- Accueil
- Volume 27 (2023)
- Numéro 5 : Agrigenomics for Food and Health 2022 C...
- The effectiveness of melanin from the fungi Sclerotium cepivorum, Aspergillus niger, and Albifimbria verrucaria for use in dye-sensitive solar cells (DSSCs)
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
The effectiveness of melanin from the fungi Sclerotium cepivorum, Aspergillus niger, and Albifimbria verrucaria for use in dye-sensitive solar cells (DSSCs)

Document(s) associé(s)
Version PDF originaleRésumé
L'efficacité de la mélanine des champignons Sclerotium cepivorum, Aspergillus niger et Albifimbria verrucaria pour une utilisation dans les cellules solaires sensibles aux colorants (CSACs)
Description du sujet. De nouveaux pigments naturels sensibilisants respectueux de l'environnement avec des couleurs appropriées pour les cellules solaires sensibles aux colorants (CSACs) sont nécessaires. Ici, des structures mélanisées pour obtenir des pigments formés par Aspergillus niger, Sclerotium cepivorum et Albifimbria verrucaria ont été utilisées.
Objectifs. Le but de cette étude était de construire des cellules solaires CSACs pour étudier le fonctionnement de ces mélanines extraites de ces champignons.
Méthode. L'extraction de mélanine à partir de structures fongiques a été réalisée à l'aide de KOH-HCl et de NaOH-HCl et appliquée dans les CSACs.
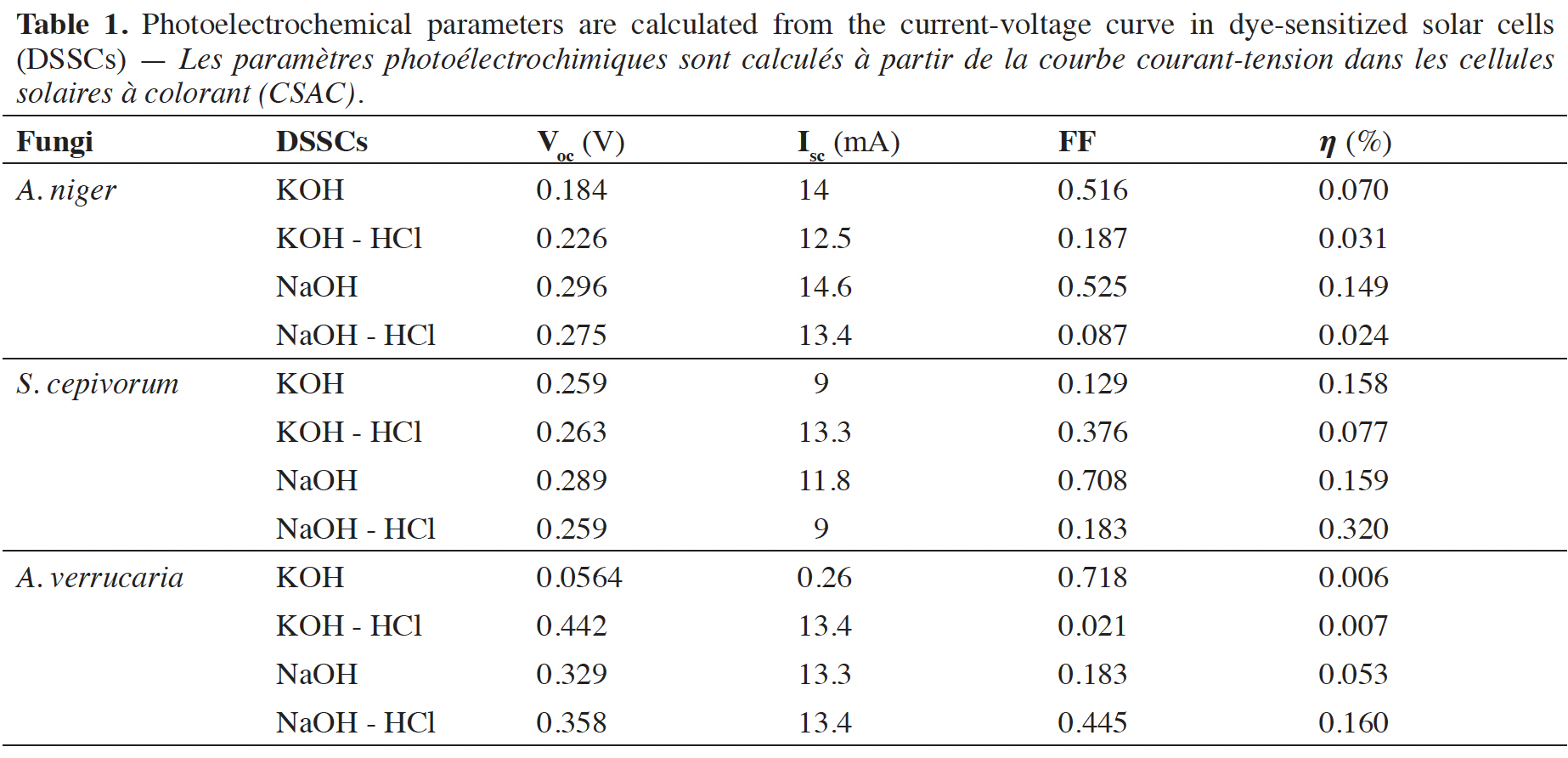
Résultats. La mélanine de A. verrucaria avait le meilleur FF (0,718), suivie de celle de S. cepivorum et A. niger (0,708 et 0,525). La mélanine de S. cepivorum avait l'efficacité la plus élevée (0,320 %), suivie par A. verrucaria (0,160 %) et A. niger (0,149 %).
Conclusions. Les courbes I-V et V-P des tests d'évaluation ont montré que la mélanine fongique se comportait comme un semi-conducteur. En conséquence, le développement de dispositifs de cellules solaires de type CSAC utilisant des extraits de différentes sources de mélanine fongique semble prometteur.
Abstract
Description of the subject. Novel sensitizer environmentally friendly natural pigments with suitable colors for dye-sensitive solar cells (DSSCs) are required. Here, melanized structures to obtain pigments formed by Aspergillus niger, Sclerotium cepivorum, and Albifimbria verrucaria, were used.
Objectives. The goal of this study was to build DSSC solar cells to study the operation of these extracted melanins from these fungi.
Method. Melanin extraction from fungal structures was carried out using KOH-HCl and NaOH-HCl and applied in the DSSC.
Results. The melanin from A. verrucaria had the best fill factor (FF) (0.718), followed by that from S. cepivorum and A. niger (0.708 and 0.525). Sclerotium cepivorum melanin had the highest effectiveness (0.320%), followed by A. verrucaria (0.160%) and A. niger (0.149%).
Conclusions. The I-V and V-P curves in the evaluation tests showed that fungal melanin behaved as a semiconductor; as a result, the development of DSSC-type solar cell devices employing extracts from different sources of fungal melanin appears promising.
Table des matières
Received 13 January 2023, accepted 29 August 2023, available online 9 October 2023.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1Considering the urgent demand for environmentally friendly energy sources (Kusumawati et al., 2021), new choices for natural pigment as a sensitizer in Dye-Sensitized Solar Cells (DSSCs) are required to reduce reliance on conventional silicon sources. Melanin is a complex, high-molecular-weight pigment made of a polymer of phenolic or indolic molecules which are among the most chemically stable materials (Casadevall et al., 2000; Valdés-Santiago et al., 2021; El-Naggar & Saber, 2022). The melanin from S. cepivorum previously displayed signals at 400-442, 500-600, and 655-700 nm, suggesting that it might be employed for light harvesting (Valdés-Santiago et al., 2021). However, there is no experimental proof of the use of fungus melanin from this or other microscopic fungi in DSSCs. Each component in a DSSC plays a significant role and works in tandem to achieve a high-power conversion efficiency. Since Albifimbria verrucaria, Sclerotium cepivorum, and Aspergillus niger are ascomycetes, they are known to produce DHN-melanins or allomelanins (Wheeler, 1983; Funa et al., 1999; Plonka & Grabacka, 2006) and those molecules can be found in the mycelium during sporulation, mainly in the resistance fungal structures for reproduction, survival or dispersal (Romero-Martinez et al., 2000; Kües & Fischer, 2006; Treseder & Lennon, 2015). In the present research, we probed the suitability of fungal melanins present in these organisms, not previously reported, as a natural photosensitizer of DSSCs.
2. Methodology
2.1. Strains and culture conditions
2Albifimbria verrucaria (Av-LVS, Biofunctional Metabolites Lab Collection), Sclerotium cepivorum (Sc-LPM, Valdés-Santiago et al., 2021); and Aspergillus niger (ATCC 1015) strains were selected according to their melanized structures pigmentation. All strains were grown using Potato Dextrose Agar (PDA) and Sabouraud Dextrose Agar (SDA) media and their mycelial growth was compared. Fungal melanized structures were recovered as follows: ten sclerotia from S. cepivorum and A. verrucaria were placed in plates to induce its germination and production of new sclerotia. Spore suspension of A. niger was collected with 5 ml of triton 0.01%, using a Digralsky stick, and 100 µL of 106 cells·ml-1 were cultured on Petri plates by dispersed with the same stick. Albifimbria verrucaria and S. cepivorum mycelium in active growth (4 mm diameter) were placed at the Petri plate center and radial mycelial growth was measured daily for seven days. Three independent biological repetitions with three replicates were performed in all assays.
2.2. Melanin extraction
3Pigment brown extraction was extracted from spores of A. niger and sclerotia of A. verrucaria and S. cepivorum. Fungal melanized structures were recovered with 5 ml of Triton 0.01%, centrifuged at 5,000 rpm·5 min-1, followed by two washes with distilled water supernatant removed. A volume of 5 ml of 2 M NaOH or 1 M KOH was added and incubated at room temperature for 24 h. After agitation in the vortex for 5 min, the sample was centrifugated at 5,000 rpm·5 min-1, the pigment was recovered from supernatant by vacuum filtration using a Millipore membrane (0.45 µm, Cat. No. HAWG047S6). Later, melanin was removed from membranes by washing with 3 ml of 2 M NaOH or 1 M KOH. To precipitate melanin, 3 ml of 2 M HCl was added to another group of samples. Dissolved melanin and acidified samples were probed in DSSC.
2.3. DSSC construction
4For the development of the DSSC, glasses coated on one of their faces with a conductive film of indium tin oxide ITO were used. Before starting the assembly, the grease chains on the conductive surfaces were eliminated, using 70% alcohol. In the preparation of the semiconductor paste, 6 g of TiO2 was used, mixed with 10 ml of acetic acid, and 1 ml of Triton X-100, which was mixed in a mortar until a uniform paste was obtained and left to rest for 15 min. For the preparation of the anode, the paste was placed on the conductive surface of one of the glass plates in such a way that it completely covered said surface, the paste was allowed to dry completely, to later place the melanin extract was left again. dry off. As a cell activator, a solution of 20 ml of distilled water with 0.2 g of KCl and 0.2 g of NaCl was prepared.
5In the manufacture of the cathode, 5 g of carbon was dissolved in distilled water and deposited on the conductive side of a second glass plate. Finally, for the assembly of the solar cell, the KCl solution with NaCl was added to the plate containing the extract, and the two plates were joined, on the side where both were stained with a small gap between them.
2.4. Current-voltage (I-V) curves
6Subsequently, the DSSCs are connected to an electrical circuit to perform voltage and current measurements. For this, a variable resistance was connected in parallel to the solar cell to take the different readings with which the graphs will be built. For current readings, the circuit is closed and for voltage, the circuit is opened. The readings were taken with a multimeter, connected to the electrical circuit through alligators which are connected at the ends of the cells and are held with clips to have better support and do not have movement.
7The tests that were carried out on the solar cells sensitized by melanin extracted from three different species of fungi were carried out between 12:00 am and 2:00 pm to take the readings at the point with the greatest amount of radiant energy from the sun. When exposed to solar radiation, the voltage (Voc) and current (Isc) generated by the cells were measured using a multimeter.
3. Results
3.1. Mycelial growth in PDA and SDA
8Mycelial growth using both culture media (PDA and SDA) of all strains was compared, and no difference was found, according to ANOVA and Tukey analysis with a significance of 95% (Figure 1). However, SDA medium favored an early development of the fungal melanized structures, and therefore, this medium was selected.
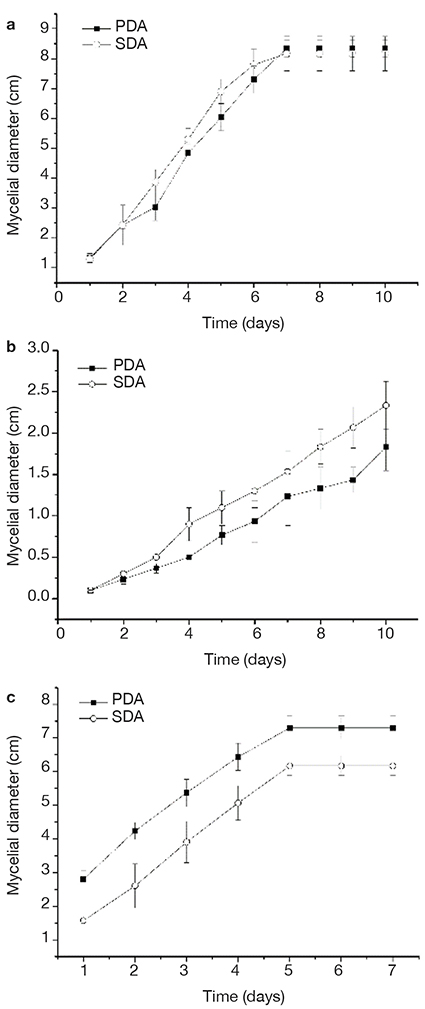
Figure 1. Mycelial growth in potato dextrose agar (PDA) and Sabouraud Dextrose Agar (SDA) of Albifimbria verrucaria (a), S. cepivorum (b), Aspergillus niger (c) — Croissance mycélienne dans la gélose au dextrose de pomme de terre (GDP) et dans la gélose au dextrose de Sabouraud (GDS) de Albifimbria verrucaria (a), S. cepivorum (b), Aspergillus niger (c).
Significant differences in the means (one-way ANOVA, p < 0.0005) — différences significatives dans les moyennes (ANOVA unidirectionnelle, p < 0,0005).
3.2. Photoelectric conversion efficiency of melanin from A. niger, A. verrucaria, and S. cepivorum
9The fill factor (FF) and efficiency () of the solar cells mentioned in this article were estimated based on the maximum voltage, open-circuit voltage, maximum current, and short-circuit current, which were determined from the I-V curve. To calculate the fill factor and the efficiency of the DSSC, the equations described by Tiwari & Dubey (2010) were used, according to the author, the fill factor is equal to the maximum current (Imax) times the maximum voltage (Vmax), between the open circuit voltage (Voc) times short circuit current (Isc), described in Equation (1). The theoretical maximum fill factor (FF) is 1:
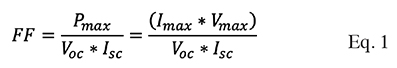
10And on the other hand, the efficiency of the cell is equal to the open circuit voltage times the short circuit current times the fill factor, between the solar radiation times the area, described in Equation (2):
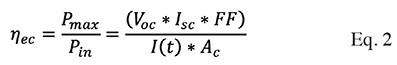
11The values of maximum current and maximum voltage were obtained from each of the cells assembled with each of the evaluated fungal dyes and, these data were used for calculating the fill factor as well as the efficiency of the manufactured devices (Figure 2).
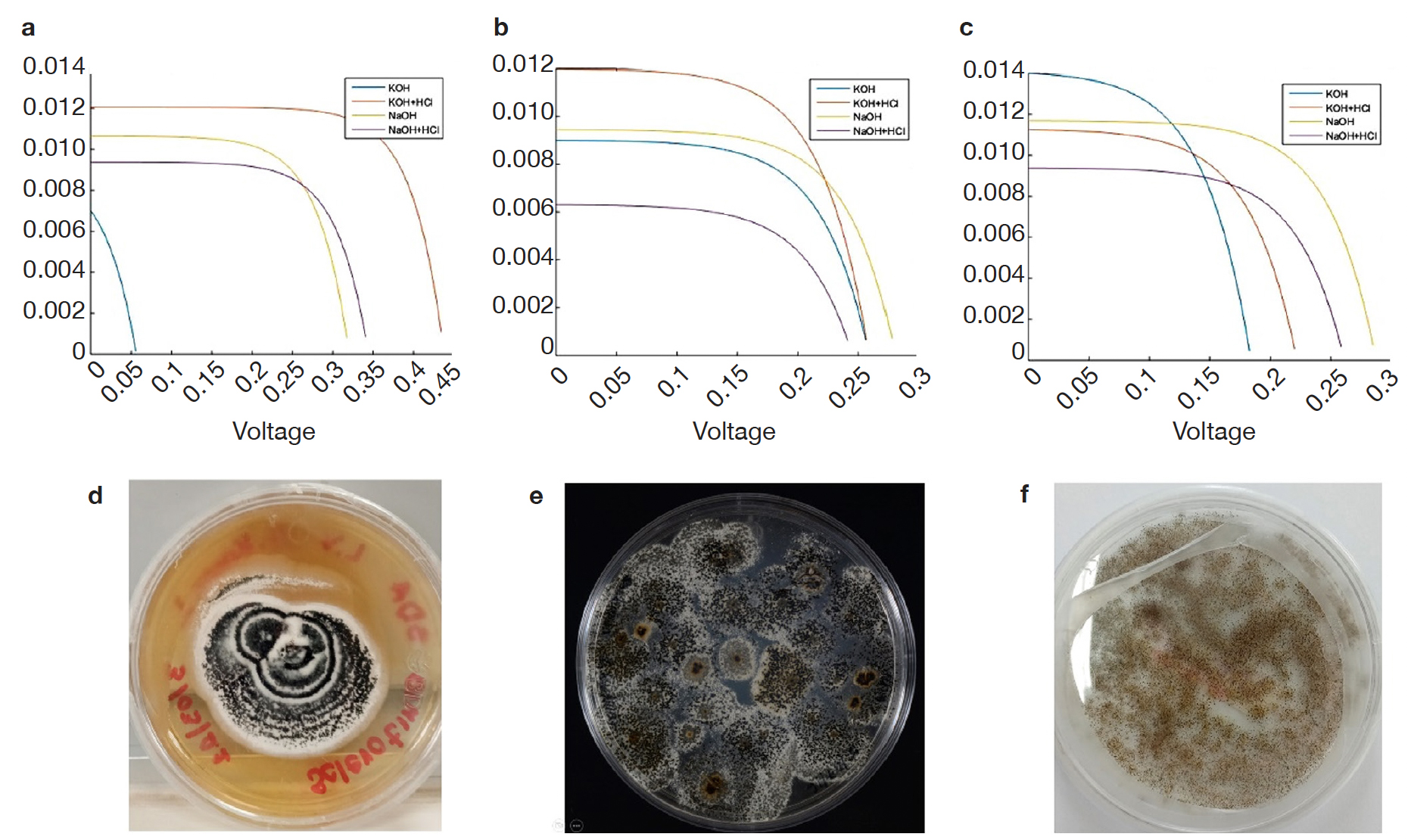
Figure 2. Current-voltage curves of melanized fungal structures and colonial morphology: Albifimbria verrucaria (a, d), S. cepivorum (b, e), Aspergillus niger (c, f) — Courbes courant-tension des structures fongiques mélanisées et morphologie coloniale : Albifimbria verrucaria (a, d), S. cepivorum (b, e), Aspergillus niger (c, f).
12Therefore, the results shown in table 1 indicate that the sensitized organic solar cells that used KOH for extraction and as solvent are the ones that present the best performance in terms of FF and efficiency ().
4. Discussion
13The higher efficiency of the organic solar cells recorded and analyzed in this research is 0.3 %, since the photon conversion efficiency as a function of current depends on the collection of the incident light, specifically, it depends on the intensity. The survivability of this sensitizer is intriguing since, when compared to silicon solar cells, it is less expensive and more readily available, making it a potential replacement. However, the application of natural pigments as photosensitizers can translate into alternatives for the development of low-cost and environmentally friendly DSSCs. Plants and fungi, and microorganisms such as fungi, yeast, molds, bacteria, cyanobacteria, and microalgae, possess a range of important pigments: those related to the photosynthetic process and those produced as secondary metabolites, all with the potential to be used as sensitizers. Furthermore, the use of these sources of sensitizers has the advantage of having a shorter growth time, which means a shorter time to obtain the pigments, a high production of biomass, and the potential to scale up the production using a bioreactor at controlled conditions. Fungi are known to possess various kinds of pigments such as carotenoids, melanins, and quinones, among others (Dufosse et al., 2014). In the comparison of the photoconversion efficiency of pigments as sensitizers between algae and microalgae (photosynthetic organisms), bacteria and fungi (non-photosynthetic organisms), the efficiency of fungal pigments is intermediate (from 0.26% to 2.3%) in comparison with the higher efficiency of photosynthetic pigments (0.001% to 4.6%) with that of bacterial pigments (0.004% to 1.67%) according to Orona-Navar et al. (2021).
14Indicated values for solar efficiency for green algae are 0.1 % with 0.397 of FF and 0.69 for algal pigments from the brown seaweed Undaria pinnatifida (Calogero et al., 2014). However, an extract of the fruiting bodies of Cortinarius spp. presented higher efficiency (0.64%) and FF (65.9%) (Zalas et al., 2015). Pigments from the green algae, Cladophora sp., were FF, 0.60%, and efficiency of 0.085% (Lim et al., 2015).
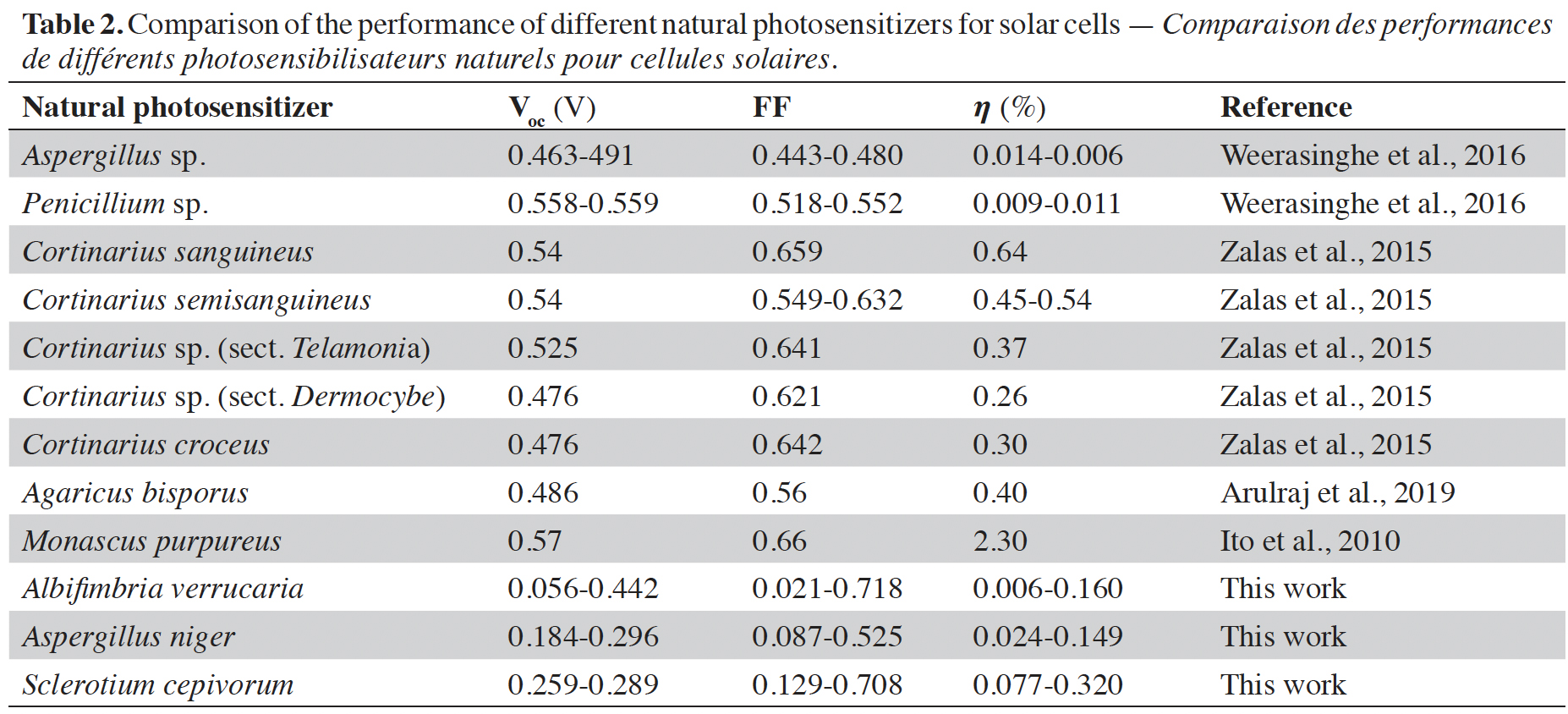
15The performance of various natural photosensitizers including those developed in this article and that have been used in organic solar cells is compared in table 2. The values previously reported by other researchers around the world and those found in this article are in the range indicated by the authors in Orona-Navar et al. (2021). Although the values of open circuit voltage (Voc), fill factor (FF), and efficiency () obtained are not so high, the truth is that they provide a possibility to develop dyes with natural photosensitizers derived from fungi to offer biodegradable and environmentally friendly alternatives. The fill factor values achieved in our work are higher than those achieved by previously reported results. The values of the open circuit voltage are lower but by implementing them in series it is possible to increase the voltage reached by the solar cell. Finally, the conversion efficiency values reached in our work are within the values reached in other previously published reports.
16To illustrate the photovoltaic conversion efficiency (PCE) values that the different natural photosensitizers can offer, figure 3 has graphically compiled the possible ranges for different natural dyes used in solar cells. The highest reported photovoltaic conversion efficiency for fungi-based dyes is 2.3%. However, through performance refinement, higher efficiencies are likely to be achieved when the dye properties are fully exploited, as occurred even with the silicon solar cells now in use. Although the development of these dyes is still in its infancy to be taken to the commercialization phase, natural photosensitizers represent an interesting alternative to organic dyes thanks to their wide availability, low-cost production, implementation in modules, and environmental friendliness. Since photovoltaic conversion efficiency represents the percentage of solar energy that is converted into useful electricity, researchers are continually proposing technological alternatives to improve efficiency derived from solar cell components such as the dye used. The dyes discussed in this article seek to eliminate the presence of metals in the dye and seek to increase conversion efficiency. Organic chromophores such as 3,3'-dithioalkyl-2,2'-bithiophene (SBT) used as dyes have been synthesized and optimized with co-adsorbents such as chenodeoxycholic acid (CDCA) reaching 9.46% efficiency, but it is of a synthetic type and was not obtained from natural products (Lin et al., 2020). In our case, the performance of the natural dye has not been optimized. One strategy is to extend the light conversion spectrum and molar extinction coefficient to increase the number of photons that can be converted to electrons by adjusting associated kinetic and electrical processes including electron injection, relaxation vibration of the excited dyes, and the excitation of the dye. The advantage of the dye obtained with fungi in these solar cells is the strong interaction between it and TiO2, as has been reported in Narayan (2012).
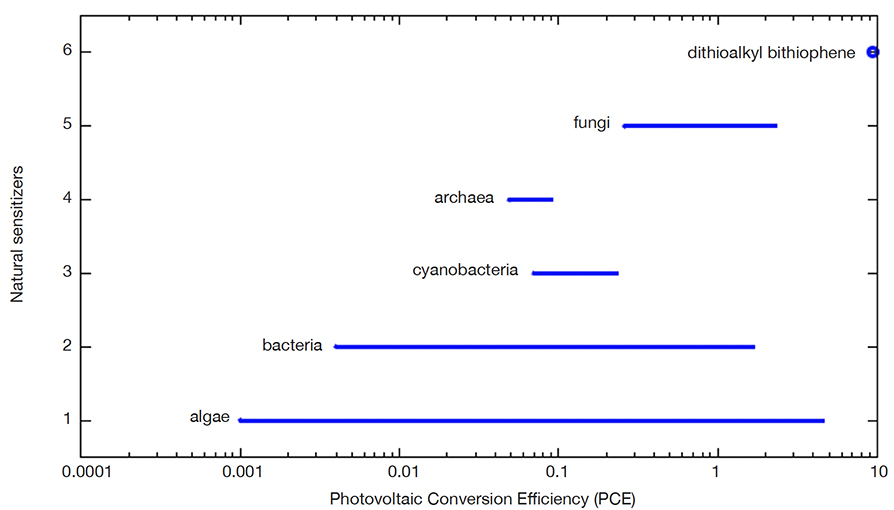
Figure 3. Solar photovoltaic conversion efficiency (PCE) ranges for different natural photosensitizers — Plages d’efficacité de conversion solaire photovoltaïque (ECP) pour différents photosensibilisateurs naturels.
17Metal-free dyes such as natural photosensitizers have unique properties related to solar cell electrochemistry, tunable photon absorption, low cost, and tunable molecular engineering. The efficiency of dyed organic solar cells can reach values up to 14% when co-sensitizers and co-absorbers are used (Kakiage et al., 2015), which was not developed in this preliminary study. The dye must present high optical absorption properties and preferably with a simple structure and be easy to synthesize. The main idea of this work was to present the application of other fungal species not yet reported in the literature that could provide dyes to be exploited in the implementation of dye-sensitized organic solar cells. The performance of the solar cells implemented in this work can be improved in future work by considering methodologies to improve the stability of the natural dyes obtained, which depends on physical and chemical effects that may be present during the operation of the solar cell and that change during their useful life (Kabir et al., 2022), and offer an alternative on the design of non-toxic and ecologically friendly DSSC’s.
5. Conclusions
18This study investigated the potential use of melanin extracted from three fungal species, Sclerotium cepivorum, Aspergillus niger, and Albifimbria verrucaria, as sensitizers in dye-sensitive solar cells (DSSCs). The potential to improve the performance of DSSCs through the evaluation of the photovoltaic characteristics of these natural pigments was tested. The results showed that the melanin from A. verrucaria exhibited the highest fill factor (FF) of 0.718, followed by S. cepivorum (0.708) and A. niger (0.525). In terms of effectiveness, S. cepivorum melanin demonstrated the highest value (0.320%), followed by A. verrucaria (0.160%) and A. niger (0.149%). The study indicated these fungal melanins behaved as a semiconductor in the DSSCs, suggesting their potential for use in solar cell devices, including benefits such as their widespread availability, low-cost production, and the creation of an environmentally friendly device. However, the efficiency values obtained from these allomelanins were relatively modest compared to other sensitizers, and therefore, further optimization to enhance the photovoltaic performance of these natural pigments needs to be investigated to fully exploit their capabilities in solar cell technology.
Bibliographie
Arulraj A. et al., 2019. Photovoltaic performance of natural metal free photo-sensitizer for TiO2 based dye-sensitized solar cells. Optik, 181, 619-626, doi.org/10.1016/j.ijleo.2018.12.104
Calogero G. et al., 2014. Brown seaweed pigment as a dye source for photoelectrochemical solar cells. Spectrochim. Acta A Mol. Biomol. Spectrosc., 117, 702-706, doi.org/10.1016/j.saa.2013.09.019
Casadevall A., Rosas A.L. & Nosanchuk J.D., 2000. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol., 3, 354-358, doi.org/10.1016/S1369-5274(00)00103-X
Dufosse L. et al., 2014. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol., 26, 56-61, doi.org/10.1016/j.copbio.2013.09.007
El-Naggar N.E.-A. & Saber W.E.I.A., 2022. Natural melanin: current trends, and future approaches, with especial reference to microbial source. Polymers, 14(7), 1339, doi.org/10.3390/polym14071339
Funa N. et al., 1999. A new pathway for polyketide synthesis in microorganisms. Nature, 400, 897-899, doi.org/10.1038/23748
Ito S. et al., 2010. Fabrication of dye-sensitized solar cells using natural dye for food pigment: Monascus yellow. Energy Environ. Sci., 3, 905-909, doi.org/10.1039/c000869a
Kabir F. et al., 2022. Instability of dye-sensitized solar cells using natural dyes and approaches to improving stability – an overview. Sustainable Energy Technol. Assess., 52, 102196, doi.org/10.1016/j.seta.2022.102196
Kakiage K. et al., 2015. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. ChemComm, 51(88), 15894-15897, doi.org/10.1039/C5CC06759F
Kües U. & Fischer R., 2006. Growth, differentiation and sexuality. Vol. 1. Heidelberg, Germany: Springer Science & Business Media, doi.org/10.1007/3-540-28135-5
Kusumawati Y., Hutama A.S., Wellia D.V. & Subagyo R., 2021. Natural resources for dye-sensitized solar cells. Heliyon, 7(12), e08436, doi.org/10.1016/j.heliyon.2021.e08436
Lim A. et al., 2015. Higher Performance of DSSC with dyes from Cladophora sp. as mixed cosensitizer through synergistic effect. J. Biophys., 2015, 510467, doi.org/10.1155/2015/510467
Lin F.-S. et al., 2020. Metal-free efficient dye-sensitized solar cells based on thioalkylated bithiophenyl organic dyes. J. Mater. Chem. C, 8(43), 15322-15330, doi.org/10.1039/d0tc02310h
Narayan M.R., 2012. Review: dye sensitized solar cells based on natural photosensitizers. Renewable Sustainable Energy Rev., 16, 208-215, doi.org/10.1016/j.rser.2011.07.148
Orona-Navar A. et al., 2021. Alternative sources of natural pigments for dye-sensitized solar cells: algae, cyanobacteria, bacteria, archaea and fungi. J. Biotechnol., 332, 29-53, doi.org/10.1016/j.jbiotec.2021.03.013
Plonka P.M. & Grabacka M., 2006. Melanin synthesis in microorganisms — biotechnological and medical aspects. Acta Biochim. Pol., 53, 429-443, doi.org/10.18388/abp.2006_3314
Romero-Martinez R. et al., 2000. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun., 6, 3696-3703, doi.org/10.1128/iai.68.6.3696-3703.2000
Tiwari G.N. & Dubey S., 2010. Fundamentals of photovoltaic modules and their applications. Cambridge, UK: Royal Society of Chemistry, 101, doi.org/10.1039/9781849730952
Treseder K.K. & Lennon J.T., 2015. Fungal traits that drive ecosystem dynamics on land. Microbiol. Mol. Biol. Rev., 2, 243-262, doi.org/10.1128/MMBR.00001-15
Valdés-Santiago L. et al., 2021. Application of two-photon microscopy to study Sclerotium cepivorum Berk sclerotia isolated from naturally infested soil and produced in vitro. Curr. Microbiol., 78(2), 749-755, doi.org/10.1007/s00284-020-02341-4
Weerasinghe W.A.B.S., Weerakkody W.J.S.K. & Perera G.A.K.S., 2016. Performance of fungal dyes in dye sensitized solar cells as photosensitizers. In: Proceedings of 15th Agricultural Research Symposium (AGRESS), 28-29 June 2016, Makandura, Wayamba University of Sri Lanka, 441-444.
Wheeler M.H., 1983. Comparisons of fungal melanin biosynthesis in ascomycetous, imperfect and basidiomycetous fungi. Trans. Br. Mycol. Soc., 81(1), 29-36, doi.org/10.1016/S0007-1536(83)80200-9
Zalas M., Gierczyk B., Bogacki H. & Schroeder G., 2015. The Cortinarius fungi dyes as sensitizers in dye-sensitized solar cells. Int. J. Photoenergy, 2015, 653740, doi.org/10.1155/2015/653740






