- Accueil
- Volume 27 (2023)
- Numéro 5 : Agrigenomics for Food and Health 2022 C...
- Zimapan dam in Mexico, a reservoir of extended-spectrum-beta-lactamase-producing bacteria and arsenic resistance
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
Zimapan dam in Mexico, a reservoir of extended-spectrum-beta-lactamase-producing bacteria and arsenic resistance

Document(s) associé(s)
Version PDF originaleRésumé
Barrage de Zimapan au Mexique, réservoir de bactéries productrices de bêta-lactamases à spectre étendu et de résistance à l'arsenic
Description du sujet. En milieu aquatique, une exposition prolongée aux métaux lourds et/ou aux antimicrobiens sélectionne des bactéries résistantes, augmentant ainsi le risque pour la santé de la population.
Objectifs. Évaluer la résistance aux antimicrobiens et à l'arsenic (As), la présence de systèmes de dépendance et la production de biofilm dans les bacilles à Gram-négatif isolés du barrage de Zimapan.
Méthode. Des échantillons d'eau de surface ont été prélevés sur 10 sites dans la zone ouest du barrage. Des plaques CHROMagar™ ESBL et mSuperCARBA™ ont été utilisées pour isoler des bacilles à Gram-négatif fermentant (FGB) et non fermentant (NFGB) résistants aux bêta-lactamines et aux carbapénèmes, respectivement. La résistance a été vérifiée par la méthode de diffusion sur gélose et le test de synergie double disque. Les gènes des bêta-lactamases à spectre étendu (BLSE), des carbapénémases et des systèmes de dépendance ont été détectés par la réaction en chaîne par polymérase. La production de biofilm et la résistance à l'As et/ou au céfotaxime (CTX) ont été mesurées par spectrophotométrie. La tolérance à l’As a été déterminée par des dilutions en série sur de la gélose LB en ajoutant diverses concentrations de Na3AsO2.
Résultats. Sur 47 souches résistantes aux bêta-lactamines, 77,5 % produisaient des BLSE, les gènes blaCTX-M (38 % et 62 %) et blaTEM-2 (41 % et 51 %) étant détectés respectivement dans les zones de pisciculture et de loisirs. Une résistance significative à l’imipénème et au méropénème a été détectée, bien qu’aucun gène de carbapénémase n’ait été détecté. Les gènes du système addictif relE, pnd, ccdA et vagC étaient les plus fréquents. Dix-neuf souches ont montré une résistance jusqu'à 183 ppm de Na3AsO2, 11 souches jusqu'à 400 ppm et 3 souches jusqu'à 2 000 ppm. La production de biofilm a été détectée dans 83 % des souches.
Conclusions. La contamination du barrage de Zimapan par des bactéries multirésistantes, formant un biofilm, tolérantes à l'As et au CTX avec différents systèmes de dépendance aux plasmides nécessite des stratégies urgentes de prévention et de contrôle environnemental.
Abstract
Description of the subject. In aquatic environments, prolonged exposure to heavy metals and/or antimicrobials selects for resistant bacteria, increasing the health risk for the population.
Objectives. To evaluate antimicrobial and arsenic (As) resistance, presence of addiction systems and biofilm production in Gram-negative bacilli isolated from the Zimapan dam.
Method. Surface water was sampled from 10 sites in the western area of the dam. CHROMagar™ ESBL and mSuperCARBA™ plates were used to isolate fermenting (FGB) and non-fermenting (NFGB) Gram-negative bacilli resistant to beta-lactams and carbapenems, respectively. Resistance was verified by the agar-diffusion method and double-disk synergy test. Genes for extended spectrum beta-lactamases (ESBL), carbapenemases and addiction systems were detected by PCR. Biofilm production and resistance to As and/or cefotaxime (CTX) were measured by spectrophotometry. The tolerance As was determined by serial dilution in LB agar adding variable concentrations of Na3AsO2.
Results. Of 47 beta-lactams resistant strains, 77.5% produced ESBL, with blaCTX-M (38% and 62%) and blaTEM-2 (41% and 51%) genes being detected in pisciculture and recreational areas, respectively. Significant resistance to imipenem and meropenem was detected, although no carbapenemase genes were detected; relE, pnd, ccdA, and vagC addiction system genes were the most frequent. Nineteen strains showed resistance up to 183 ppm of Na3AsO2, 11 strains at 400 ppm and 3 strains up to 2,000 ppm. Biofilm production was detected in 83% of the strains.
Conclusions. Contamination of the Zimapan dam with multidrug-resistant, biofilm-forming, As- and CTX-tolerant bacteria with different plasmid addiction systems requires urgent prevention and environmental control strategies.
Table des matières
Received 12 February 2023, accepted 30 May 2024, available online 18 June 2024.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1In Mexico, the Zimapan dam maintains recreational and pisciculture areas with species of tilapia, carp, and bass (Figure 1) (Flores, 2017). Contamination of this dam by arsenic (As) has been reported in the water and sediments (Armienta et al., 1997; Armienta et al., 2001; Mendez & Armienta, 2003; Perez et al., 2003; Pérez, 2004; Osuna-Martinez et al., 2021), while population monitoring showed risks of cardiovascular disease by exposure to As in children and residents from the Zimapan region (Torres-Arellano et al., 2020). Microorganisms can adapt to different levels of As concentration (Mellado et al., 2011; Muñoz-Silva et al., 2019). This selective pressure causes the development of chromosomal or plasmid mechanisms for decontamination and/or tolerance to As, which has been reported to be linked to antimicrobial resistance (Kaur et al., 2011). In this sense, chromosomal genes arsRBC of the arsenic operon have been reported in Pseudomonas aeruginosa, thereby conferring increased resistance to sodium arsenate and sodium arsenite (Prithivirajsingh et al., 2001). In addition, bacterial plasmids conferring arsenic resistance encode specific pumps that extrude arsenite (AsIII). In Gram-negative bacteria, the efflux pump consists of a complex formed by an ATPase (ArsA) associated with a membrane anion channel (ArsB). Furthermore, arsenate (AsV) is converted to arsenite by a soluble reductase (ArsC). Proteins ArsB and ArsC, but not the ATPase, are also found in Gram-positive bacteria (Cervantes, 1995). The transformation of As as a bacterial defense mechanism and its association with the production of biofilms provides a suitable environment for fish pathogens to resist antimicrobial treatments (Cai & Arias, 2017; Pandey & Kumar, 2021). The prophylactic and metaphylactic use of antimicrobials in aquaculture is associated with resistance because they are not specific to aquaculture and persist and spread in the aquatic environment (Cabello, 2006; Burridge et al., 2010; FAO, 2017), promoting co-resistance and cross-resistance in human pathogens (Kerry et al., 1996; Rhodes et al., 2000; Furushita et al., 2003; EMA, 2014). In Mexico, the most used antibiotics in aquaculture to counteract infections by bacteria of the genus Vibrio are oxytetracycline, florfenicol, ormethoprim - sulfamethoxazole, sarafloxacin and enrofloxacin (Santiago et al., 2009). Aquaculture systems are "genetic reactors" of new resistance profiles and genetic exchange (Sørum, 2008; Cabello et al., 2013; Watts et al., 2017). In sediments, bacteria have shown to possess plasmid-coded beta-lactamases (Sousa et al., 2011; Yang et al., 2013; Czekalski et al., 2014; Chen et al., 2015) or plasmid addiction systems that remain in the bacteria even in the absence of selection (Tsang, 2017), this shows the need to evaluate antimicrobial resistance in the Zimapán dam, as well as to characterize the capacity of biofilm formation of the resistant isolates. The obtained data will be helpful for designing prevention measures in areas where pisciculture, recreational activities and water consumption are carried out.
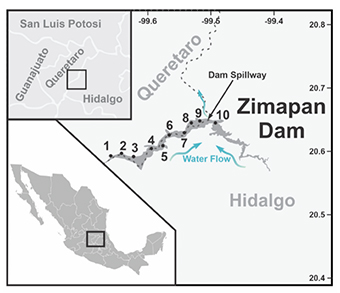
Figure 1. Map of the Zimapan dam showing the sampling sites. Sites 1-3 are in the hot springs (recreational zone), sites 4-7 are located along the central part of the dam, and sites 8-10 are located around the fish farms — Carte du barrage de Zimapan montrant les sites d’échantillonnage. Les sites 1 à 3 sont situés dans les sources chaudes (zones de loisirs), les sites 4 à 7 sont situés le long de la partie centrale du barrage et les sites 8 à 10 sont situés autour des piscicultures.
2. Materials and methods
2.1. Sampling and isolation of bacterial strains
2The Zimapan dam is located in the so-called Infiernillo canyon, formed mainly by the San Juan and Tula rivers, spilling out to form the Moctezuma River (Figure 1). The dam floods land in the municipalities of Zimapan, Tasquillo and Tecozautla, in the state of Hidalgo, and the municipality of Cadereyta de Montes in the state of Querétaro. The dam has an approximate area of 2,600 ha, is elongated and has an exit (arch) in the center. Fishing and aquaculture activities are among the productive activities carried out around the Zimapan dam, followed by agriculture and recreational areas.
3For this study, surface water was sampled from 10 sites along the western zone (Figure 1), numbered from upstream (site 1) to the central outlet (site 10). Sites 1-3 are in the hot springs (recreational zone), sites 4-7 are located along the central part of the west side, with higher agricultural activities, and sites 8-10 are where the fish farms are located.
4For the intake of surface water, the protocol established by the Mexican standard NMX-AA-042-SCFI-2015 for WATER ANALYSIS was followed (https://www.gob.mx/cms/uploads/attachment/file/166147/nmx-aa-042-scfi-2015.pdf), but the amount of water obtained was increased (500 ml, 0.5 m depth). Surface water taken from all sampling locations were placed in preprepared sterile glass bottles and stored in a refrigerator at 4 ℃ as soon as possible for processing within 24 h. All water samples were plated on MacConkey Agar to detect Gram-negative bacilli as biomarkers of dam water quality (Ríos-Tobón et al., 2017) and grouped as fermenting (FGB) and non-fermenting (NFGB) Gram-negative bacilli. The quality of the water samples was classified according to what was reported by Muñoz-Rojas et al. (2016): A; completely satisfactory (0 Colony Forming Unit·milliliter-1 [CFU·ml-1]), B; satisfactory and represents low risk (1-10 CFU·ml-1, C; marginally unsatisfactory (10-100 CFU·ml-1), D; unsatisfactory with high risk (100-1,000 CFU·ml-1), E; unacceptable with very high risk (> 1,000 CFU·ml-1).
5Subsequently, each bacterial isolate was grown in LB broth (Luria Bertani), incubating for 24 h at 37 °C. They were then grown in chromogenic and selective culture media of CHROMagar™ ESBL and mSuperCARBA™, to detect bacteria resistant to beta-lactams and carbapenems, respectively, following the manufacturer's specifications.
2.2. Phenotypic detection of Extended Spectrum Beta-Lactamases (ESBL)
6The agar diffusion method was used to evaluate resistance to the following antimicrobials: ceftazidime (30 µg) [CAZ] and cefotaxime (30 µg) [CTX], both with or without clavulanic acid (10 µg) [CLA], meropenem (10 µg) [MEM] and imipenem (10 µg) [IMP]. A double disk synergy test (DDST) was performed to confirm ESBL. A difference greater than or equal to 5 mm in inhibition halos between CAZ-CLA and CAZ discs or between CTX-CLA and CTX was interpreted as positive.
2.3. Detection of antimicrobial resistance genes (ARGs)
7Genomic DNA was extracted using the Wizard Genomic kit (Promega), following the manufacturer's instructions. PCR was performed for the detection of the ESBL genes blaSHV (encodes for a β-lactamase enzyme known as SHV [sulfhydryl variable]), blaTEM (encodes for a β-lactamase enzyme known as TEM [Temoneira]), and blaCTX-M (encodes for a β-lactamase enzyme known as CTX-M [cefotaximase]) (Galani et al., 2002; Aarestrup et al., 2003; Edelstein et al., 2003). In addition, Multiplex PCR was performed for the amplification of the following genes that confer resistance to carbapenems: blaVIM (encodes for a metallo-β-lactamase enzyme known as VIM [Verona integron-encoded metallo-β-lactamase]), blaOXA (encodes for an oxacillinase enzyme), blaIMP, blaSPM (encodes for a metallo-β-lactamase enzyme known as São Paulo metallo-β-lactamase [SPM]), blaNDM (encodes for New Delhi metallo-β-lactamase [NDM]), and blaKPC (encodes for Klebsiella pneumoniae carbapenemase [KPC]) (Poirel et al., 2011). The amplified products were run on 2% agarose gel, stained with Gel Green, in 1X TBE buffer at 80 volts for 1 h, and the results were analyzed on the iBright CL1000.
2.4. Characterization of plasmid addiction systems
8Plasmid DNA was extracted using the Purelink™ Quick Plasmid Miniprep Kit (ThermoFisher Scientific) according to the manufacturer's specifications. Five genes of plasmid addiction systems regulated by protein-antitoxins were amplified by PCR: pemK (plasmid emergency maintenance), ccdA/B (coupled cell division), relB/E (relaxed control of stable RNA synthesis), parD/E (encode the antitoxin PemI identical to Kis, killing suppressor), vagC/D (virulence-associated protein), and three plasmid systems regulated by antisense RNA: hok/sok (host killing and suppression of killing genes from plasmid), srnB/C (stable RNA degradation) and pndC/A (promotion of nucleic acid degradation) (Mnif et al., 2010).
2.5. Characterization of As and beta-lactam tolerance
9The tolerance was determined by serial dilution in a plate with LB agar adding variable concentrations of Na3AsO2 [sodium arsenite] (25; 50; 100; 150; 183; 400; 600; 1,200; 1,400; 1,600; 1,800 and 2,000 ppm). The bacterial cultures were plated at 0.5 McFarland. LB plates without As were inoculated to be used as controls. They were incubated at 37 ºC for 72 h (Campos et al., 2007).
10To measure the effect on bacterial growth with cefotaxime and Na3AsO2, the optical density was measured in 96-well plates (absorbance at 520/540 nm in X-Mark BioRad spectrophotometer). The strains were placed in LB broth with cefotaxime (300 µg·ml-1) and As (400 ppm), As alone, cefotaxime alone, and with neither of those components and the optical density was measured every 2 h for 24 h. Each strain was cultivated in duplicate, and negative controls were mounted on each plate.
2.6. Production of biofilm
11The production of biofilm was assessed according to Kwiecińska-Pirog et al. (2020). Overnight cultures of the strains (2 µL) were added to TSB (Tryptic Soy Broth) supplemented with 1% glucose (198 µL). The biofilm was quantified by measuring the optical density after staining by crystal violet at 570 nm. The P. aeruginosa strain (M-PA01) was used as a positive control, and sterile broth culture as a negative control to verify sterility and non-specific binding. The cut-off point was calculated using the negative control (NC), considering negative strains those with values ≤ average (0.084) + 3σ (0.007) of the negative control and biofilm producers with values > than the cut-off (0.106).
3. Results
12In 10 sampled sites in the Zimapan dam, 47 strains were identified as resistant to beta-lactams, of which 68% (32/47) were FGB, and 32% (15/47) were NFGB. The distribution of these 47 strains was not homogeneous along the sampling site (Figure 2), with the largest number of strains found in the middle of the west dam (sites 4-6), where most of the agricultural activities around the dam is taking place.
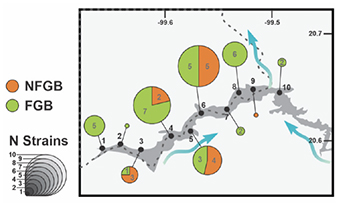
Figure 2. Distribution of the strains identified as resistant to beta-lactams in the different sampling sites of the Zimapan Dam — Répartition des souches identifiées comme résistantes aux bêta-lactamines dans les différents sites de prélèvement du barrage de Zimapan.
13Of these 47 resistant strains, 77.5% were positive for ESBL by CHROMagar™ ESBL and DDST, which coincides with a high frequency of resistance to CAZ and CTX (Figure 3a) and a high frequency of genes blaCTX-M y blaTEM-2 genes (Figure 3b). Significant resistance to imipenem and meropenem was found in these strains (Figure 3a), although the studied carbapenemase genes were not found. In addition, 38% of strains carrying blaCTX-M and 41% of blaTEM-2 were located in the pisciculture area (around and inside cages). The remaining 62% of blaCTX-M- and 51% of blaTEM-2-carrying bacteria were found in the recreational area.
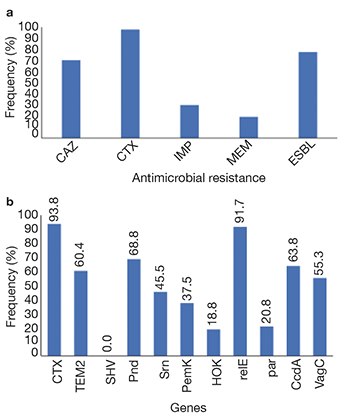
Figure 3. Frequency of antimicrobial resistance and addiction system: a. phenotypic resistance to 3rd generation of cephalosporins, carbapenems and ESBLs; b. frequency of antimicrobial resistance genes and plasmid addiction genes — Fréquence de la résistance aux antimicrobiens et des systèmes d’addiction : a. résistance phénotypique aux céphalosporines de 3è génération, carbapénèmes et BLSE ; b. fréquence des gènes de résistance antimicrobiens et des gènes de dépendance aux plasmides.
14The isolated bacteria presented different plasmid addiction systems in both areas, with relE, pnd, ccdA, and vagC being the most frequent (Figure 3b). Of these, 41%, 33%, 53%, and 46%, respectively, were found in pisciculture areas, while 51%, 67%, 47%, and 54% of these genes, respectively, were found in the recreational area. Only 8% of the plasmid addiction system relE were found in the strains from the central sites of the west dam.
15Many of the strains showed resistance up to 183 ppm of As (19 strains), but a smaller proportion grew still at 400 ppm (11 strains), with 3 strains withstanding up to 2,000 ppm (Figure 4). FGB showed a different growth pattern than the NFGB in the negative controls (Figure 5a and Figure 6A), but the growth pattern was affected under the effect of either or both As and CTX. Nevertheless, the strains showed very slow growth in both groups of bacteria at an early phase, after 8-12 h of incubation, the strains started to adapt and increase their growth, reaching significant levels, with optical densities 1.0-1.4 for FGB (Figures 5b and 5c), and 1.1-1.6 for NFGB (Figure 6b and 6c), showing, first that when exposed to these compounds, the bacteria is able to turn on mechanisms that let them not only survive but even grow up to significant densities, and second, that all of the strains isolated for being resistant to beta-lactams (as seen in the presence of CTX) are also resistant of As, which was not used for selecting these bacteria, showing a clear association between both types of resistance. The worse inhibition was seen when both compounds were present (Figure 5d and Figure 6d), which could be affecting the bacteria at multiple systems, making their growth slower, although, not killing them.
16Regarding the production of biofilm, 83% (39/47) of the strains presented biofilm production (Figure 7), showing very similar proportions in both groups but observing more variations in the optical densities in the NFGB.
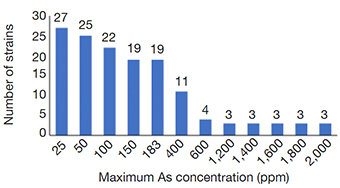
Figure 4. Capacity of As resistance at increasing concentrations among the 47 isolated strains. Bars are showing the number of strains still able to grow at each particular As concentration — Capacité de résistance à l’arsenic à des concentrations croissantes chez les 47 souches isolées. Les barres indiquent le nombre de souches encore capables de croître à chaque concentration particulière d’As.
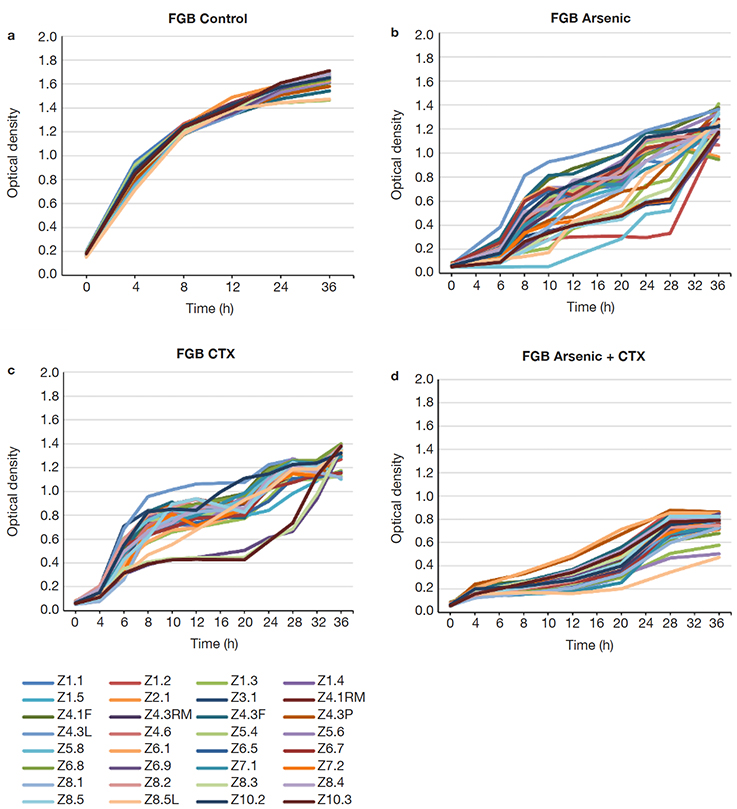
Figure 5. Growth curves of the FGB strains isolated in this study: a. LB broth, b. LB broth + Na3AsO2 (400 ppm), c. LB broth + cefotaxime (300 µg.ml-1), d. LB broth + Na3AsO2 (400 ppm) + cefotaxime (300 µg.ml-1) — Courbes de croissance des souches FGB isolées dans cette étude : a. bouillon LB, b. bouillon LB + Na3AsO2 (400 ppm), c. bouillon LB + céfotaxime (300 µg.ml-1), d. bouillon LB + Na3AsO2 (400 ppm) + céfotaxime (300 µg.ml-1).
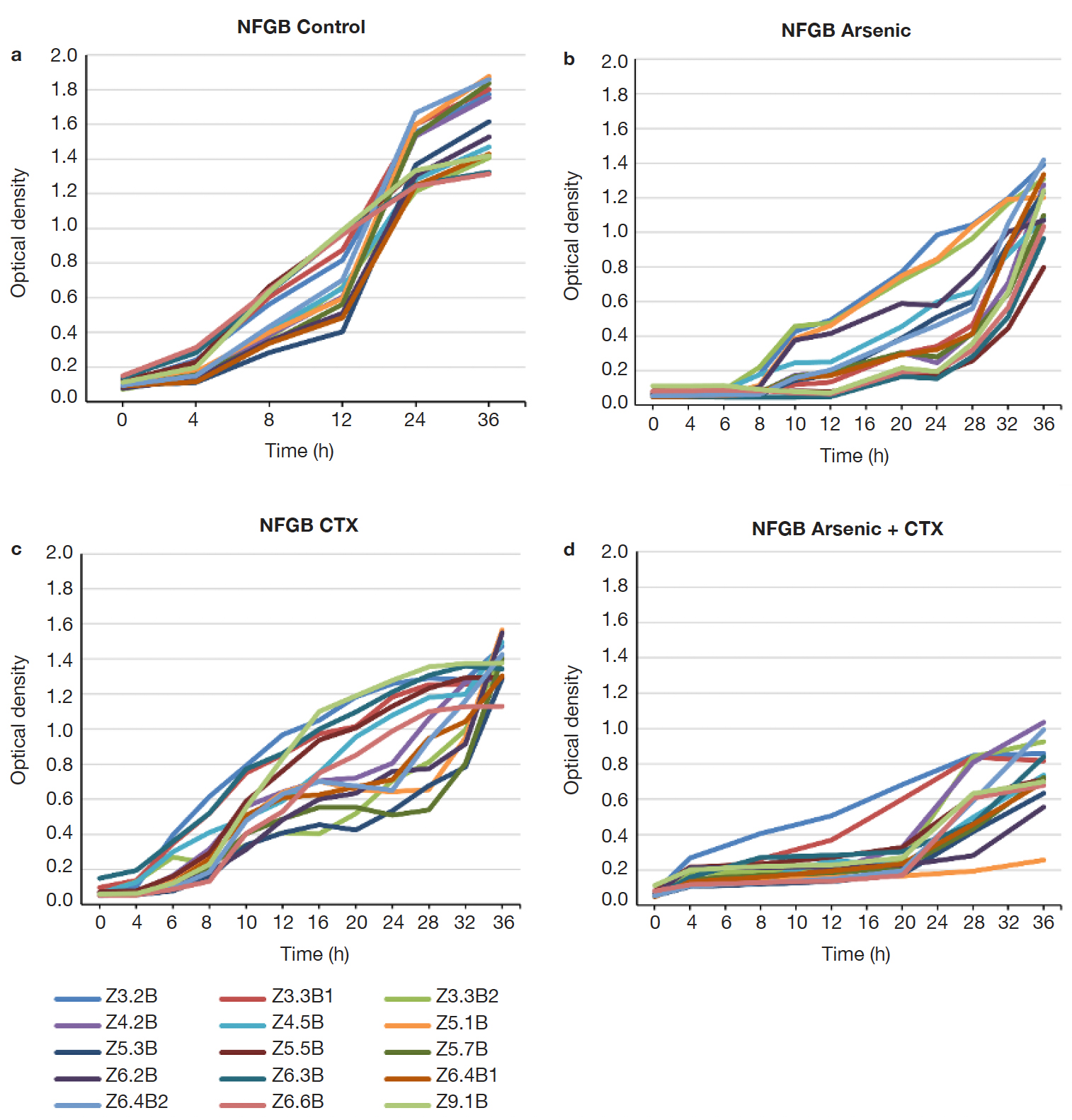
Figure 6. Growth curves of the NFGB strains isolated in this study: a. LB broth, b. LB broth + Na3AsO2 (400 ppm), c. LB broth + Cefotaxime (300 µg.ml-1), d. LB broth + Na3AsO2 (400ppm) + Cefotaxime (300 µg.ml-1) — Courbes de croissance des souches NFGB isolées dans cette étude : a. Bouillon LB, b. Bouillon LB + Na3AsO2 (400 ppm), c. Bouillon LB + Céfotaxime (300 µg.ml-1), d. Bouillon LB + Na3AsO2 (400 ppm) + Céfotaxime (300 µg.ml-1).
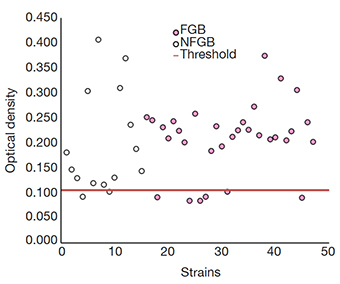
Figure 7. Detection of biofilm production in the studied strains by the staining technique — Détection de la production de biofilm dans les souches étudiées par la technique de coloration.
4. Discussion
17Antimicrobial resistance is a public health challenge in the Zimapan dam, where recreational and aquaculture activities are carried out in an environment contaminated with multiresistant bacterial strains that carry ESBL and are tolerant to As. The World Health Organization establishes Enterobacteria and NFGB resistant to third generation cephalosporins and carbapenems as a critical priority (Cherak et al., 2021). This problem in Zimapan could be due to sublethal concentrations of antibiotics and As that creates a selective environment for resistance. Trace levels of antibiotics are reported worldwide in surface waters due to high human and veterinary use of antimicrobials that are inefficiently eliminated in wastewater (Jonkers et al., 2020).
18Various types of plasmids are associated with ESBL in humans, animals, and the environment (Cottell et al., 2011; Dhanji et al., 2011a and 2011b; Randall et al., 2012), but their spread along the Zimapan dam could be explained by the presence of different plasmid addiction systems in the tested strains, coinciding with Doumith et al. (2012) who reported that ESBLs encoded by transformable, multiresistant plasmids and with multiple addiction systems, favors their spread and maintenance in the environment.
19Bacterial survival in environments contaminated with As such as Zimapan implies that bacteria use metal ion pumping mechanisms encoded in chromosomes and/or plasmids, in addition to transforming the ions in non-toxic compounds, which results in the form of remediation of contaminated sites. However, tolerance to heavy metals, like As, has been correlated to antimicrobial resistance and could become a worse threat to the treatment of infections in plants, animals, and humans (Kaur et al., 2011).
20Another interesting aspect is the formation of biofilm in the Zimapan strains, which allows the coexistence of micro niches with different physiological requirements, where contaminants interact, having a high accumulation capacity of heavy metals from the surrounding environment, being able to actively influence their absorption, desorption, and transformation that determine the fate of As in the environment (Barral-Fraga et al., 2018). In addition, biofilm-associated bacteria are highly resistant to antibiotics, and efflux pumps are widely implicated in antibiotic resistance because they can expel most clinically relevant antimicrobials but also play a role in biofilm formation (Alav et al., 2018).
5. Conclusions
21This preliminary study shows the presence of multidrug-resistant bacteria, tolerant to As and CTX and able to form biofilm in the Zimapan dam, evidencing the need for a multidisciplinary approach to propose control and prevention strategies for public health problems that may arise from agriculture, fish farming and recreational activities that are held in this dam. The problem is even worse knowing that the water of the dam is used for human consumption.
22The regular monitoring of the presence of resistant bacteria, the detection of antimicrobials and heavy metals, like As, are needed to make a more detailed evaluation of this problem. Also, restrictive measures in the use of antimicrobials by the farmers and fish farmers are needed, as well as education interventions in the communities surrounding the dam, are necessary for everyone to be part of the solution.
Bibliographie
Aarestrup F.M. et al., 2003. Antimicrobial susceptibility and occurrence of resistance genes among Salmonella enterica serovar Weltevreden from different countries. J. Antimicrob. Chemother., 52(4), 715-718, doi.org/10.1093/jac/dkg426
Alav I., Sutton J.M. & Rahman K.M., 2018. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother., 73(8), 2003-2020, doi.org/10.1093/jac/dky042
Armienta M.A. et al., 1997. Arsenic contamination of groundwater at Zimapán, Mexiko. Hydrogeol. J., 5(2), 39-46.
Armienta M.A. et al., 2001. The role of arsenic-bearing rocks in groundwater pollution at Zimapán Valley, México. Environ. Geol., 40, 571-581.
Barral-Fraga L. et al., 2018. Mutual interaction between arsenic and biofilm in a mining impacted river. Sci. Total Environ., 636, 985-998, doi.org/10.1016/j.scitotenv.2018.04.287
Burridge L. et al., 2010. Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture, 306(1), 7-23, doi.org/10.1016/j.aquaculture.2010.05.020
Cabello F.C., 2006. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ. Microbiol., 8(7), 1137-1144, doi.org/10.1111/j.1462-2920.2006.01054.x
Cabello F.C. et al., 2013. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol., 15(7), 1917-1942, doi.org/10.1111/1462-2920.12134
Cai W. & Arias C.R., 2017. Biofilm formation on aquaculture substrates by selected bacterial fish pathogens. J. Aquat. Anim. Health, 29(2), 95-104, doi.org/10.1080/08997659.2017.1290711
Campos V.L., Valenzuela C., Alcorta M. & Mondaca M., 2007. Aislamiento de bacterias resistentes a arsenico desde muestras de rocas volcanicas de la quebrada camarones, region Parinacota: Chile. Gayana, 71(2), 150-155, doi.org/10.4067/S0717-65382007000200003
Cervantes C., 1995. Bacterial resistance to arsenic compounds. Rev. Latinoam. Microbiol., 37(4), 387-395.
Chen H. et al., 2015. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: occurrence, bioaccumulation and human dietary exposure. Mar. Pollut. Bull., 90(1-2), 181-187, doi.org/10.1016/j.marpolbul.2014.10.053
Cherak Z., Loucif L., Moussi A. & Rolain J.-M., 2021. Carbapenemase-producing Gram-negative bacteria in aquatic environments: a review. J. Global Antimicrob. Resist., 25, 287-309, doi.org/10.1016/j.jgar.2021.03.024
Cottell J. et al., 2011. Complete sequence and molecular epidemiology of IncK epidemic plasmid encoding blaCTX-M-14. Emerg. Infect. Dis., 17(4), 645-652, doi.org/10.3201/eid1704.101009.
Czekalski N., Gascón Díez E. & Bürgmann H., 2014. Wastewater as a point source of antibiotic-resistance genes in the sediment of a freshwater lake. ISME J., 8(7), 1381-1390.
Dhanji H. et al., 2011a. Variation in the genetic environments of blaCTX-M-15 in Escherichia coli from the faeces of travellers returning to the United Kingdom. J. Antimicrob. Chemother., 66(5), 1005-1012, doi.org/10.1093/jac/dkr041
Dhanji H. et al., 2011b. Isolation of fluoroquinolone-resistant O25b:H4-ST131 Escherichia coli with CTX-M-14 extended-spectrum β-lactamase from UK river water. J. Antimicrob. Chemother., 66(3), 512-516, doi.org/10.1093/jac/dkq472
Doumith M. et al., 2012. Characterization of plasmids encoding extended-spectrum β-lactamases and their addiction systems circulating among Escherichia coli clinical isolates in the UK. J. Antimicrob. Chemother., 67(4), 878-885, doi.org/10.1093/jac/dkr553
Edelstein M. et al., 2003. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob. Agents Chemother., 47(12), 3724-3732, doi.org/10.1128/aac.47.12.3724-3732.2003
EMA (European Medicines Agency), 2014. Answers to the requests for scientific advice on the impact on public health and animal health of the use of antibiotics in animals. London: EMA, https://www.ema.europa.eu/en/documents/other/answers-requests-scientific-advice-impact-public-health-and-animal-health-use-antibiotics-animals_en.pdf, (18-12-2014).
FAO, 2017. El uso de antimicrobianos en la acuicultura en América Latina: desafíos y perspectivas futuras. Roma: FAO, https://www.fao.org/family-farming/detail/es/c/1035498/, (22/11/2017).
Flores F., 2017. Es un mito que en Zimapán haya aguas negras. El Universal, https://www.eluniversalqueretaro.mx/municipios/18-03-2017/es-un-mito-que-en-zimapan-haya-aguas-negras/, (18/03/2017).
Furushita M. et al., 2003. Similarity of tetracycline resistance genes isolated from fish farm bacteria to those from clinical isolates. Appl. Environ. Microbiol., 69(9), 5336-5342, doi.org/10.1128/AEM.69.9.5336-5342.2003
Galani I. et al., 2002. Transferable plasmid mediating resistance to multiple antimicrobial agents in Klebsiella pneumoniae isolates in Greece. Clin. Microbiol. Infect., 8(9), 579-588, doi.org/10.1046/j.1469-0691.2002.00391.x
Jonkers T.J.H. et al., 2020. Development of a high-throughput bioassay for screening of antibiotics in aquatic environmental samples. Sci. Total Environ., 729, 139028, doi.org/10.1016/j.scitotenv.2020.139028
Kaur S., Kamli M.R. & Ali A., 2011. Role of arsenic and its resistance in nature. Can. J. Microbiol., 57(10), 769-774, doi.org/10.1139/w11-062
Kerry J. et al., 1996. Spatial distribution of oxytetracycline and elevated frequencies of oxytetracycline resistance in sediments beneath a marine salmon farm following oxytetracycline therapy. Aquaculture, 145(1-4), 31-39, doi.org/10.1016/S0044-8486(96)01353-1
Kwiecińska-Piróg J. et al., 2020. Biofilm formation reducing properties of manuka honey and propolis in Proteus mirabilis rods isolated from chronic wounds. Microorganisms, 8(11), 1823, doi.org/10.3390/microorganisms8111823
Mellado C., Campos V. & Mondaca M.A., 2011. Distribución de genes de resistencia a arsénico en bacterias aisladas de sedimentos con concentraciones variables del metaloide. Gayana (Concepción), 75(2), 131-137, doi.org/10.4067/S0717-65382011000200001
Mendez M.O. & Armienta M.A., 2003. Arsenic phase distribution in Zimapán mine tailings, Mexico. Geofis. Int., 42(1), 131-140, doi.org/10.22201/igeof.00167169p.2003.42.1.366
Mnif B. et al., 2010. Molecular characterization of addiction systems of plasmids encoding extended-spectrum β-lactamases in Escherichia coli. J. Antimicrob. Chemother., 65(8), 1599-1603, doi.org/10.1093/jac/dkq181
Muñoz-Rojas et al., 2016. Métodos económicos para la cuantificación de microorganismos. In: Instituciones de educación superior. La labor investigadora e innovadora en México. Cheyenne, WY, USA : Science Associated Editors, 67-84.
Muñoz-Silva L., Olivera-Gonzales P., Santillan Torres M. & Tamariz-Angeles C., 2019. Microorganismos tolerantes a metales pesados del pasivo minero Santa Rosa, Jangas (Perú). Rev. Peruana Biol., 26(1), 109-118, doi.org/10.15381/rpb.v26i1.15912
Osuna-Martínez C.C., Armienta M.A., Bergés-Tiznado M.E. & Páez-Osuna F., 2021. Arsenic in waters, soils, sediments, and biota from Mexico: an environmental review. Sci. Total Environ., 752, 142062, doi.org/10.1016/j.scitotenv.2020.142062
Pandey P.K. & Kumar V.S., 2021. Biofilm in aquaculture production. In: Pandey P.K. & Parhi J., eds. Advances in fisheries biotechnology. Singapore: Springer Nature Singapore, 401-422.
Pérez Moreno F., 2004. Dinámica del arsénico en aguas subterráneas de pozos y sedimentos del distribuidor general de agua potable de Zimapán. PhD thesis : Universidad Autonoma del Estado de Hidalgo, Pachuca de Soto (Mexico).
Perez F. et al., 2003. Chemical characterization of groundwaters in wells and a water distributor of Zimapan State of Hidalgo, Mexico. Hidrobiológica, 13(2), 95-102.
Poirel L., Walsh T.R., Cuvillier V. & Nordmann P., 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis., 70(1), 119-123, doi.org/10.1016/j.diagmicrobio.2010.12.002
Prithivirajsingh S., Mishra S.K. & Mahadevan A., 2001. Detection and analysis of chromosomal arsenic resistance in Pseudomonas fluorescens strain MSP3. Biochem. Biophys. Res. Commun., 280(5), 1393-1401, doi.org/10.1006/bbrc.2001.4287
Randall L. et al., 2012. Virulence genes in blaCTX-M Escherichia coli isolates from chickens and humans. Res. Vet. Sci., 93(1), 23-27, doi.org/10.1016/j.rvsc.2011.06.016
Rhodes G. et al., 2000. Distribution of oxytetracycline resistance plasmids between aeromonads in hospital and aquaculture environments: implication of Tn1721 in dissemination of the tetracycline resistance determinant Tet A. Appl. Environ. Microbiol., 66(9), 3883-3890, doi.org/10.1128/aem.66.9.3883-3890.2000
Ríos-Tobón S., Agudelo-Cadavid R.M., & Gutiérrez-Builes L.A., 2017. Patógenos e indicadores microbiológicos de calidad del agua para consumo humano. Rev. Fac. Nac. Salud Pública, 35(2), 236-247, doi.org/10.17533/udea.rfnsp.v35n2a08
Santiago M.L., Espinosa A. & del Carmen Bermúdez M., 2009. Uso de antibióticos en la camaronicultura. Rev. Mex. Cienc. Farm., 40(3), 22-32.
Sørum H., 2008. Antibiotic resistance associated with veterinary drug use in fish farms. In: Lie O., ed. Improving farmed fish quality and safety. Cambridge, England: Woodhead Publishing Ltd; Boca Raton, FL, USA: CRC Press, 157-182.
Sousa M. et al., 2011. Gilthead seabream (Sparus aurata) as carriers of SHV-12 and TEM-52 extended-spectrum beta-lactamases-containing Escherichia coli isolates. Foodborne Pathog. Dis., 8(10), 1139-1141, doi.org/10.1089/fpd.2011.0866
Torres-Arellano J.M. et al., 2020. Natriuretic peptides and echocardiographic parameters in Mexican children environmentally exposed to arsenic. Toxicol. Appl Pharmacol., 403, 115164, doi.org/10.1016/j.taap.2020.115164
Tsang J., 2017. Bacterial plasmid addiction systems and their implications for antibiotic drug development. Postdoc J., 5(5), 3-9.
Watts J.E.M., Schreier H.J., Lanska L. & Hale M.S., 2017. The rising tide of antimicrobial resistance in aquaculture: sources, sinks and solutions. Mar. Drugs, 15(6), 158, doi.org/10.3390/md15060158
Yang J. et al., 2013. Marine sediment bacteria harbor antibiotic resistance genes highly similar to those found in human pathogens. Microb. Ecol., 65, 975-981.






