merah et al., 2012; Sayed-Ahmad, 2018; Roche et al., 2019).
32In 2017, climatic conditions were unfavorable for sterol production during seed filling (Table 1), resulting in lower levels of accumulation for sterols (Table 3). For example, cycloartenol (the first sterol formed in seeds) content in 2017 was almost half that in 2014. The same trend was observed for oils and fatty acids in this year. Indeed, the low water availability probably affected lipid synthesis. Moreover, for phytosterols, these conditions mostly affected the synthesis of squalene, a precursor of sterol synthesis (Merah et al., 2012), probably by downregulating the enzymes involved in squalene production and transformation: Squalene Synthetase, Squalene Epoxidase, and CycloArtenol Synthase. Unfortunately, squalene levels were not determined in this study.
33Tocopherol contents and compositions were influenced by year of cultivation (Table 3). These results are consistent with those of several studies reporting that tocopherol levels are dependent not only on extraction method (Dąbrowski et al., 2017; Sayed-Ahmad, 2018), but also on genotype and environment (Ciftci et al., 2012; Dąbrowski et al., 2017; Sayed-Ahmad, 2018).
34The production of chia seeds in Europe should help to meet the long-term objective of achieving 6 balance through the use of a new source of healthy oil. Indeed, chia oil has a low entirely appropriate for meeting this goal. Moreover, our results are based on the cultivation of a variety released for cultivation in Europe. In addition, the ingestion of chia seeds (in accordance with European Union recommendations) can decrease cardiovascular risks, contribute to the prevention of inflammatory disease and hypertension, decrease hepatocellular and intestinal damage, and exert hypoglycemic and LDL cholesterol-lowering effects (Mohd-Ali et al., 2012; Chañi et al., 2018; Da Silva et al., 2019; Grancieri et al., 2019; Kulczyński et al., 2019).
5. Conclusions
35This study investigated the impact of year of cultivation on oil, protein and ash contents, fatty acid composition, sterol and tocopherol contents and compositions in chia (Salvia hispanica L.) seed, in France. This is the first study to investigate the effect of year of cultivation on a cultivar selected for use in Europe. Significant effects were observed for all seed quality traits. Oil, protein and ash contents were significantly affected, in different manners, by site and climatic conditions (years). Fatty acid, sterol and tocopherol contents and profiles also depended on climatic conditions. Low rainfall in 2017 resulted in lower oil content and changes to the content and composition of fatty acids, sterols and tocopherols. It therefore seems likely that quality traits in chia are affected by climatic conditions. Further investigations are required to confirm these findings with other genotypes.
Bibliographie
Abad A. & Shahidi F., 2020. Compositional characteristics and oxidative stability of chia seed oil (Salvia hispanica L). Food Prod. Process. Nutr., 2, 9, doi.org/10.1186/s43014-020-00024-y
Amato M. et al., 2015. Nutritional quality of seeds and leaf metabolites of chia (Salvia hispanica L.) from southern Italy. Eur. Food Res. Technol., 241, 615-625, doi.org/10.1007/s00217-015-2488-9
Arpitha M., Rajesh M., Devika Rani K. & Ramachandra Setty S., 2019. Evaluation of anti-osteoarthritic activity of chia seeds (Salvia hispanica) in rats. World J. Pharm. Pharm. Sci., 8, 745-756, doi.org/10.20959/wjpps20196-13779
Ayerza R., 1995. Oil content and fatty acid composition of chia (Salvia hispanica L.) from five northwestern locations in Argentina. J. Am. Oil Chemists' Soc., 72, 1079-1081, doi.org/10.1007/BF02660727
Ayerza R. & Coates W., 2004. Composition of chia (Salvia hispanica) grown in six tropical and subtropical ecosystems of South America. Trop. Sci., 44, 131-135, doi.org/10.1002/ts.154
Ayerza R. & Coates W., 2011. Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.). Ind. Crops Prod., 34, 1366-1371, doi.org/10.1016/j.indcrop.2010.12.007
Bilalis D. et al., 2016. Chia (Salvia hispanica) fodder yield and quality as affected by sowing rates and organic fertilization. Commun. Soil Sci. Plant Anal., 47, doi.org/10.1080/00103624.2016.1206921
Blanchet R., 1987. Principaux caractères agronomiques et physiologiques déterminant la production des graines de tournesol. Rev. Fr. Corps Gras, 2, 75-80.
Bochicchio R. et al., 2015. Effect of sowing density and nitrogen top-dress fertilisation on growth and yield of chia (Salvia hispanica L.) in a Mediterranean environment: first results. Ital. J. Agron., 10, 163-166, doi.org/10.4081/ija.2015.640
Câmara A.K.F.I. et al., 2020. Reducing phosphate in emulsified meat products by adding chia (Salvia hispanica L.) mucilage in powder or gel format: a clean label technological strategy. Meat Sci., 163, 108085, doi.org/10.1016/j.meatsci.2020.108085
Capitani M.I., Spotorno V., Nolasco S.M. & Tomás M.C., 2012. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. LWT - Food Sci. Technol., 45, 94-102, doi.org/10.1016/j.lwt.2011.07.012
Chañi E. et al., 2018. Long-term dietary intake of chia seed is associated with increased bone mineral content and improved hepatic and intestinal morphology in Sprague-Dawley rats. Nutrients, 10, 922, doi.org/10.3390/nu10070922
Ciftci O.N., Przybylski R. & Rudzińska M., 2012. Lipid components of flax, perilla, and chia seeds. Eur. J. Lipid Sci. Technol., 114, 794-800, doi.org/10.1002/ejlt.201100207
Creus A., Ferreira M.R., Oliva M.E. & Lombardo Y.B., 2016. Mechanisms involved in the improvement of lipotoxicity and impaired lipid metabolism by dietary α-linolenic acid rich Salvia hispanica L. (S. alba) seed in the heart of dyslipemic insulin-resistant rats. J. Clin. Med., 5, doi.org/10.3390/jcm5020018
Da Silva B.P. et al., 2019. Effects of chia (Salvia hispanica L.) on oxidative stress and inflammation in ovariectomized adult female Wistar rats. Food Funct., 10, 4036-4045, doi.org/10.1039/C9FO00862D
Dąbrowski G., Konopka I., Czaplicki S. & Tańska M., 2017. Composition and oxidative stability of oil from Salvia hispanica L. seeds in relation to extraction method. Eur. J. Lipid Sci. Technol., 119, 1600209, doi.org/10.1002/ejlt.201600209
de Falco B., Amato M. & Lanzotti V., 2017. Chia seeds products: an overview. Phytochem. Rev., 16, 745-760, doi.org/10.1007/s11101-017-9511-7
de Falco B. et al., 2018. Metabolomic analysis by UAE-GC MS and antioxidant activity of Salvia hispanica (L.) seeds grown under different irrigation regimes. Ind. Crop Prod., 112, 584-592, doi.org/10.1016/j.indcrop.2017.12.030
Enes B.N. et al., 2020. Chia seed (Salvia hispanica L.) effects and their molecular mechanisms on unbalanced diet experimental studies: a systematic review. Food Sci., 85, 226-239, doi.org/10.1111/1750-3841.15003
European Commission, 2009. Commission decision of 13rd October 2009 authorising the placing on the market of chia seed (Salvia hispanica) as novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union, L294/14, 14-15.
European Commission, 2013. Commission implementing decision of 22th January 2013 authorising an extension of use of chia (Salvia hispanica) seed as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union, L21/34, 34-35.
Fabre J.F., Lacroux E., Cerny M. & Mouloungui Z., 2015. Barriers to the release of flaxseed oil bodies and ways of overcoming them. OCL, 22(6), D607.
Ferreira M.R., Oliva M.E., Aiassa V. & D’Alessandro M.E., 2020. Salvia hispanica L. (chia) seed improves skeletal muscle lipotoxicity and insulin sensitivity in rats fed a sucrose-rich diet by modulating intramuscular lipid metabolism. J. Funct. Foods, 66, 103775, doi.org/10.1016/j.jff.2019.103775
Gosukonda V., 2020. Comparative analysis of nitrogen-to-protein conversion factors for determining net protein content in six superfoods. J. Microbiol. Biotechnol. Food Sci., 9, 856-860, doi.org/10.15414/jmbfs.2020.9.4.856-860
Grancieri M., Martino H.S.D. & de Mejia E.G., 2019. Chia seed (Salvia hispanica L.) as a source of proteins and bioactive peptides with health benefits: a review. Compr. Rev. Food Sci. Food Saf., 18, 480-499, doi.org/10.1111/1541-4337.12423
Gravé G. et al., 2019. Accumulation of components of interest in seed of chia (Salvia hispanica L). OCL, 26, 50, doi.org/10.1051/ocl/2019037
Grimes S.J., Phillips T.D., Capezzone F. & Graeff-Hönninger S., 2019. Impact of row spacing, sowing density and nitrogen fertilization on yield and quality traits of chia (Salvia hispanica L.) cultivated in southwestern Germany. Agronomy, 9, 136, doi.org/10.3390/agronomy9030136
ISO 749:1977. Oilseed residues — Determination of total ash. Geneva, Switzerland: ISO.
ISO 5509:2000. Animal and vegetable fats and oils — Preparation of methyl esters of fatty acids. Geneva, Switzerland: ISO.
ISO 5983-1:2005. Animal feeding stuffs — Determination of nitrogen content and calculation of crude protein content. Part 1: Kjeldahl method. Geneva, Switzerland: ISO.
ISO 12966-3:2006. Animal and vegetable fats and oils — Gas chromatography of fatty acid methyl esters — Part 3: Preparation of methyl esters using trimethylsulfonium hydroxide (TMSH). Geneva, Switzerland: ISO.
Ixtaina V.Y., Nolasco S.M. & Tomás M.C., 2012. Oxidative stability of chia (Salvia hispanica L.) seed oil: effect of antioxidants and storage conditions. J. Am. Oil Chemists' Soc., 89, 1077-1090, doi.org/10.1007/s11746-011-1990-x
Karkanis A.C. et al., 2018. Efficacy and selectivity of pre- and post-emergence herbicides in chia (Salvia hispanica L.) under Mediterranean semi-arid conditions. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 46, 183-189, doi.org/10.15835/nbha46110979
Kulczyński B. et al., 2019. The chemical composition and nutritional value of chia seeds—current state of knowledge. Nutrients, 11, 1242, doi.org/10.3390/nu11061242
Mas A. et al., 2020. Defatted chia flour as functional ingredient in sweet cookies. How do processing, simulated gastrointestinal digestion and colonic fermentation affect its antioxidant properties? Food Chem., 316, 126279, doi/org.10.1016/j.foodchem.2020.126279
Merah O., 2001. Carbon isotope discrimination and mineral composition of three organs in durum wheat genotypes grown under Mediterranean conditions. CR Acad. Sci., Ser. III, 324, 355-363, doi.org/10.1016/S0764-4469(01)01307-5
Merah O. et al., 2012. Genetic analysis of phytosterol content in sunflower seeds. Theor. Appl. Genet., 125, 1589-1601, doi.org/10.1078/0176-1617-00273
Mohd Ali N. et al., 2012. The promising future of chia, Salvia hispanica L. J. Biomed. Biotechnol., 171956, doi.org/10.1155/2012/171956
Mosse J., 1990. Nitrogen-to-protein conversion factor for ten cereals and six legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and to seed protein content. J. Agric. Food Chem., 38, 18-24, doi.org/10.1021/jf00091a004
Muñoz-González I. et al., 2019. Chia (Salvia hispanica L.) a promising alternative for conventional and gelled emulsions: technological and lipid structural characteristics. Gels, 5, doi.org/10.3390/gels5020019
Nguyen Q.H. et al., 2020. Fatty acid composition and oil content during coriander fruit development. Food Chem., 326, 127034, doi.org/10.1016/j.foodchem.2020.127034
Petit-Pigeard R., 2002. Règlementation, expertise, mise sur le marché. Les enjeux de la propriété intellectuelle : brevet, certificat d’obtention végétale. OCL, 9(2), doi/org.10.1051/OCL.2002.0150
Roche J. et al., 2006. Management of environmental crop conditions to produce useful sunflower oil components. Eur. J. Lipid Sci. Technol., 108, 287-297, doi.org/10.1002/ejlt.200500310
Roche J., Mouloungui Z., Cerny M. & Merah O., 2016. Fatty acid and phytosterol accumulation during seed development in three oilseed species. Int. J. Food Sci., 51, 1820-1826, doi.org/10.1111/ijfs.13153
Roche J., Mouloungui Z., Cerny M. & Merah O., 2019. Effect of sowing dates on fatty acids and phytosterols patterns of Carthamus tinctorius L. Appl. Sci., 9, 2839, doi.org/10.3390/app9142839
Sandoval-Oliveros M.R. & Paredes-López O., 2013. Isolation and characterization of proteins from chia seeds (Salvia hispanica L.). J. Agric. Food Chem., 61, 193-201, doi.org/10.1021/jf3034978
Sayed Ahmad B., 2018. Étude de l’agroraffinage de graines d’Apiaceae, Lamiaceae et Chenopodiaceae pour la production de molécules biosourcées en vue d’application en industrie cosmétique. Thèse de doctorat : Institut National Polytechnique, Toulouse (France).
Segura-Campos M.R. et al., 2014. Chemical and functional properties of chia seed (Salvia hispanica L.) gum. Int. J. Food Sci., 2014, 241053, doi.org/10.1155/2014/241053
Zettel V. & Hitzmann B., 2018. Applications of chia (Salvia hispanica L.) in food products. Trends Food Sci. Technol., 80, 43-50, doi.org/10.1016/j.tifs.2018.07.011






