- Portada
- volume 12 (2008)
- numéro 3
- Effect of growth regulators and explant origin on in vitro propagation of Ceratonia siliqua L. via cuttings
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Effect of growth regulators and explant origin on in vitro propagation of Ceratonia siliqua L. via cuttings

Notes de la rédaction
Received on May 2, 2007, accepted on November 13, 2007
Résumé
Effet des régulateurs de croissance et de l'origine de l'explant sur la culture in vitro du caroubier (Ceratonia siliqua L.) par le microbouturage. Une méthode de micropropagation du caroubier (Ceratonia siliqua L.) à partir de microboutures comportant un ou deux bourgeons axillaires est décrite. Le débourrement et le développement des pousses sont obtenus sur le milieu MS additionné de BAP (0,5 mg.l-1), d'AIB (0,1 mg.l-1) et de GA3 (0,5 mg.l-1). Les pousses feuillées sont multipliées sur le milieu de MS avec de la BAP à raison de 2 mg.l-1 puis enracinées sur le milieu de MS dont les macro-éléments et les micro-éléments ont été dilués de moitié et additionnés de charbon actif (2 mg.l-1) et d'AIB (2mg.l-1). Les meilleurs résultats sont obtenus avec les explants herbacés, issus d'arbres jeunes. Des différences significatives de résultats sont obtenus aux stades d'initiation, de multiplication et d'enracinement. Elles sont dues à l'origine des explants et aux différentes concentrations hormonales.
Abstract
The present work was undertaken to develop a basic and simple protocol for micropropagation of Ceratonia siliqua. Axillaries bud sprouting and shoot development were stimulated on MS supplemented with BAP (0.5 mg.l-1), IBA (0.1 mg.l-1) and GA3 (0.5 mg.l-1), shoot multiplication was obtained on MS supplemented with BAP (2 mg.l-1) and rooting of microshoots was achieved on MS supplemented with IBA (2 mg.l-1) and charcoal (2 mg.l-1). The best results were obtained with herbaceous explants taken from juvenile trees. Significant differences in proliferation, multiplication and rooting due to the type and origin of explant and to the concentration of growth regulators were found.
Tabla de contenidos
1. INTRODUCTION
1The carob tree (Ceratonia siliqua L.) belonging to the family Cesalpiniaceae, is widely used in the Mediterranean regions (Rejeb, 1989; Tous et al., 1990) and cultivated for ornamental and industrial purposes (Girolamo et al., 2002). The carob is mainly used in pharmaceutical and in liquor industries (Van Uden, 1981; Maria et al., 1997; Haslberg, 2000).
2There is a great intraspecific variability with a large number of cultivars of carob (Mitrakos, 1987). The high phenotypic variation between cultivars has important implications for selection, cultivation practices and establishment of new plantations to improve productivity of this crop (Battle et al., 1997) which is hampered by its high morphological variability (Naghmouchi et al., 2004) and its high long reproductive cycle.
3To optimise the productivity of plantations, it is essential to plant a maximum number of female plants. This could be achieved through in vitro culture method for cloning superior carob plants of each sex.
4Most studies on carob tree have focused on applied aspects, such as agricultural, industrial and commercial. Nevertheless, there are some aspects on carob flower phenology, fruiting and pollination (Tucker, 1992; Retana et al., 1994; Bosch et al., 1996; Ortiz et al., 1996; Arista et al., 1999).
5Organogenesis in carob has been reported in the past, Martins-Loucao et al. (1981) obtained calli and shoot regeneration from cotyledon cultures. Sebastian et al. (1986) and Romano et al. (2002) reported a micropropagation protocol from seedlings and mature trees, using Murashige and Skoog (1962) medium (MS) supplemented with zeatin for shoot multiplication and MS medium supplemented with indole-3-butyric acid (IBA) for root induction. Bhalerao et al. (1992) tested young male inflorescences on MS medium supplemented with 6-benzylaminopurine (BAP) and casein hydrolysate. On this medium, the floral buds were transformed into shoot buds, and developed into various types of shoots. After transferring to MS medium supplemented with BAP and kinetin, the shoots elongated and formed 2-3 leaf pairs. Belazi et al. (1994) cultured nodal segments of seedlings in Quoirin & Lepoivre (1977) medium added with IBA, GA3 and IBA at different concentrations.
6Adventitious root formation is essential for successful vegetative propagation of many woody plants in a cutting. However, in several tree species, rooting is still a major problem. Furthermore, rooting ability declines with age (Dimitris et al., 2002). Some progress have been made in rooting of different species using different chemical or natural compounds in the rooting media such as auxins combined with phenolic compounds (Onay et al., 2003; Fotso et al., 2004).
7Root formation of ligneous species is regulated by a great number of factors, and to a great extent by auxins (Shwab et al., 1988; Baksha et al., 2003) and by addition of charcoal in the media which is indispensable to reduce the effect of polyphenols secreted by the microcuttings.
8Our work aimed at developing an in vitro vegetative propagation method of selected trees of C. siliqua and founding optimal conditions for shoot proliferation and root formation.
2. MATERIAL AND METHODS
2.1. Explant origin, sterilization protocol and cultivation
9Nodal segments of carob were harvested from trees growing in the Botanical Garden of the Institute of Research in Rural Engineering, Water and Forestry in Tunisia.
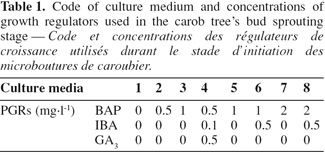
10The material collected from February to April, from herbaceous and semi-ligneous parts of the stem of mother-trees, was used as initial explant for in vitro propagation of carob trees of different ages (5, 12 and 25 years).
11These explants were washed with water and detergent (Teepol). They were shaken successively for 5 minutes in 70% ethanol, 20 minutes in a NaClO solution and 20 minutes in a 0.1% HgCl2 solution. Finally, explants were rinsed three times with sterile distilled water and cultured on agar nutrient medium.
2.2. Media and culture conditions
12Cuttings of 20 mm long (one node with a single or two axillary buds) were excised and individually placed in 20x150 mm pyrex test tubes containing 15 ml of MS basal medium (Murashige & Skoog, 1962) supplemented with 30 g.l-1 sucrose and solidified with agar (8 g.l-1). The pH of medium was adjusted to 5.6 with HCl 0.1N or NaOH 0.1N before sterilization by autoclaving at 121°C for 105 minutes.
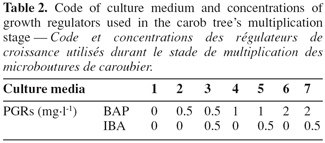
13Growth regulators: 6-Benzylaminopurine (BAP), Indol-3-butyric acid (IBA) and Gibberelic acid (GA3) were added in media according to the experimental stage. After inoculation, cultures were incubated under a 16:8 h photoperiod of cool-white light at 1 250 Lux.
2.3. Shoot culture
14In vitro regenerated shoots, longer than 20 mm, were excised from the microcuttings and maintained by subculturing every five weeks on a shoot multiplication medium: MS salts supplemented with IBA (0, 0.1 and 0.5 mg.l-1) and BAP (0, 0.5, 1 and 2 mg.l-1).
15Observations were made after five weeks including percentage of axillary bud sprouting, shoot lengh, shoot numbers and multiplication rate.
2.4. Rooting induction
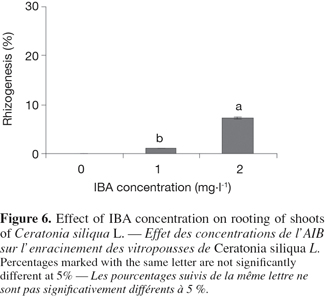
16Multiplying microshoots were cultured on rooting medium. The rooting process was separated into two treatments:
17– Shoots were cultured on darkness on the rooting medium that contained macro and micronutrients in half-strength with 30 g.l-1 sucrose and 8 g.l-1 agar, supplemented with IBA (0, 1 and 2 mg.l-1)
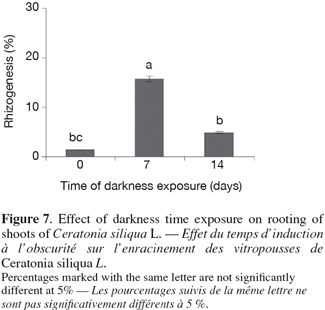
18– Shoots were transferred to the basal medium and incubated under the same light and temperature regime as shoot multiplication cultures.
19Data on rooting percent were recorded after five weeks of transfer in rooting medium.
2.5. Statistical design
20Forty-eight microcuttings were tested on initiation medium and 24 shoots were tested on each multiplication and rooting medium.
21Data from each experimental stage were analysed separately by an analysis of variance and the means compared with Duncan's multiple-range test at P < 0.05.
3. RESULTS
3.1. Effect of plant growth regulators and explant origin on axillary bud sprouting and shoots development
22Nodal segments placed on MS agar medium started to form shoots within two to three weeks. Fast axillary bud sprouting was achieved on MS medium supplemented with different concentrations of BAP and IBA.
23All tested BAP concentrations stimulated the fast developpment of new shoots from axillary buds of nodal segments.
24Experiments showed that the major treatment was significantly different from the control. MS medium supplemented with BAP (0.5 mg.l-1) plus IBA (0.1 mg.l-1) and GA3 (0.5 mg.l-1) gives a higher rate of bud sprouting and promoted effectively formation of longer shoots in nodal segments (Figure 1).
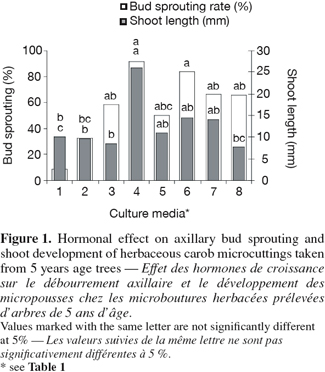
25The MS medium supplemented with higher concentration of BAP (1 and 2 mg.l-1) stimulated formation of shoots. However, the shoots were shorter than those obtained with MS added with BAP (0.5 mg.l-1), IBA (0.1 mg.l-1) and GA3 (0.5 mg.l-1) (Figure 1).
26Nodal segments, taken from herbaceous and semi-ligneous parts of trees at different ages, and cultured on MS medium supplemented with BAP and IBA produced new shoots within two to three weeks.
27Results showed that bud sprouting decreased with increasing the age of mother tree and with lignification of the explant.
28There is a significant difference in response of the different explants inoculated on the better selected medium: MS plus BAP (0.5 mg.l-1), IBA (0.1 mg.l-1) and GA3 (0.5 mg.l-1) (Figure 2).
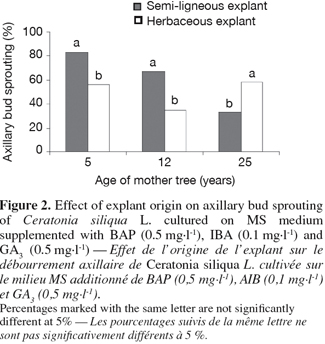
29Bud sprouting rates and shoot length of herbaceous microcuttings were higher than that of semi-ligneous microcuttings (Figures 2 and 3).
3.2. Effect of plant growth regulators and explant origin on shoot multiplication of Ceratonia siliqua
30The highest multiplication rate and the high number of shoots regenerated by microshoot were obtained on MS medium supplemented with 2 mg.l-1 BAP (Figure 4).
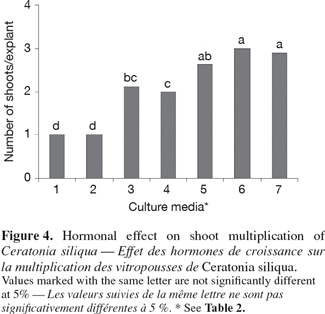
31In general, the number of shoots produced on media supplemented with BAP increased with increasing BAP concentration.
32Our experiments with explants taken from trees of different ages and different parts of mature trees of C. siliqua showed that age of explants and lignification of material are two important factors influencing shoot multiplication (Figure 5).
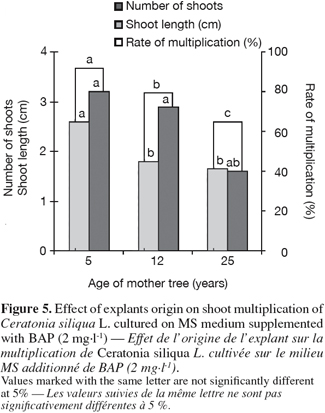
33Shoots culture was produced from all tested explants. However, multiplication rates of explants issued from herbaceous parts were higher than those from semi-ligneous parts. Also microshoots produced from nodal segments taken from young trees (five years) exhibited higher multiplication rates and produced numerous and longer shoots than the other experimented materials.
3.3. Effect of explant origin, darkness exposure time, growth regulators and charcoal on rooting
34Shoots excised from multiplying cultures and transferred on rooting medium started to form adventitious roots within three weeks after darkness exposure.
35Figure 6 shows results on root formation with different concentrations of IBA.
36When no IBA was supplemented in the medium, no roots were formed.
37In order to promote maximum rooting, an exposure time for one week was required for explants of carob with 2 mg.l-1 IBA (Figure 7). The percentage of rooted shoots and the number of roots increased with increasing time of exposure, whereas the long time of exposure (more than 7 days) decreased rooting and the explants became chloritic.
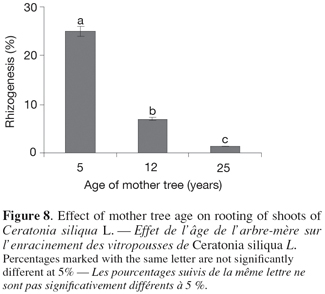
38Shoots issued from nodal segments taken from young trees gave highest percentage of rhizogenesis (Figure 8). Shoots derived from juvenile parts of mature trees exhibited also good rooting response. Twenty-five percents of shoots formed adventitious roots within three to five weeks.
39The best rate of rhizogenesis was observed with charcoal (2 mg.l-1). The addition of charcoal ameliorates the development of roots and callus.
4. DISCUSSION
40Recent advances in micropropagation of forest trees have opened great opportunities for mass propagation of selected valuable genotypes (Chalupa, 1993).
41Selection of explants, composition of nutrient media, concentration of phytohormones and methods used for micropropagation had significant effects on shoot multiplication and rooting rates of carob explants.
42Shoot elongation depends on the medium used. The addition of GA3, IBA and BAP ameliorates bud sprouting and shoot elongation. The best elongation was shown on media with BAP. Shoots obtained on free BAP medium presented short internodes and quickly lost their ability to elongate. At 0.5 mg.l-1 BAP, the leaves were curled. Those formed on media with BAP were normal and no apparent difference was observed between shoots.
43The development of microshoots had a similar tendency as bud sprouting. The best results were obtained with 2 mg.l-1 for herbaceous material collected from juvenile trees.
44On media without growth regulators, nodal segments produced only one shoot.
45The best shoot multiplication was obtained on media supplemented with 2 mg.l-1 BAP or with BAP (2 mg.l-1) supplemented with IBA (0.5 mg.l-1).
46Shoot multiplication and elongation were also greatly affected with juvenility of explants.
47It was demonstrated that technique of in vitro culture is used for rejuvenation of mature trees (Vieitez, 1994; Chalupa, 2000, 2002). The capacity of trees to be propagated vegetatively decreases with age; mature trees have usually a low capacity to be vegetative propagation (Brhadda et al., 2003).
48Bud sprouting ability has been found to be closely related to cuttings origin in a number of other tree species. For example, Olea europea bud sprouting was the same in juvenile and adult material (Abousalim et al., 2005).
49Our experiments with different explants origin of C. siliqua indicate that explants taken from juvenile parts from mature trees and those taken from juvenile trees, exhibit juvenile characteristics. Cultures initiated from these explants showed a high capacity for shoot formation and fast shoot proliferation.
50A difference between herbaceous and semi-ligneous explants was found. Herbaceous explants are at a younger stage of development than semi-ligneous ones. A young developmental stage has often been found to be more optimal for shoot regeneration than older stages, which may be explained by differences in anatomical and physiological properties.
51The potential of cuttings of C. siliqua to form adventitious roots decreased with increasing plant age. Furthermore mature trees are characterized by decreasing capacity for vegetative propagation. However, different parts of mature tree are often in different degree of maturity.
52Despite the recalcitrance to vegetative propagation of C. siliqua, our results indicate that the species can be rooted by addition of IBA on the induction medium. Auxins are involved in the process of adventitious root formation. In many woody plants, IBA is commonly used to promote root initiation (Onay et al., 2003; Bhatt, 2004). In our study, the absence of IBA in the rooting medium did not lead to root formation. Several authors have shown that auxin is only required during the initiation phase, and becomes inhibitory for root out growth (Shwab et al., 1988; Elhamdouni et al., 2000; Chalupa, 2002). This inhibition of rooting is often accompanied with callus formation. The presence of callus on the shoots increased time for rooting as well as the number of roots formed. Studies on in vitro rooting of explants of Eucalyptus (Fazal et al., 2003) also showed an increased callus formation with increased IBA concentrations.
53Charcoal is indispensable to induce good development of roots, clamping the reaction of tannins. In fact the tannin constitutes a physiological inhibitor (Souayah et al., 2002; Custódio et al., 2005).
54The low rates of rhizogenesis were found with other species (Wallali et al., 1993). The difficulty to root in vitro can be overcome by rejuvenation of materials by different methods (Franclet et al., 1982; Howard et al., 1989). With carob, Sebastian et al. (1986) demonstrate the importance of multiplication medium on rhizogenesis and the presence of GA3 in the shoot multiplication medium suppressed rooting.
55Rooting experiments demonstrated the good rooting capacity of microshoots originated from juvenile parts of mature trees.
56This study induces plantlets regeneration from explants of C. siliqua. These regenerated plantlets could be further transferred to the natural conditions.
5. CONCLUSION
57Plant growth regulators, age of mother tree and type of explant clearly affected shoot development from nodal explants cultivated in vitro and shoot multiplication.
58In vitro propagation of mature or juvenil carob trees can be achieved by the use of MS medium with BAP, IBA and GA3 on the initiated medium and BAP, or BAP supplemented with IBA on the multiplication medium.
59It can be concluded that the presence of IBA and charcoal in the medium is indispensable to induce a good development of roots, clamping the reaction of tannins. Thus, this medicinal plant has a considerable potential morphogenic capacity. This potential can be optimized by searching the performing factors in each stage of the breeding technique: age of the mother tree and type and concentration of plant growth regulators, and by optimizing the different stages.
60The problems associated with the great variability of carob (C. siliqua L.) and the long stage of juvenility can be now overcome, using in vitro microcutting or other techniques like meristem culture and direct and indirect organogenesis.
61List of abbreviations
62BAP: 6- Benzylaminopurine
63GA3: Gibberelic acid
64HgCl2: Bichlorure mercure
65IBA: Indol-3-butyric acid
66MS: Murashige and Skoog (1962)
67NaClO: Hypochlorite sodium
68GRs: Growth Regulators
Bibliographie
Abousalim A., Brhadda N. & Wallali L.D., 2005. Essais de prolifération et d'enracinement de matériel issu de rajeunissement par bouturage d'oliviers adultes (Olea europea L.) et de germination in vitro : effets de cytokinine et d'auxines. Biotechnol. Agron. Soc. Environ., 9, 237-240.
Arista M., Ortiz P. & Talavera S., 1999. Apical pattern of fruit production in the racemes of Ceratonia siliqua (Leguminosae: Caesalpinioideae): role of pollinators. Am. J. Bot., 8, 1708-1716.
Baksha R. et al., 2003. Effect of auxin, sucrose and pH level on in vitro rooting of callus induced microshoots of sugarcane (Saccharum officinarum). J. Biol. Sci., 3, 915-920.
Battle I. & Tous J., 1997. Carob tree (Ceratonia siliqua L.). Promoting the conservation and use of under-utilised and neglected crops. Roma: Institute of Plant Genetics and Crop Plant Research; Gatersleben: International Plant Genetic Resource Institute (IPGRI).
Belazi M., Bolen M.R. & Boxus P., 1994. Régénération in vitro et acclimatation du caroubier (Ceratonia siliqua L.). Quel avenir pour l'amélioration des plantes. Paris : Ed. AUPELF-UREF, 223-227.
Bhalerao V.P. & Chinchanikar G.S., 1992. In vitro transformation of floral buds to vegetative shoots in Ceratonia siliqua L. Biovigyanam, 18, 82-88.
Bhatt I.D. & Dhar U., 2004. Factors controlling micropropagation of Myrica esculenta buch. -Ham. ex D. Don: a high value wild edible of Kumaun Himalaya. Afr. J. Biotechnol., 3, 534-540.
Bosch J., García del Pino F., Ramoneda J. & Retana J.,1996. Fruiting phenology and fruit set of carob, Ceratonia siliqua L. (Caesalpiniaceae). Israel J. Plant Sci., 44, 359-368.
Brhadda N., Abousalim A., Loudiyi D. & Benali D., 2003. Effect of culture medium on micropropagation of olive (Olea europea) cv. Morrocan Picholine. Biotechnol. Agron. Soc. Environ., 7, 177-182.
Chalupa V., 1993. Vegetative propagation of oak (Quercus robur and Quercus petrea) by cutting and tissue culture. Ann. Sci. For., 50, 295-307.
Chalupa V., 2000. In vitro propagation of mature trees of pedunculate oak (Quercus robur L.). J. For. Sci., 46, 537-542.
Chalupa V., 2002. In vitro propagation of mature trees of Sorbus aucuparia L. and field performance of micropropagated trees. J. For. Sci., 48, 529-535.
Custódio L., Carneiro M.F. & Romano A., 2005. Microsporogenesis and anther culture in carob tree (Ceratonia siliqua L.). Sci. Hortic., 104, 65-77.
Dimitris P. & Panagiotis K., 2002. Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxidants. Food Technol. Biotechnol., 42, 105-108.
Elhamdouni E.M., Lamarti A. & Badoc A., 2000. Micropropagation des cultivars " Chandler " et " Tudla " de fraisier (Fragaria *Ananassa Duch). Bull. Soc. Pharm. Bordeaux, 139, 91-104.
Fazal R., Mussarrat J. & Ihsan I., 2003. Mass propagation in Eucalyptus camaldulensis Dehn. Asian J. Plant Sci., 2, 184-187.
Fotso A., Tchinda N.D., Duclaire M. & Ndoumou D.O., 2004. Propagation de Ricinodendron heudelotii par bouturage in vitro. Fruits, 10, 351-358.
Franclet A. & Boulay M., 1982. Micropropagation of frost-resistant eucalypt clones. Aust. Forest Res., 13, 83-89.
Girolamo R. & Laura D., 2002. Evaluation and preservation of genetic resources of carob (Ceratonia siliqua L.) in southern of Italy for pharmaceutical use. Breed. Res. Aromat. Med. Plants, 9, 367-372.
Haslberg C.D., 2000. Vegetative growth and flower and fruit development in carob trees (Ceratonia siliqua L.) with special emphasis on environmental conditions at marginal production sites in south Portugal. Thesis: University of Barcelona, Department of Biological Sciences (Spain).
Howard B.H., Jones O.P. & Vasek J., 1989. Long-term improvement in the rooting of plum cuttings following apparent rejuvenation. J. Hortic. Sci., 64, 147-156.
Maria G., Barbagallo R., Di Lorenzo R.M. & Crescimanno F.G., 1997. Characterization of carob germplasm (Ceratonia siliqua L.) in Sicily. J. Hortic. Sci., 72, 537-543.
Martins-Loucao M.A. & Rodriguez-Barrueco C., 1981. Establishment of proliferating callus from roots, cotyledons and hypocotyls of carob (Ceratonia siliqua L.) seedlings. Pflanzenphysiol, 103, 297-303.
Mitrakos K., 1987. The botany of Ceratonia. In: Fito P. & Mulet A., eds. Proceedings of the second international carob symposium, September/October 1986, Generalitat Valenciana, Conselleria d'Agricultura I Pesca, Valencia, Spain, 209-218.
Murashige T. & Skoog F., 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant, 15, 473-497.
Naghmouchi S., Khouja M.L. & Boussaid M., 2004. La variabilité morphologique du caroubier (Ceratonia siliqua L.) : un patrimoine biologique pour la Tunisie. Journées Inter-Universitaires « Paysage et patrimoine », Troisièmes rencontres « Horticulture et paysages », Chott Mariem, Tunis.
Onay A. et al., 2003. In vivo and in vitro micrografting of pistachio (Pistacia vera L.). Turk. J. Biol., 27, 95-100.
Ortiz P., Arista M. & Talavera S., 1996. Producción de néctar y frecuencia de polinizadores en Ceratonia siliqua L. (Caesalpiniaceae). An. Jard. Botan. Madrid, 54, 540-546.
Quoirin M. & Lepoivre P., 1977. Etude de milieux adaptés aux cultures in vitro de Prunus. Acta Hortic., 78, 437-442.
Rejeb M.N., 1989. Mécanismes physiologiques d'adaptation à la sécheresse du caroubier. Rev. Réseau Amélior. Prod. Agric. Milieu Aride, 1, 47-55.
Retana J., Ramoneda J., Garcia Del Pino F. & Bosh J., 1994. Flowering phenology of carob, Ceratonia siliqua L. (Caesalpiniacea). J. Hortic. Sci., 69, 97-103.
Romano A., Barros S. & Martins-Loucao M.A., 2002. Micropropagation of the Mediterranean tree Ceratonia siliqua. Plant Cell Tissue Organ Cult., 68, 35-41.
Sebastian K.T. & McComb J.A., 1986. A micropropagation system for carob (Ceratonia siliqua L.). Sci. Hortic., 28, 127-131.
Shwab L. & Martins-Loucao M.A., 1988. Shoot formation in Ceratonia siliqua hypocotyls callus. In: Fito Maupoey P. & Mulet Pons A., coord. Proceedings of the 2d international carob symposium. Valencia, Spain: Servei d'Estudis Agraris i Comunitaris, 245-253.
Souayah N. et al., 2002. Breeding improvement of Laurus nobilis L. by conventional and in vitro propagation techniques. Breed. Res. Aromat. Med. Plants, 3, 47-50.
Tous J. & Battle I., 1990. El algarrobo. Madrid: Ed. Mundi-Prensa.
Tucker S., 1992. The developmental basis for sexual expression in Ceratonia siliqua (Leguminosae: Caesalpinioideae: Cassieae). Am. J. Bot., 79, 318-327.
Van Uden N., 1981. Industrial bioconversion of carob and other carbon sources from plants. Port. Acta Biol., 16, 11-14.
Vieitez A.M., Sanchez M.C., Amo-Marco J.B. & Ballester A., 1994. Forced flushing of branch segments as a method for obtaining reactive explants of mature Quercus robur trees for micropropagation. Plant Cell Tissue Organ Cult., 37, 113-120.
Wallali L.D. & Abousalim A., 1993. Olive tree propagation. In: Tantaoui – El Araki A., ed. Proceedings of the 1st CNCPRST- CSIS seminar on oleaginous plants, October 19-21, Rabat, Morocco, 63-67.
Para citar este artículo
Acerca de: Souheila Naghmouchi
Institute of Research in Rural Engineering, Water and Forestry (INRGREF). Rue Hédi Karray. BP 10. TN-2080 Ariana (Tunisia) – University of Sciences. Campus universitaire. TN-1060 Tunis (Tunisia).
Acerca de: Mohamed Larbi Khouja
Institute of Research in Rural Engineering, Water and Forestry (INRGREF). Rue Hédi Karray. BP 10. TN-2080 Ariana (Tunisia). E-mail: Khouja.medlarbi@iresa.agrinet.tn
Acerca de: Mohamed Nejib Rejeb
Institute of Research in Rural Engineering, Water and Forestry (INRGREF). Rue Hédi Karray. BP 10. TN-2080 Ariana (Tunisia).
Acerca de: Mohamed Boussaid
Institute National of Sciences Applied to Technology (INSAT). Centre urbain Nord. BP 676. TN-1080 Tunis (Tunisia).






