- Accueil
- Volume 28 (2024)
- Numéro 1
- β-glycosidase activity associated with the formation of aroma compounds in native non-Saccharomyces yeasts isolated from cocoa bean fermentation
Visualisation(s): 0 (0 ULiège)
Téléchargement(s): 0 (0 ULiège)
β-glycosidase activity associated with the formation of aroma compounds in native non-Saccharomyces yeasts isolated from cocoa bean fermentation

Résumé
Activité de β-glycosidase associée à la formation de composés aromatiques parmi les levures natives non-Saccharomyces isolées de la fermentation du cacao
Description du sujet. L'utilisation de levures non-Saccharomyces pour améliorer la perception des saveurs fines du cacao a été peu explorée.
Objectifs. Dans cette étude, nous avons étudié l'apport des levures non-Saccharomyces productrices de β-glycosidase isolées de la fermentation spontanée du cacao à la qualité et à la production d'arômes lors de la fermentation alcoolique d'un milieu à base de pulpe de cacao.
Méthode. Le criblage de l'activité β-glycosidase et l'identification moléculaire d'isolats de levures non-Saccharomyces provenant de fermentations de fèves de cacao amazoniennes ont été réalisés sur des plaques de gélose avec de l'esculine comme substrat et par analyse de séquence d'ADNr 5,8S ITS, respectivement. Les levures productrices de β-glycosidases ont été utilisées comme starter pour des micro-fermentations alcooliques d'un milieu à base de pulpe de cacao, incubées à 30 °C pendant 48 h. La quantification des acides organiques, de l'éthanol et des sucres a été obtenue par HPLC et les composés aromatiques après fermentation de la pulpe de cacao ont été identifiés par GC-FID et GC-MS.
Résultats. Vingt-six isolats de levures non-Saccharomyces ont été sélectionnés par activité β-glycosidase, comprenant des espèces telles que Pichia kudriavzevii (n = 15), Pichia sp. (n = 2), Pichia sporocuriosa (n = 1), Candida orthopsilosis (n = 3), Issatchenkia sp. (n = 1), I. orientalis (n = 1) et P. kudriavzevii/I. orientalis (n = 3). Toutes les souches de levure ont présenté une croissance rapide dans un milieu de pulpe de cacao, une faible production d'éthanol, une production élevée d'acide organique et une consommation de glucose plus élevée que le fructose. Ces levures produisaient trente composés volatils différents. Les principaux groupes comprenaient les alcools (3), les esters (19), les terpènes (4), les phénols (2), les acides organiques (2) et les aldéhydes (3), dont 67 % avaient des arômes fruités et floraux. Pichia sp., P. kudriavzevii/I. orientalis et Issatchenckia sp. étaient les plus grands producteurs de composés volatils. Notamment, cette étude a identifié pour la première fois des composés volatils aux arômes fruités auparavant non associés aux fermentations du cacao.
Conclusions. Ces données démontrent le potentiel des levures non-Saccharomyces productrices de β-glycosidase pour améliorer les arômes floraux et fruités, suggérant leur utilisation prometteuse comme culture de départ pour la fermentation du cacao.
Abstract
Description of the subject. The use of non-Saccharomyces yeast for improving fine cocoa flavor perception has been poorly explored.
Objectives. In this study, we investigated the contribution of the non-Saccharomyces yeasts producing β-glycosidase isolated from the spontaneous fermentation of cocoa to the quality and production of aromas during the alcoholic fermentation of a medium based on cocoa pulp.
Method. The screening for β-glycosidase activity and molecular identification of non-Saccharomyces yeast isolates from Amazonian cocoa bean fermentations were performed on agar plates with esculin as a substrate and by 5.8S ITS rDNA sequence analysis, respectively. The yeasts producing β-glycosidases were used as a starter culture for alcoholic micro-fermentations of a medium based on cocoa pulp, incubated at 30 °C for 48 h. The quantification of organic acids, ethanol, and sugars was obtained by HPLC, and aroma compounds after cocoa pulp fermentation were identified by GC-FID and GC-MS.
Results. Twenty-six non-Saccharomyces yeast isolates were selected by β-glycosidase activity, comprising species such as Pichia kudriavzevii (n = 15), Pichia sp. (n = 2), Pichia sporocuriosa (n = 1), Candida orthopsilosis (n = 3), Issatchenkia sp. (n = 1), I. orientalis (n = 1), and P. kudriavzevii/I. orientalis (n = 3). All yeast strains exhibited rapid growth in cocoa pulp medium, low ethanol production, high organic acid production, and higher glucose consume than fructose. These yeasts produced thirty different volatile compounds. The main groups included alcohols (3), esters (19), terpenes (4), phenols (2), organic acids (2), and aldehydes (3), with 67% of these having fruity and floral aromas. Pichia sp., P. kudriavzevii/I. orientalis and Issatchenckia sp. were the largest producers of volatile compounds. Notably, this study identified volatile compounds with fruity aromas previously unassociated with cocoa fermentations for the first time.
Conclusions. These data demonstrate the potential of β-glycosidase-producing non-Saccharomyces yeasts to improve floral and fruity aromas, suggesting their promising use as a starter culture for cocoa fermentation.
Table des matières
Received 4 April 2023, accepted 19 March 2024, available online 28 March 2024.
This article is distributed under the terms and conditions of the CC-BY License (http://creativecommons.org/licenses/by/4.0)
1. Introduction
1Cocoa beans (Theobroma cacao L.) are the main ingredient used in chocolate production. Chocolate is very popular worldwide due to its unique flavor and organoleptic properties (Castro-Alayo et al., 2019). Cocoa fermentation is a spontaneous process carried out by a complex microbial community and the first stage for developing the precursors of chocolate flavors (Santander-Muñoz et al., 2019). During fermentation, the successive action of yeast, lactic acid bacteria and acetic acid bacteria is responsible for ethanol, lactic acid and acetic acid production, respectively (Serra et al., 2019; Gutiérrez-Ríos et al., 2022).
2Starter cultures (Batista et al., 2016; Koné at al., 2016; Pereira et al., 2017; Ouattara et al., 2020; Viesser et al., 2021; Sandoval-Lozano et al., 2022), chemical and/or enzymatic catalysis improve the sensorial quality of the cocoa beans during fermentation (Delgado-Ospina et al., 2020; De Vuyst & Leroy, 2020). Yeasts, individually or in microbial consortia, have emerging applicability in cocoa-fermentation processes worldwide, in order to improve the fine aroma of cocoa and the resulting chocolate (Gutiérrez-Ríos et al., 2022). In cocoa bean fermentation, the yeasts generate both flavor precursor molecules and flavor-active compounds through secondary metabolite production contributing to the floral and fruity notes (Dzialo et al., 2017; Díaz-Muñoz & De Vuyst, 2022). Naturally existing yeast in cocoa fermentation can be divided into Saccharomyces and non-Saccharomyces yeast (Ho et al., 2014). Non-Saccharomyces yeasts are not independently responsible for sugar-ethanol conversion and can improve the aroma profile mainly through their ability to secrete enzymes and produce desirable secondary metabolites (Sadoudi et al., 2012).
3The cocoa flavor is related to the volatile organic compounds (VOCs), which are composed of a complex mixture of over 500 chemical compounds, mainly pyrazines, esters, aldehydes, ketones, alcohols, terpenes and esters amines, amides and acids (Castro-Alayo et al., 2019). Accordingly, some studies have selected strains based on VOC production with aroma characteristics (Batista et al., 2016; Sandoval-Lozano et al., 2022). The enzymatic conversions inside the cocoa beans by endogenous enzymes to the flavor precursors production such as peptides, amino acids and reducing sugars are well understood. On the other hand, there has been much research on the importance of yeast for flavor modulation of cocoa (Figueroa-Hernández et al., 2019; Díaz-Muñoz & De Vuyst, 2022). Nevertheless, the association of enzyme derived from yeasts, especially from the non-Saccharomyces yeast on the formation of active-flavor compounds formation is not fully established in the cocoa fermentation (Koné et al., 2016; De Vuyst & Leroy, 2020).
4In wine fermentation, the use of β-glycosidase-producing yeasts has shown a positive influence on the increase in VOCs, such as 2-ethylhexanol (Ruppert et al., 2021) and isoamyl alcohol (Sadoudi et al., 2012), which confer a fruity aroma to volatile-active molecules. It was also suggested that under fermentation conditions, non-Saccharomyces yeasts express better β-glycosidase enzyme activity compared to S. cerevisiae (Hu et al., 2016).
5The ability to produce aromas may be associated with the production of enzymes such as β-glycosidases, which hydrolyze glycosidic bonds present in compounds such as terpenes and polyphenols that are naturally present in plant products in glycosylated form (Fia et al., 2005; Pérez et al., 2011). In cocoa fermentation, only one study has related the functional biodiversity of yeasts from Criollo Colombian cocoa fermented beans, such as Hyphopichia burtonii, Trichosporon asahii var. asahii, Wickerhamomyces anomalus and Pichia kudriavzevii with a β-glycosidase activity; however, their potential for aroma production was not investigated (Delgado-Ospina et al., 2020).
6Although post-harvest cocoa transformation from seeds to cocoa beans has been widely studied in prior works (Figueroa-Hernández et al., 2019; Díaz-Muñoz & De Vuyst, 2022; Gutiérrez-Ríos et al., 2022), the potential of β-glycosidase-producing non-Saccharomyces yeasts for biochemical changes and improving aroma has not been demonstrated. Based on this focus, we investigated the effect of non-Saccharomyces yeast isolates from Amazonian cocoa bean fermentations with suitable β-glycosidase activity as starter cultures for aroma enhancers, using cocoa pulp as a substrate.
2. Materials and methods
2.1. Yeast isolation and culture-dependent analysis
7The cocoa beans sampling and fermentation process was performed as previously described (Serra et al., 2019). Cocoa fruits were harvested during the main harvest of 2017 in the municipalities of Tomé-Açu, Medicilândia, Placas from the state of Pará, Brazil and Ilheús, Bahia state, Brazil (a major Brazilian cocoa producer in 2017). The yeast count and isolation were performed according to the protocol described by Ardhana & Fleet (2003) and Camu (2007). For each sampling point, fermented cocoa beans were aseptically withdrawn on days 0, 2, 4, and 6 of the process. Each sample (20 g) was homogenized with 180 ml of 0.1% buffered-peptone water, followed by 10-fold serial dilutions until 10-5. Aliquots (0.1 ml) of each dilution were inoculated on a Dichloran Rose-Bengal agar base medium (Himedia, India) containing 100 mg·l-1 chloramphenicol (DRBC). Cultures were incubated on DRBC agar at 30 °C for 4 days. The yeast colonies were grouped based on morphological characteristics (color and shape) on DRBC agar, fermentability of carbohydrates (glucose, fructose, lactose and maltose), resistance to different temperatures (25, 35 and 45 °C), tolerance to different concentrations of ethanol (5, 10 and 15% ethanol) and growth at different pH (2.5, 3.5 and 5), according to the protocol described by Daniel et al. (2009). The morphologically different and representative colonies were selected and incubated on malt extract agar medium at 30 °C for two days. All isolated samples were stored at -80 °C in yeast extract peptone glucose (YEPG) broth with 20% (v/v) glycerol until analysis.
2.2. Selection of ß-glycosidases-producing yeasts
8The selection of yeasts producing β-glycosidases was carried out using esculin as substrate (Gaensly et al., 2015) in an esculin glycerol (EG) agar medium (1 g·l-1 esculin, 0.3 g·l-1 ferric chloride, 1 g·l-1 hydrolyzed casein, 25 g·l-1 yeast extract, 8 ml·l-1 glycerol, 20 g·l-1 agar) (Pérez et al., 2011). All the isolated yeasts from a single colony biomass were activated and grown on YEPG broth at 30 °C for 24 h. Subsequently, 10 µl of the resulting culture with an average OD600nm of 0.8 were transferred to a plate containing EG agar and incubated at 30 ºC for 48 h. The strains that produce β-glycosidase enzyme cleave the substrate and produce a dark-brown-colored halo. The diameters of halo were classified using the following levels: weak (14-17 mm), medium (18-22 mm), and strong (≥ 23 mm) (Pérez et al., 2011). The diameters of the brown halo were measured in millimeters. A non-inoculated plate was used as a negative control. All assays were performed in triplicate.
2.3. Identification of ß-glycosidase-producing yeasts
9Twenty-six isolates that produce β-glycosidase enzyme were selected for molecular identification via a sequence analysis of the 5.8S ITS rRNA gene. Each yeast in YEPG agar was collected and re-suspended in 50 µL of ultrapure water. The suspension was heated in a thermal cycler at 95 °C for 15 min and 1 µl was used as a DNA template in PCR. The primer pairs ITS1 and ITS4 (Carvalho Neto et al., 2017) were used to amplify ITS region in a Veriti thermal cycler (Applied Biosystems, Paisley, UK). The reaction mixture (55 µl) was performed including 5.5 µl of 10x PCR buffer, 2 µl of MgCl2 (50 mM) (Promega, Madison, WI, USA), 1.21 µl of dNTP Mix (10 mM) (Invitrogen, Carlsbad, CA, USA), 4 µl of each primer ITS 1 and ITS 4, 0.4 µl of 5 U·µl-1 Platinum Taq DNA polymerase (Invitrogen, Carlsbad, USA). The cycle amplification used was an initial denaturation at 95 °C for 5 min, followed by 35 cycles at 95 °C for 30 s, annealing at 55 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. Amplicons were loaded and separated by electrophoresis in 1.5% (w/v) agarose gels. The bands were visualized by ethidium bromide staining and photographed under UV light. The PCR products were sequenced using an ABI3730 XL DNA sequencer (Biosystems, Foster City, USA). The sequences obtained were used in a similarity search and compared with sequences available in the GenBank database using the BLAST tool for sequence comparison.
2.4. Evaluation of the growth curve
10Aliquots of yeast isolates in YEPG broth were inoculated in YEPD agar and incubated at 30 °C for 24 h. From each colony, new cultures were again prepared in 10 ml of YEPG broth and incubated at 30 °C until 107 cells·ml-1 (OD600nm of approximately 0.7 and 0.9). For the growth-curve assay, 10 µl of the cell culture was inoculated into 1,000 µl of YEPG broth. At each growth time, 200 µl of this culture was transferred to a 96-well polyethylene microplate. The monitoring of the cell concentration was followed by measuring at 600 nm in a spectrophotometer (Biotek, Biosystems, Brazil). The growth curve assay was carried out in triplicate over 96 h.
2.5. Enzyme production kinetics of ß-glycosidase from yeast isolates
11Cultures of the β-glycosidase-producing isolates were previously prepared in YPEG broth, with incubation at 30 °C for 24 h, using a biomass from one colony. Each yeast in the YPEG medium was aliquoted (20 µl) in a sterile microplate containing 180 µl of the EG broth medium. The microplate was incubated at 30 °C for 24 h. The absorbance at 600 nm was measured in a spectrophotometer at 0, 6, 12 and 24 h. An EG medium without inoculum was used as a negative control. All measurements were performed in triplicate.
2.6. Aroma production by selected yeast in cocoa pulp-based medium
12Cocoa pulp-based medium. Cocoa fruits were harvested during the main harvest of 2017 in the municipality of Tomé-Açu, from the state of Pará, Brazil. Fruits (~10 kg) were washed, brushed, sanitized (sodium hypochlorite solution at 200 ppm). After opening, the beans were mechanically pulped (mechanical pulper with 10 l capacity, Weq, Brazil) in a laminar flow hood to avoid contamination. The cocoa pulp medium was obtained by dilution with mineral water in a proportion of 1:1 (w/v), and immediately pasteurized at 90 ºC for 5 min. The storage was performed at the same temperature in polyethylene recipients, previously sanitized and then refrigerated in an ice bath. Afterwards, the cocoa pulp medium was autoclaved at 121 ºC for 15 min. The ºBrix and pH were carried out after the thermal processing. A manual refractometer (Yh equipment, model RHB-32ATC, Shenzhen, China) and digital pH meter (QUIMIS, model Q-261A21, Diadema, Brazil) were properly calibrated and used for ºBrix and pH measurements, respectively. All measurements were performed in triplicate.
13Fermentation conditions. Micro-fermentations were carried out using 26 isolated yeasts, individually inoculated in a sterilized cocoa-pulp medium. Each yeast was previously activated in a YEPG broth medium. The medium (30 ml) was inoculated with 10% (v/v) of inoculum concentration (107 to 108 cells·ml-1) and incubated at 30 °C for 48 h. Uninoculated cocoa-pulp medium was used as a negative control in the same conditions. The fermentation obtained was stored at -20 °C for further analysis. The growth among the strains during the fermentation was monitored by surface inoculation in YEPG agar.
14Quantification of organic acids, ethanol and sugars by HPLC. The organic acid, ethanol and sugar concentrations were determined after 48 h of fermentation, using a method previously described by Pereira et al. (2013). Twenty-six alcohol-fermented samples were diluted in ultrapure water. Then the aqueous extracts were microfiltered on a cellulose acetate membrane with pore sizes of 0.22 µm (Sartorius Stedim, Goettingen, Germany). The ethanol, organic acids (acetic, citric, succinic, lactic and propionic) and reducing sugars (glucose and fructose) were determined using a HPLC (HP series 1200, Hewlett-Packard Company, USA) equipped with a refractive index detector (HPG1362A, Hewlett-Packard Company, USA). The separation of the compounds was performed with an Aminex HPX-87H column (300 x 7.8 mm, 9 µm) (Bio-Rad Laboratories, Hercules, USA) under isocratic conditions, using phase 5 mM H2SO4 at 60 °C and a flow rate of 0.6 ml·min-1. Aliquots of 10 μl of the previously prepared samples were injected. The compounds were identified by comparison of the retention times with authentic standards and their concentration determined by the external standard method. The calibration curves were constructed using different concentrations (0.1 to 2 g·l-1, n = 6) of authentic standards of reducing sugars, organic acids, and ethanol purchased from Sigma-Aldrich (Annex 1). The results were expressed in g·l-1.
15Analysis of volatile compounds by GC flame ionization detector (FID). The extraction and quantification of the VOCs was performed in headspace, in accordance with the protocol previously described in Pereira et al. (2014). Five milliliters of supernatants fermentation were transferred into a 20 ml headspace vial containing 0.25 g of NaCl. The vials were heated to 60 °C for 5 min via agitation. Afterwards, air was collected with a syringe and injected manually. The separation of the VOCs was performed using a GC system (Shimadzu model 17A, Tokyo, Japan) equipped with FID at 230 °C and a capillary column (HP-5, 30 m × 0.32 mm) for 15 min. The operating conditions were as follows: helium carrier gas (flow rate of 1 ml·min-1), column temperature from 40 to 150 °C at a rate of 20 °C·min-1. The temperature program employed was set to start at 40 °C, hold for 5 min, gradually increasing to 150 °C at 20 °C·min-1 rate and holding at 150 °C for 5 min. The injector temperature was maintained at 230 °C under split mode of 1:5 rate. The concentration of the identified compounds was expressed in μg·ml-1 as ethanol equivalents. VOCs were identified by comparing the peak retention times of commercial analytical standards from Sigma. The standards used were eleven alcohols (methanol, ethanol, 1-pentanol, 1-hexanol, 1-heptanol, 1-octanol, 1-decanol, 2-hexanol, 2-octanol, n-butanol and 3-methyl-1-butanol), nine esters (ethyl acetate, propyl acetate, ethyl propionate, ethyl isobutyrate, ethyl hexanoate, isoamyl acetate, isobutyl acetate, n-butyl acetate and hexyl acetate), five ketones (2,3- butanedione, 2-pentanone, 2-hexanone, 2-octanone, and 2-heptanone), two aldehydes (acetaldehyde and benzaldehyde) and one organic acid (acetic acid) and five terpenes (R-(+)-limonene, (R)-(-)–linalool, (-)-terpinen-4-ol, alfa-terpineol and D-carvone).
16Identification and quantification of volatile compounds by GC-MS. The VOCs were identified using the protocol established with a headspace solid-phase microextraction method (HS-SPME) (Carvalho Neto et al., 2017). The HS-SPME fiber was composed of 5% Carboxen (CARB)/95% Polydimethylsiloxane (PDMS) (Supelco, St. Louis, MI, USA). Aliquots (5 ml) from samples were placed in 20 ml headspace vials in duplicate. The vials were heated at 60 °C for 10 min without stirring, followed by exposure of the SPME fiber for 15 min in a COMBI-PAL system. The compounds adsorbed by the fiber were desorbed into the GC injection system at 260 °C. Analysis of the VOCs was performed on a GC/MS-gun TQ, Series 8040 and 2010, Plus GC-MS (Shimadzu, Tokyo, Japan) coupled to a mass selective detector, HP 5972 (Hewlett Packard Enterprise, CA, USA). The compounds were separated on the 95% PDMS/5% PHENYL column (30 m x 0.25 mm, 0.25 mm film thickness). The column oven temperature was maintained at 60 °C for 10 min, followed by heating ramps of 4 and 10 °C·min-1 until reaching the temperatures of 100 and 200 °C, respectively. Helium was used as a carrier gas at a flow rate of 1 ml·min-1. The total run time for each analysis was 50 min. Mass spectra were obtained by electron impact at 70 eV in scan mode with a mass range of 30-500 m/z. The identification of the volatile compounds was done by comparison to the mass spectra described in the National Institute of Standard and Technology-NIST database (Nist'98) and Wiley7n.
2.7. Statistical analysis
17The data obtained were analyzed by one-way analysis of variance using the software STATISTIC (Statsoft, Tulsa, USA) followed by the Tukey’s test. The graphs and tables were prepared using Excel software (Microsoft, 2017) and Numbers, version 5.1 (Apple Inc, EUA). The similarities between the yeast isolates and the major VOCs identified were verified using cluster analysis by Ward's method, and principal component analysis using version 3.8 of the Past software (Hammer et al., 2001).
3. Results
3.1. Isolation and molecular identification of β-glycosidase-producing yeasts
18A total of 119 yeasts were isolated during the cocoa-fermentation process in three municipalities of the Pará (Placas, Medicilândia and Tomé-Açu) and Bahia (Ilhéus) states in Brazil. Twenty-six isolates showed intense brown coloration after 48 h of growth on EG agar (β-glycosidase activity) (Table 1) and were selected for further molecular identification and fermentation of the cocoa pulp. All except two isolates showed a medium level (18-22 mm) of β-glycosidase activity. The other 93 yeast isolates that did not exhibit β-glycosidase activity (data not shown) were stored.
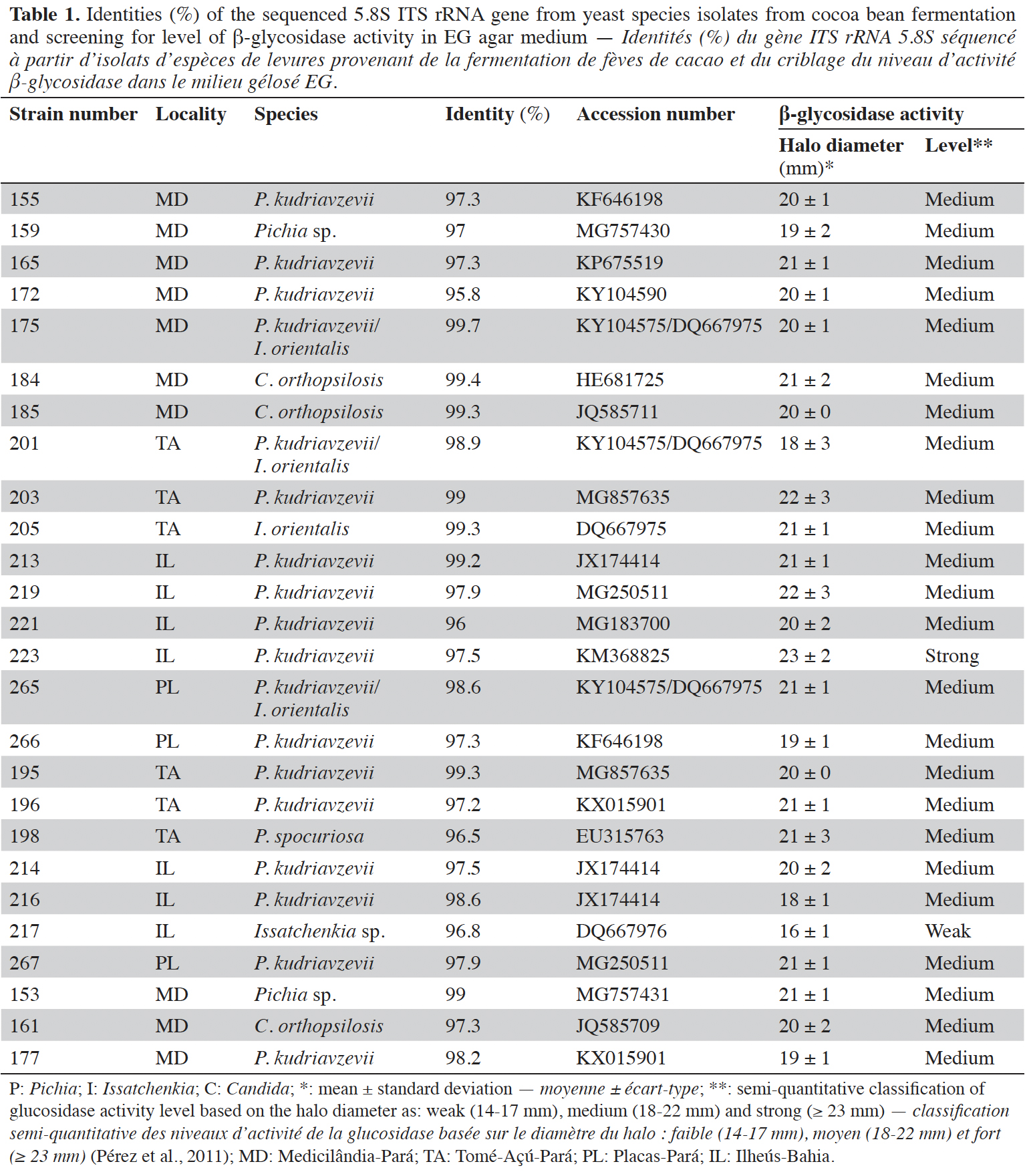
19The amplifications of the ITS rRNA gene obtained from the twenty-six selected yeast isolates resulted in amplicons with single bands observed in 1.5% (w/v) agarose gel, corresponding to 450 bp (data not shown). As shown in table 1, the identity percentages ranged from 95.8 to 99.7%. The isolates belonged to the species Pichia kudriavzevii (n = 15), Pichia sp. (n = 2), Pichia sporocuriosa (n = 1), Candida orthopsilosis (n = 3), Issatchenkia sp. (n = 1), I. orientalis (n = 1) and P. kudriavzevii/I. orientalis (n = 3).
3.2. Growth kinetics and production of β-glycosidase enzymes from yeast isolates
20The β-glycosidase enzyme activity and the color change observed after cleavage of esculin with seven yeast strains representative of each species is variable, depending on the species of the yeast isolate (Annex 2). Color change was observed after 24 h for all species, indicating production of the β-glycosidase enzyme. Candida orthopsilosis, I. orientalis, P. kudriavzevii and P. spocuriosa strains showed a large increase in absorbance during the first 12 h, whereas the other isolates showed enzyme activity between 12 and 24 h of growth.
21The growth trends for the strains were similar (Annex 3). All yeasts reached maximum growth at 24 h. Concurrently, higher enzyme activity was observed during the exponential phase of cell growth, indicating that β-glycosidase activity is linked to microbial growth.
3.3. Cocoa pulp fermentation by ß-glycosidases-producing yeasts
22Production of sugars, organic acids and ethanol. The 26 yeast strains that produce β-glycosidase were used as inoculum in the cocoa-pulp fermentation for VOC production. The growth curve for representative isolates of each species shows that the isolates adapted well to the proposed fermentation system using the cocoa pulp medium (pH 4.6 ± 0.1 and 8.2 ± 0.1 °Brix) (Annex 4). In the first 24 h, there was a rapid growth of yeasts, especially P. kudriavzevii/I. orientalis and Pichia sp., which reached counts of 10.3 and 9.2 Log (CFU·ml-1) respectively, followed by a slight decline in the yeast population.
23After 48 h of fermentation, the concentration of sugar, ethanol, and organic acids in the uninoculated and inoculated cocoa-pulp was evaluated, and the results are shown in table 2. The glucose, fructose and ethanol concentration at the end of fermentation in the uninoculated sample was 4.74, 5.55 and 1.43 g·l-1, respectively. For the inoculated samples, the glucose and fructose concentration ranged between 0.31 to 3.66 g·l-1 and 3.06 to 5.85 g·l-1, respectively. The utilization rate of soluble sugars ranged from 4.8 °Brix (P. kudriavzevii, strain 165) to 7.1 °Brix (Pichia sp., strain 153). Ethanol concentration ranging from 0.98 to 3.87 g·l-1.
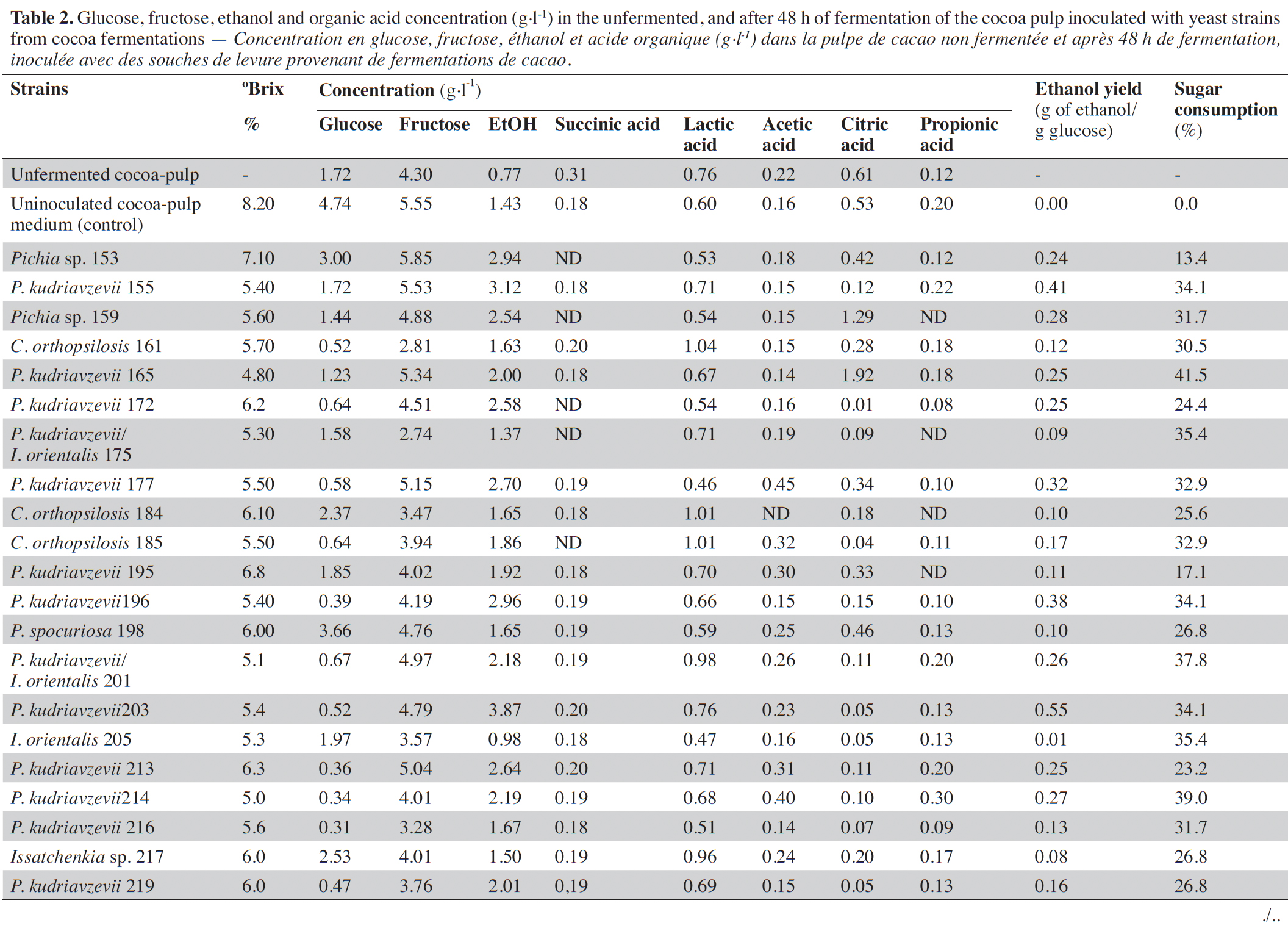
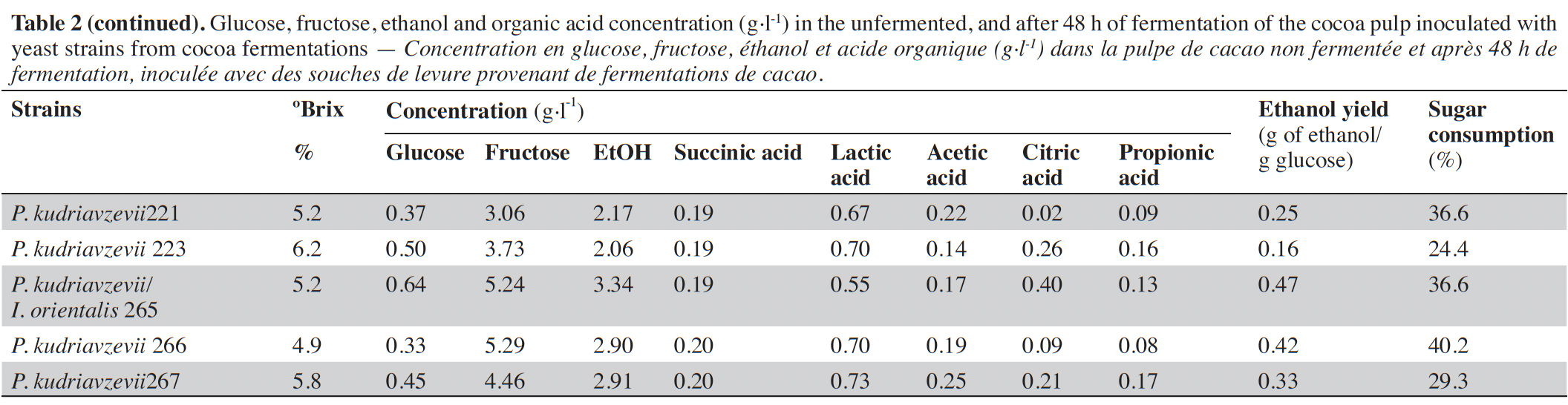
24The unfermented and uninoculated cocoa-pulp medium showed high concentrations of lactic acid (0.76 and 0.6 g·l-1, respectively) and citric acid (0.61 and 0.53 g·l-1, respectively), while other acids were found in low concentrations (≤ 0.31 g·l-1).
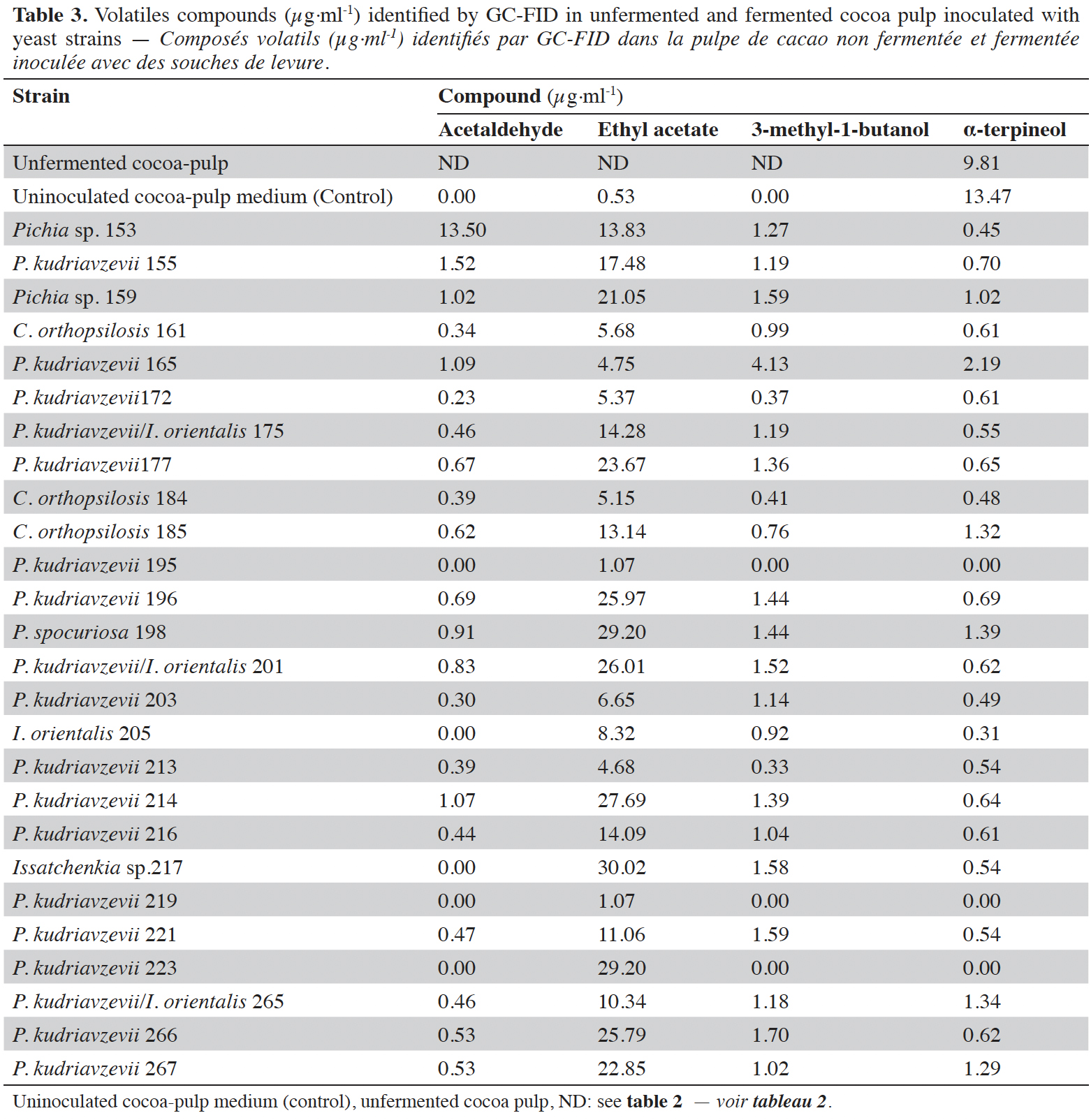
25Volatile compounds. In this study, two analytical techniques (GC-FID and SPME-GC-MS) were used to identify the VOCs produced during cocoa-pulp fermentation with β-glycosidases-producing yeasts. The major VOCs produced by micro-fermentation with the 26 identified yeast isolates were detected by GC-FID, including acetaldehyde, ethyl acetate, 3-methyl-1-butanol and α-terpineol (Table 3). Furthermore, 3-methyl-1-butanol (isoamyl alcohol), ethyl hexanoate, ethyl (Z)9-octadecenoate, ethyl octanoate, ethyl decanoate, 2,4-Diacetoxypentane, ethyl acetate, 2-phenyl-ethyl acetate, phenyl acetaldehyde and acetaldehyde were also identified during cocoa-pulp fermentation. The α-terpineol was the compound present in highest concentrations (13.47 µg·ml-1 ethanol equivalent) in the uninoculated control, followed by ethyl acetate (0.53 µg·ml-1 ethanol equivalent). In the inoculated fermentation, ethyl acetate was the compound produced in the highest quantities by most isolates, with concentrations ranging from 1.07 to 30.02 µg·ml-1.
26Alternatively, the SPME-GC-MS method was used to identify a higher number of VOCs in the inoculated processes, with seven yeast strains representative of each identified species. The prerequisite for the selection of isolates was higher β-glycosidase enzyme activity. Table 4 presents the seven classes of VOCs identified at the end of the fermentation, as well as the aroma descriptors. A total of 36 compounds were identified by both methods and grouped into alcohols (3), esters (19), terpenes (4), phenols (2), organic acids (2), aldehydes (3), others (3).
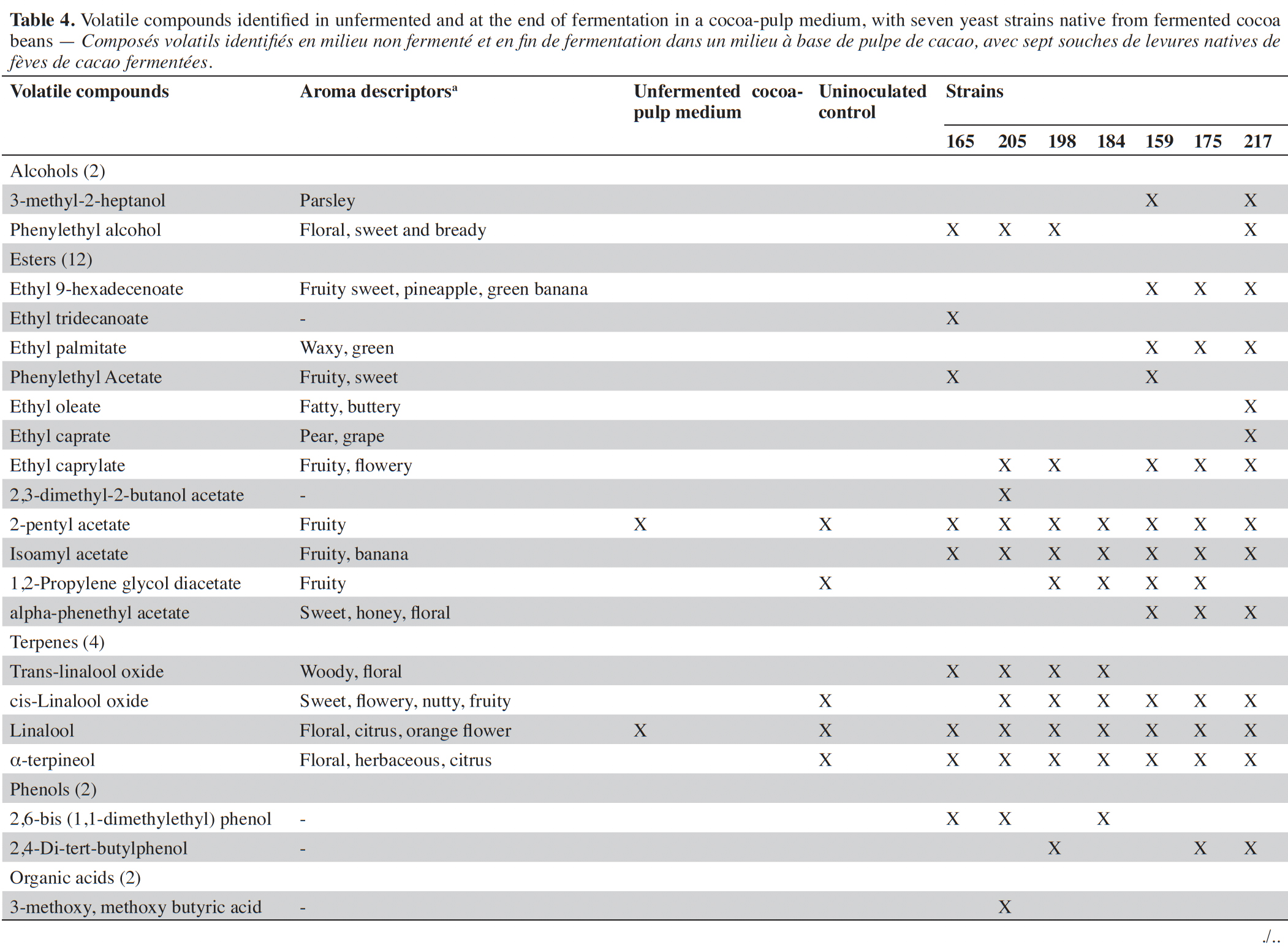
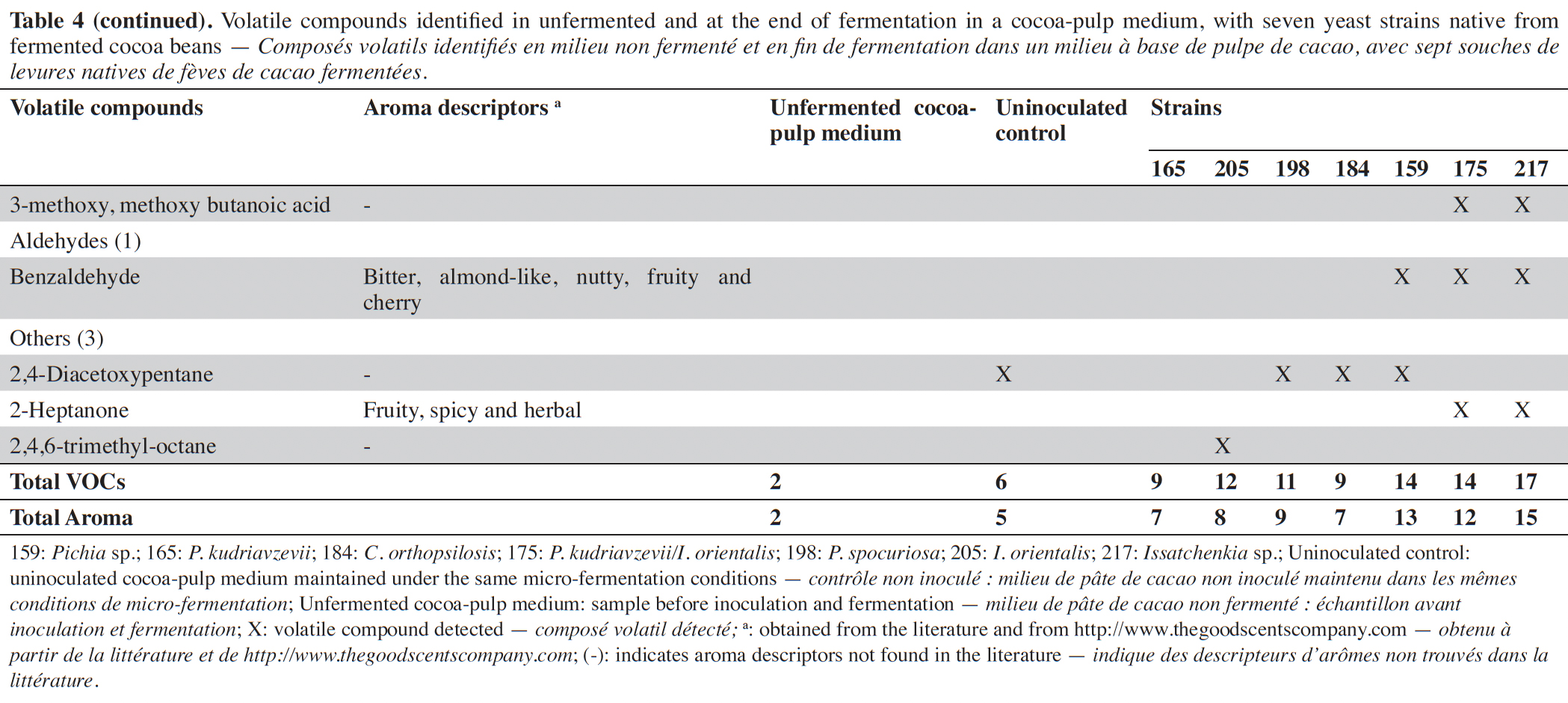
27To visualize the relationship between yeast isolates and aroma production, a cluster and a multivariate analysis were performed using the major VOCs obtained (Figure 1). The first two principal components account for 88.5 and 5.9% of the variance, respectively, explaining 94.5% of the total variance. The dendrogram and the plot scores suggest the occurrence of two groups. In group one, there are yeast strains that presented a low potential for ethyl acetate production and higher concentrations of 3-methyl-1-butanol. Candida orthopsilosis and I. orientalis are representative species of this group. In contrast, group 2 presented yeast strains with higher ethyl acetate production. This group was mainly composed of P. kudriavzevii, P. spocuriosa, Issatchenckia sp. and P. kudriavzevii/I. orientalis species.
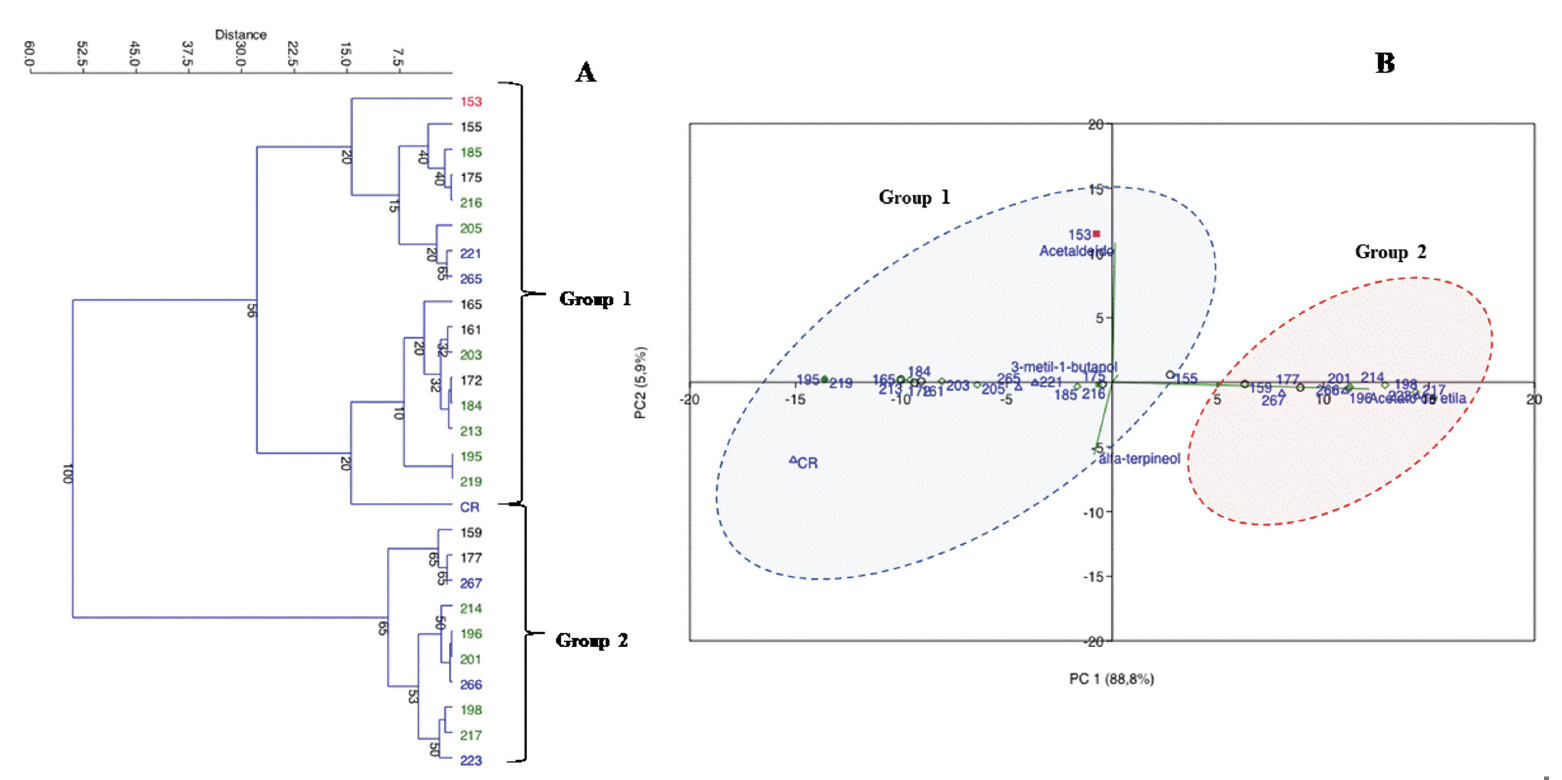
Figure 1. Cluster analysis results (A) and principal component analysis results (B) showing the intraspecies relationship of the isolates that produce β-glycosidases, based on the production of aromas during fermentation in the cocoa-pulp medium. The cluster and PCA analysis highlight the segregation of strains into two main groups — Résultats de l’analyse en clusters (A) et de l’analyse en composantes principales (B) montrant la relation intraspécifique des isolats produisant des β-glycosidases, basée sur la production d’arômes pendant la fermentation dans le milieu à base de pulpe de cacao. Les analyses en cluster et ACP mettent en évidence la séparation des souches en deux groupes principaux.
4. Discussion
4.1. Isolation and molecular identification of β-glycosidase-producing yeasts
28The molecular identification of β-glycosidase-producing yeasts isolated in this study agreed with many studies that reported the predominance of non-Saccharomyces yeasts, P. kudriavzevii, Pichia sp. and C. orthopsilosis in cocoa fermentation from various countries, such as Colombia, Ghana, Côte d’Ivoire and Brazil (Pereira et al., 2013; Koné et al., 2016; Serra et al., 2019; Delgado-Ospina et al., 2020). P. kudriavzevii 223 and Issatchenkia sp. 217 showed a strong and weak level of β-glycosidase activity, respectively, while the other twenty-four isolates showed a medium activity. Delgado-Ospina et al. (2020) reported a stronger β-glycosidase activity in P. kudriavzevii, H. burtonii, T. asahii var. asahii and W. anomalus isolated from Criollo Colombian fermented cocoa beans. However, the relationship between the ability of cocoa yeast to produce β-glycosidase and their impacts on the flavor richness of cocoa fermentation has not been studied and this constitutes an untapped potential for development of new cocoa bean flavors (Gutiérrez-Ríos et al., 2022).
29β-glycosidase enzymes are widely used in the fermented beverage mainly because they are able to hydrolyze glycosidic substrates, and the potential for aromas release with floral and fruity flavor (Han et al., 2023). Screening yeasts that produce this enzyme is interesting for the food industry, because it contributes to the production of food with unique aroma and flavor characteristics.
4.2. Cocoa pulp fermentation by ß-glycosidases-producing yeasts
30Organic acids, reducing sugars and ethanol production. During fermentation in the inoculated samples, the glucose was consumed first and the observed fructose consumption was relatively lower. This difference indicated that most isolates metabolized more glucose than fructose during fermentation. Yeasts are first characterized by a preference for glucose consumption due to rapid metabolization, then fructose (Pereira et al., 2012; Dzialo et al., 2017). This remaining fructose in spontaneous fermentations of cocoa beans is usually used as a carbon source for other microorganisms, such as Lactobacillus and Frutobacillus (Viesser et al., 2020).
31The ethanol concentration was 3 to 15 times lower compared to alcoholic fermentations of cocoa pulp using S. cerevisiae as a starter culture (Duarte et al., 2010). In general, P. kudriavzevii is among the highest ethanol producers in this study. The ethanol yield of four isolates was greater than 0.4 g ethanol·g-1 sugar, and the sugar consumption ranged from 13.4 to 41.5%. Many non-Saccharomyces yeasts have a limited capacity to utilize carbohydrates, such as glucose and fructose, and to produce ethanol. Wines fermented with Pichia and Candida species had ethanol contents lower than 0.4 g ethanol·g-1 sugar, and their maximum sugar consumption was 75% for P. kudriavzevii (Contreras et al., 2014).
32Initial citric acid levels decreased by approximately 75% for half of the isolates. The other isolates, such as Pichia sp. (n = 1), P. spocuriosa (n = 1), C. orthopsilosis (n = 1) and P. kudriavzevii/I. orientalis (n = 1), consumed less than 50% of the citric acid. Citric acid is one of the main organic acids present in cocoa pulp (1 to 3% of the pulp) (Gutiérrez-Ríos et al., 2022). The citric acid is usually used by yeast as an alternative source of carbon. Several citrate-positive yeast species isolated from fermenting cocoa-bean pulp, e.g. P. kudriavzevii, P. kluyveri and C. tropicalis, have also shown in vitro citric acid assimilation (Daniel et al., 2009; De Vuyst & Leroy, 2020). In contrast, a significant increase in citric acid was observed for strains 159 (Pichia sp.) and 165 (P. kudriavzevii), with these yielding 1.29 and 1.92 g·l-1, respectively. A slight increase of 15 mg·l-1 of citric acid content was observed both in the presence and absence of yeast during cocoa fermentations (Ho et al., 2014). This could be ascribed to the yeast's oxidation of ethanol to acetaldehyde and further to acetyl-CoA, generating excessive flux through the TCA cycle, leading to an accumulation of citrate and its subsequent secretion (Díaz-Muñoz & De Vuyst, 2022).
33Lactic acid concentration increased ranging from 0.66 to 1.04 g·l-1 for eighteen isolates when compared to uninoculated control. The other isolates showed lower values than the control fermentation. This acid is normally detected in cocoa pulp after fermentation (Pereira et al., 2013). However, the presence of lactic acid was detected at low concentrations before fermentation (0.3 mg·g-1) (Ardhana & Fleet, 2003).
34Strains of C. orthopsilosis (161, 184, 185), Issatchenkia sp. 217 and P. kudriavzevii/I. orientalis 201 produced the highest concentration of lactic acid in cocoa pulp medium and low ethanol yield. Studies previous reported that non-Saccharomyces yeast pure culture, as Candida tropicalis possess higher capacities for lactic acid production than S. cerevisiae during the sorghum beer production (N’Guessan et al., 2010; Alloue-Boraud et al., 2015).
35In wines, non-Saccharomyces yeasts with low ethanol yield are frequently used for the acidification of low-acidity wines due to their ability of producing lactic acid and to reduce ethanol during alcoholic fermentation process. The production of the lactic acid is linked to the presence of sugars, to oxygen availability and the viable cell concentration. Under these conditions the carbon from sugar metabolism can be used for organic acids and glycerol production, resulting in lower ethanol production (Zhu et al., 2020).
36The ability to produce lactic acid by non-Saccharomyces yeasts associated with tolerance to acidic conditions are interesting characteristics to biopolymers production, such as polylactide polymers, due these yeasts were able to resist the lactic acid recovery process that occurs in acidic conditions, differently of the lactic acid bacteria (Sauer et al., 2010; Matsushika et al., 2016).
37The concentrations of acetic acid for fourteen isolates were higher than the concentration of 0.16 g·l-1 detected in the uninoculated control. The maximum value obtained was 0.45 g·l-1 while, in the other isolates, similar or lower concentrations than the control were found.
38Sixteen isolates produced a small increase in the succinic acid concentrations (0.01 to 0.02 g·l-1) compared to the uninoculated control. Interestingly, succinic acid was not detected in cocoa-pulp fermentation inoculated with Pichia sp., P. kudriavzevii, P. kudriavzevii/I. orientalis and C. orthopsilosis. Ho et al. (2014) also observed a minimum increase in succinic acid during spontaneous cocoa fermentation. Regarding propionic acid, most isolates consumed up to 50% of this acid and only two strains (155 and 214) showed a slight increase in the production of this acid compared to the uninoculated control from 0.02 and 0.10 g·l-1, respectively. To date, propionic acid has not been reported in cocoa-pulp fermentations. Most organic acids are the result of yeast metabolism. The citric acid and succinic acid are intermediates of the tricarboxylic acid cycle, whereas acetic acid and lactic acid result from glycolysis and anaerobic fermentation. However, propionic acid is the only acid that does not result from yeast metabolism (De Vuyst & Leroy, 2020).
39Volatile compounds production. Regarding the VOCs detected by GC-FID, the concentration of ethyl acetate increased during fermentation ranged from 2 to 56 times compared to uninoculated cocoa pulp maintained under the same fermentation conditions. The highest concentrations (> 20 µg·ml-1) of this compound were produced by Pichia sp., P. kudriavzevii, Issatchenckia sp. and P. sporocuriosa species. Pichia kudriavzevii isolates were able to produce ethyl acetate, ranging from 1.07 to 29.20 µg·ml-1.
40Acetaldehyde was produced at low concentrations (0-0.5 µg·ml-1ethanol equivalent) by 54% of the isolates and between 0.5 and 1.5 µg·ml-1 by the remainder, with the exception of strain 153. This strain, identified as Pichia sp., was highlighted for the highest production of acetaldehyde (13.5 µg·ml-1). Acetaldehyde is a central intermediate between pyruvate and ethanol and is the most abundant aldehyde in alcohol-fermented products. Low concentrations of acetaldehyde confer a desirable fruity aroma in products such as wine. However, concentrations above 130 µg·g-1 can generate undesirable aromas, such as green apples. Temperatures of 30 °C for wine fermentation increase the production of this compound (Dzialo et al., 2017). Cocoa fermentations in the initial stages have temperatures between 30 and 35 °C (Pereira et al., 2012), while the temperature used in the fermentation process was 30 °C.
41The concentration of α-terpineol after fermentation was reduced by around six times compared to the concentration initially detected in the uninoculated control. Normally, terpenes are not metabolized by yeast. However, it is possible that some yeast species can co-metabolize or degrade terpenes (King & Dickinson, 2000). The highest concentration of 3-methyl-1-butanol 4.13 µg·ml-1 was observed in the sample inoculated with P. kudriavzevii (strain 165). In terms of the other yeast fermentations, 69% were quantified at concentrations ranging from 0.9 to 1.7 µg·ml-1 and in 27%, the quantified concentrations were lower than 0.8 µg·ml-1.
42Interestingly, fermented cocoa pulp inoculated with β-glycosidase-producing yeast showed a richer variety of flavor-active compounds compared to controls (uninoculated and unfermented samples), such as esters (e.g. ethyl 9-hexadecenoate, isoamyl acetate, and phenylethyl acetate), terpenes (e.g. trans-linalool oxide), and higher alcohols (e.g. 3-methyl-1-butanol and phenylethyl ethanol). A total of 20 compounds were identified only in inoculated processes (Table 4). Fruity and floral were the main aromas (67%). These are of great interest for the production of cocoa beans with a fine cocoa type flavor. Secondary metabolites such as 2-butanol, isoamyl alcohol and 2-phenylethanol, as well as 2-phenylacetaldehyde and acetaldehyde, are produced by yeast, such as those that result from the metabolism of amino acids including leucine, threonine, isoleucine and phenylalanine, through the Ehrlich pathway (Díaz-Muñoz & De Vuyst, 2022). Esters are formed by a condensation reaction between acetyl-CoA and higher alcohols or acyl-CoA, and ethanol yielding to acetate esters and fatty acid ethyl esters, respectively. Acetate esters have significantly more influence on flavor than fatty acids (De Vuyst & Leroy, 2020).
43Some compounds (n = 9) have not yet been identified in cocoa samples or derivatives, i.e. 3-methyl-2-heptanol, 2,3-dimethyl-2-butanol acetate, ethyl 9-hexadecenoate, ethyl tridecanoate, (Z)-9-octadecenoate, 2,4-diacetoxypentane, 2,6-bis (1,1-dimethylethyl) phenol, 2,4-di-tert-butylphenol, 3-methoxy, methoxy butyric acid, 3-methoxy, methoxy butanoic acid, and 2,4,6-trimethyl-octane. There are no reports on these compounds and their impact on the flavor of fermented products, therefore further investigations are necessary to verify the contribution of these compounds to the final flavor of fermented cocoa pulp.
44Terpenes were the second class of VOCs detected in all the fermentations, mainly linalool, linalool oxides and α-terpineol, which contribute to a fruity and floral aroma. Linalool was also detected in the unfermented cocoa-pulp medium and in uninoculated samples. Linalool is found in glycosidic form in cocoa pulp that is transferred to the beans during fermentation or can be produced by yeast or from the metabolism of leucine (Castro-Alayo et al., 2019). It is also possible that glycosidase enzymes (α-arabinosidase, β-galactosidase, α-mannosidase) play an important role in the release of this compound (Delgado-Ospina et al., 2020; De Vuyst & Leroy, 2020).
45β-glycosidase can catalyze the hydrolysis of glucoside bonding aroma precursors thus releasing the aroma compounds, mainly the glucoside-bonding terpene aroma compounds. Hence, the non-Saccharomyces yeasts can improve the aroma quality through their ability to produce desirable secondary metabolites and β-glycosidase activity, which release aroma glycoside precursors from their glycosylated form. In alcoholic wine fermentations containing non-Saccharomyces yeasts as co-cultures, terpenes can be bio-transformed into other terpenes, which will depend on the activity of the β-glycosidase enzyme (Sadoudi et al., 2012). The biotransformation of terpenes has been reported and can occur in different ways, by reduction of geraniol to citronellol, isomerization of nerol to geraniol, and cyclization of linalool to α-terpineol (King & Dickinson, 2000). In this study, the isomerization of the oxide-cis linalool to transform, similar to the isomerization of nerol to geraniol may have occurred during fermentation due to the production of isomerases by P. kudriavzevii, P. spocuriosa, C. orthopsilosis and I. orientalis (strains 165, 198, 184 and 205, respectively).
46Issatchenckia sp. was the largest producer of compounds with aroma description, producing 15 compounds. Pichia sp. and P. kudriavzevii/I. orientalis produced a total of 13 and 12 aroma compounds, respectively; followed by P. spocuriosa, I. orientalis, C. orthopsilosis and P. kudriavzevii with 9, 8, 7 and 7 compounds, respectively. In the control, only five aroma compounds were detected, indicating that the others resulted from the metabolism of the inoculated yeasts (Figure 2).
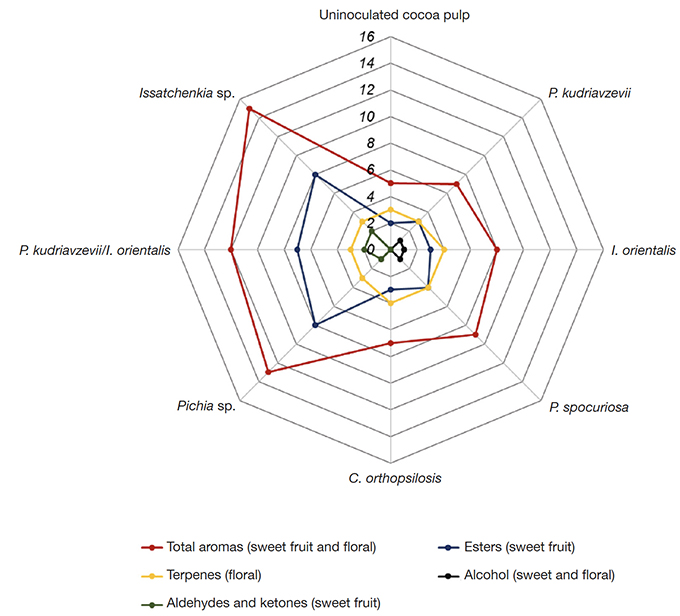
Figure 2. Aromas produced by β-glycosidase-producing yeasts during fermentation of the cocoa-pulp medium — Arômes produits par les levures productrices de β-glycosidase lors de la fermentation du milieu pulpe de cacao.
Uninoculated cocoa pulp — pulpe de cacao non inoculée : uninoculated cocoa-pulp medium maintained under the same micro-fermentation conditions — milieu de pulpe de cacao non inoculé maintenu dans les mêmes conditions de micro-fermentation.
47A positive impact on the aroma compounds in fermented cocoa with yeasts as starter cultures, including Saccharomyces cerevisiae, Pichia kluivery, Hanseniaspora uvarum, and P. kudriavzevii (Batista et al., 2016; Pereira et al., 2017; Ouattara et al., 2020; Viesser et al., 2020) was reported in comparison to those obtained through spontaneous fermentation. Viesser et al. (2020) observed a positive interaction between L. plantarum LPBF35 and P. fermentans YC5.2, resulting in an improved formation of primary (ethanol, lactic acid, and acetic acid) and secondary (2-methyl-1-butanol, isoamyl acetate, and ethyl acetate) metabolites during cocoa-bean fermentation. On the other hand, the spontaneous process showed a higher accumulation of ethanol, ethyl acetate, and 2-pentanol when compared to treatments with the addition of only lactic acid bacteria. Previous studies suggest that lactic acid bacteria do not have a significant impact on the formation of secondary metabolites during cocoa fermentation (De Vuyst & Leroy, 2020; Viesser et al., 2021) and that these metabolites are produced by native yeasts growing during the spontaneous process (Sandoval-Lozano et al., 2022). The absence of yeast during cocoa-bean fermentation causes the limited amount of higher alcohols and esters in the fermented cocoa beans (Ho et al., 2014). Regarding the application of starter cultures at an industrial level, only yeast cultures have been employed for cocoa fermentation (Figueroa-Hernández et al., 2019).
48Thirty-three VOCs produced by yeast isolates from cocoa fermentation in Indonesia were identified (Pereira et al., 2017). The species P. kudriavzevii was found to produce higher alcohols, acids and esters (17 compounds) in contrast to Candida species that produced only 11 compounds. In the cocoa fermentations from Brazil, yeast species such as Saccharomyces cerevisae, Hanseniaspora uvarum, Kluyveromyces marxianus, Pichia fermentans, Pichia kluivery and Pichia kudriavzevii have potential to produce esters and alcohols, such as isopropyl acetate, ethyl acetate, methanol, 1-propanol, isoamyl alcohol, among others (Crafack et al., 2014; Batista et al., 2016; Pereira et al., 2017; Viesser et al., 2020).
49The use of β-glycosidase-producing yeasts in wine fermentation to increase the content of desirable aroma compounds is influenced by several factors (Sadoudi et al., 2012). The biosynthesis of β-glycosidase is associated with the yeast’s growth phrase, where maximum activity is reached between 24 to 48 h (Fia et al., 2005). However, the extracellular activity of the enzyme during fermentation can be affected by environmental conditions, such as pH, sugar concentration and ethanol content (Delgado-Ospina et al., 2020). For example, in the case of the P. membranifaciens species, a reduction of β-glycosidase enzyme activity, measuring around 50 to 80%, was detected in the presence of glucose concentrations at 20%, a pH of 3, and 15% ethanol (Hu et al., 2016). Another factor is the extracellular activity of this enzyme which, in many cases, is low in yeast. On the other hand, the autolysis of yeast cells during the fermentation process contributes to the increase in activity, due to the release of intracellular enzymes. Candida species are producers of extracellular β-glycosidase, while Pichia can produce in both extracellular and intracellular manners (Fia et al., 2005; Pérez et al., 2011). During cocoa fermentation, this has a positive effect because it is a long fermentative process, so it is possible to have an increase of this activity by both intracellular and extracellular enzyme contributions.
5. Conclusions
50The presence of β-glycosidase-producing yeasts in cocoa fermentation has already been reported in the literature, including the species identified in this study. However, the relationship between β-glycosidase-producing yeasts and the production of flavors in cocoa-pulp fermentation was studied for the first time. The fermented cocoa pulp inoculated with β-glycosidase-producing yeasts showed richer flavor-active compounds such as esters, terpenes and alcohols when compared to the uninoculated and unfermented. We showed here that diversity in native β-glycosidase-producing non-Saccharomyces yeasts, in particular Pichia kudriavzevii, Pichia sp., and Issatchenchia sp., would be excellent candidates for starter cultures in the cocoa-fermentation process, enhancing the production of esters, alcohols and terpenes that confer fruity and floral aromas, two interesting characteristics to obtain beans with a flavor similar to fine cocoa. In addition, this study constitutes the first step for further investigations of the β-glycosidase characterization of yeasts on improving floral and fruity aromas in cocoa fermentation process.
Acknowledgements
51The authors thank CNPq (Brazil) for their research scholarship. We are grateful to CAMTA, in particular the Konagano family and the farmers, Mr Michinori Konagano and Mr Gerson Marques, for their technical support when collecting samples.
Bibliographie
Alloue-Boraud W.A.M. et al., 2015. Fermentation profile of Saccharomyces cerevisiae and Candida tropicalis as starter cultures on barley malt medium. J. Food Sci. Technol., 52(8), 5236-5242, doi.org/10.1007/s13197-014-1526-0
Ardhana M.M. & Fleet G.H., 2003. The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol., 86, 87-99, doi.org/10.1016/S0168-1605(03)00081-3
Batista N.N. et al., 2016. The impact of yeast starter cultures on the microbial communities and volatile compounds in cocoa fermentation and the resulting sensory attributes of chocolate. J. Food Sci. Technol., 53, 1101-1110, doi.org/10.1007/s13197-015-2132-5
Camu N., 2007. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl. Environ. Microbiol., 73(6), 1809-1824, doi.org/10.1128/AEM.02189-06
Carvalho Neto D.P. et al., 2017. Yeast diversity and physicochemical characteristics associated with coffee bean fermentation from the Brazilian Cerrado Mineiro Region. Fermentation, 3, 11, doi.org/10.3390/fermentation3010011
Castro-Alayo E.M., Idrogo-Vasquez G., Siche R. & Cardenas-Toro F.P., 2019. Formation of aromatic compounds precursors during fermentation of Criollo and Forastero cocoa. Heliyon, 5, e01157, doi.org/10.1016/j.heliyon.2019.e01157
Contreras A. et al., 2014. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol., 80, 1670-1678, doi.org/10.1128/AEM.03780-13
Crafack M. et al., 2014. Impact of starter cultures and fermentation techniques on the volatile aroma and sensory profile of chocolate. Food Res. Int., 63, 306-316, doi.org/10.1016/j.foodres.2014.04.032
Daniel H.M. et al., 2009. Yeast diversity of Ghanaian cocoa bean heap fermentations. FEMS Yeast Res., 9, 774-783, doi.org/10.1111/j.1567-1364.2009.00520.x
De Vuyst L. & Leroy F., 2020. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev., 44, 432-453, doi.org/10.1093/femsre/fuaa014
Delgado-Ospina J. et al., 2020. Functional biodiversity of yeasts isolated from Colombian fermented and dry cocoa beans. Microorganisms, 8, 1086, doi.org/10.3390/microorganisms8071086
Díaz-Muñoz C. & De Vuyst L., 2022. Functional yeast starter cultures for cocoa fermentation. J. Appl Microbiol., 133(1), 39-66, doi.org/10.1111/jam.15312
Duarte W.F. et al., 2010. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT - Food Sci. Technol., 43, 1564-1572, doi.org/10.1016/j.lwt.2010.03.010
Dzialo M.C. et al., 2017. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev., 41, S95-S128, doi.org/10.1093/femsre/fux031
Fia G., Giovani G. & Rosi I., 2005. Study of β-glucosidase production by wine-related yeasts during alcoholic fermentation. A new rapid fluorimetric method to determine enzymatic activity. J. Appl. Microbiol., 99, 509-517, doi.org/10.1111/j.1365-2672.2005.02657.x
Figueroa-Hernández C. et al., 2019. The challenges and perspectives of the selection of starter cultures for fermented cocoa beans. Int. J. Food Microbiol., 16(301), 41-50, doi.org/10.1016/j.ijfoodmicro.2019.05.002
Gaensly F. et al., 2015. Autochthonous yeasts with β-glucosidase activity increase resveratrol concentration during the alcoholic fermentation of Vitis labrusca grape must. J. Funct. Foods, 19, 288-295, doi.org/10.1016/j.jff.2015.09.041
Gutiérrez-Ríos H.G. et al., 2022. Yeasts as producers of flavor precursors during cocoa bean fermentation and their relevance as starter cultures: a review. Fermentation, 8, 331, doi.org/10.3390/fermentation8070331
Hammer Ø., Harper D.A.T. & Ryan P.D., 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electronica, 4, 1-9.
Han X. et al., 2023. Application of non-Saccharomyces yeasts with high β-glucosidase activity to enhance terpene-related floral flavor in craft beer. Food Chem., 404, 134726, doi.org/10.1016/j.foodchem.2022.134726
Ho V.T.T., Zhao J. & Fleet G., 2014. Yeasts are essential for cocoa bean fermentation. Int. J. Food Microbiol., 174, 72-87, doi.org/10.1016/j.ijfoodmicro.2013.12.014
Hu K. et al., 2016. Potential of glycosidase from non-Saccharomyces isolates for enhancement of wine aroma. J. Food Sci., 81, M935-M943, doi.org/10.1111/1750-3841.13253
King A. & Dickinson J.R., 2000. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast, 16, 499-506, doi.org/10.1002/(SICI)1097-0061(200004)16:6<499::AID-YEA548>3.0.CO;2-E
Koné M.K. et al., 2016. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int., 89, 910-917, doi.org/10.1016/j.foodres.2016.04.010
Matsushika A. et al., 2016. Identification and characterization of a novel Issatchenkia orientalis GPI-anchored protein, IoGas1, required for resistance to low pH and salt stress. PLoS ONE, 11(9), doi.org/10.1371/journal.pone.0161888
N’Guessan F.K., N’Dri D.Y., Camara F. & Djè M.K., 2010. Saccharomyces cerevisiae and Candida tropicalis as starter cultures for the alcoholic fermentation of tchapalo, a traditional sorghum beer. World J. Microbiol. Biotechnol., 26(4), 693-699, doi.org/10.1007/s11274-009-0224-y
Ouattara H.G., Elias R.J. & Dudley E.G., 2020. Microbial synergy between Pichia kudriazevii YS201 and Bacillus subtilis BS38 improves pulp degradation and aroma production in cocoa pulp simulation medium. Heliyon, 6, e03269, doi.org/10.1016/j.heliyon.2020.e03269
Pereira G.V., Miguel M.G., Ramos C.L. & Schwan R.F., 2012. Microbiological and physicochemical characterization of small-scale cocoa fermentations and screening of yeast and bacterial strains to develop a defined starter culture. Appl. Environ. Microbiol., 78, 5395-5405, doi.org/10.1128/AEM.01144-12
Pereira G.V. et al., 2013. Spontaneous cocoa bean fermentation carried out in a novel-design stainless steel tank: influence on the dynamics of microbial populations and physical-chemical properties. Int. J. Food Microbiol., 161, 121-133, doi.org/10.1016/j.ijfoodmicro.2012.11.018
Pereira G.V. et al., 2014. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. Int. J. Food Microbiol., 188, 60-66, doi.org/10.1016/j.ijfoodmicro.2014.07.008
Pereira G.V.M. et al., 2017. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT - Food Sci. Technol., 84, 290-297, doi.org/10.1016/j.lwt.2017.05.073
Pérez G. et al., 2011. A quick screening method to identify β-glucosidase activity in native wine yeast strains: application of Esculin Glycerol Agar (EGA) medium. World J. Microbiol. Biotechnol., 27, 47-55, doi.org/10.1007/s11274-010-0425-4
Ruppert V. et al., 2021. The impact of the fermentation strategy on the flavour formation of Ilzer Rose (Malus domestica borkh.) apple wine. Foods, 10(10), doi.org/10.3390/foods10102348
Sadoudi M. et al., 2012. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol., 32, 243-253, doi.org/10.1016/j.fm.2012.06.006
Sandoval-Lozano C.J., Caballero-Torres D. & López-Giraldo L.J., 2022. Screening wild yeast isolated from cocoa bean fermentation using volatile compounds profile. Molecules, 27, 902, doi.org/10.3390/molecules27030902
Santander-Muñoz M., Cortina J.R., Vaillant F.E. & Parra S.E., 2019. An overview of the physical and biochemical transformation of cocoa seeds to beans and to chocolate: flavor formation. Crit. Rev. Food Sci. Nutr., 60(10), 1593-1613, doi.org/10.1080/10408398.2019.1581726
Sauer M., Porro D., Mattanovich D. & Branduardi P., 2010. 16 years research on lactic acid production with yeast–ready for the market? Biotechnol. Genet. Eng. Rev., 27(1), 229-256, doi.org/10.1080/02648725.2010.10648152
Serra J.L. et al., 2019. Determination of the microbial community in Amazonian cocoa bean fermentation by illumina-based metagenomic sequencing. LWT - Food Sci. Technol., 106, 229-239, doi.org/10.1016/j.lwt.2019.02.038
Viesser J.A. et al., 2020. Exploring the contribution of fructophilic lactic acid bacteria to cocoa beans fermentation: isolation, selection and evaluation. Food Res. Int., 136, 109478, doi.org/10.1016/j.foodres.2020.109478
Viesser J.A. et al., 2021. Co-culturing fructophilic lactic acid bacteria and yeast enhanced sugar metabolism and aroma formation during cocoa beans fermentation. Int. J. Food Microbiol., 339, 109015, doi.org/10.1016/j.ijfoodmicro.2020.109015
Zhu X. et al., 2020. A rapid method for selecting non-saccharomyces strains with a low ethanol yield. Microorganisms, 8(5), doi.org/10.3390/microorganisms8050658






