- Portada
- volume 13 (2009)
- numéro 1
- Improving growth of shea butter tree (Vitellaria paradoxa C.F.Gaertn.) seedlings using mineral N, P and arbuscular mycorrhizal (AM) fungi
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Improving growth of shea butter tree (Vitellaria paradoxa C.F.Gaertn.) seedlings using mineral N, P and arbuscular mycorrhizal (AM) fungi

Notes de la rédaction
Received on October 22, 2007, accepted on February 12, 2008
Résumé
Amélioration de la croissance juvénile du karité (Vitellaria paradoxa) par l’utilisation d’engrais minéraux (azote et phosphore) et de champignons endomycorhiziens à vésicules et arbuscules (MVA). Pour réussir la culture de la plupart des arbres à fruits non domestiqués comme le karité (Vitellaria paradoxa C.F.Gaertn.), il est nécessaire d’en identifier les besoins nutritionnels et les conditions optimales de croissance. Les réponses de plants de karité à l’azote (N), au phosphore (P) et à l’endomycorhization (VA) ont été étudiées. Des plants de 6 mois ont été transplantés en pots, sur un sol désinfecté et pauvre en éléments nutritifs, et cultivés pendant 6 mois. Les plants ont été inoculés avec le champignon MVA Glomus intraradices Schenk & Smith. Les traitements de fertilisation ont consisté en une combinaison factorielle de trois doses de N apporté sous forme de NH4NO3-N, et de P appliqué sous forme de Ca(H2PO4)2. La fertilisation a stimulé la croissance en hauteur, le diamètre au collet et les biomasses sèches, comparativement aux traitements sans fertilisants. A ces améliorations était associée une augmentation des prélèvements totaux des parties aériennes en N et en carbone (C) (33 %), alors que les teneurs en P et en K n’ont pas été affectées. Des interactions N x P significatives ont été observées pour les biomasses sèches, et les prélèvements totaux des parties aériennes en N et C, suggérant que les réponses au N étaient influencées par la dose de P. A faible dose de P, les réponses en termes de biomasses sèches et de prélèvements totaux en N et C ont été constantes et significatives. L’augmentation de la dose de P a stimulé la croissance et les prélèvements totaux des parties aériennes en minéraux dans les cas des plants traités à faible dose de N, alors qu’aux doses plus élevées en N, la croissance en a été réduite. L’application combinée de N et de P, pour les doses respectives moyennes et élevées, a eu peu d’impact sur la croissance des plants, du fait vraisemblablement de rapports N:P déséquilibrés. En général, le taux de colonisation endomycorhizienne a été faible (≤ 12 %) et n’a pas été influencé par les facteurs étudiés. Il n’y a pas eu de réponse à l’inoculation probablement parce que les endomycorhizes formées étaient inefficaces. Les potentialités de l’utilisation des fertilisants minéraux et des champignons mycorhiziens en vue de promouvoir la croissance juvénile du karité sont discutées.
Abstract
For the successful cultivation of most undomesticated fruit trees such as shea butter tree (Vitellaria paradoxa C.F.Gaertn.), there is a need to identify their nutrient requirements and optimal growth conditions. Responses of shea seedlings to combined N and P fertilization, and to inoculation with arbuscular mycorrhizal (AM) fungi was investigated. Six months old shea seedlings were transplanted into pots and grown for six months using a sterile nutrient-deficient soil. The seedlings were inoculated with the AM fungus Glomus intraradices Schenk & Smith. The fertilization treatments consisted of a factorial combination of three levels of N supplied as NH4NO3-N and P supplied as Ca(H2PO4)2. Fertilization stimulated plant height, collar diameter and dry weights (DWs) compared with non-fertilized treatments. These improvements were associated with an increase in total shoot N and C uptake (33% increase) whereas P and K contents were not affected. There was significant N x P interaction on DWs and total shoot N and C contents, suggesting that seedling N responses were influenced by P rates. Consistent N responses in DWs, and total shoot N and C uptake were significant at the lowest P rate. P increases promoted growth and total shoot nutrient contents in low N-treated seedlings, while reducing growth in higher N rates. The combined application of medium and high rates of N and P fertilizers showed relatively low impact on seedling growth presumably because of suboptimal N:P ratios. Mycorrhizal root colonization was generally low (≤ 12%) and was not affected by any of the treatments. There was also no response to inoculation with AM fungi probably because the established mycorrhizal association was ineffective. The potential use of both mineral fertilizers and AM fungi to promote growth performance of shea seedlings are discussed.
Tabla de contenidos
1. Introduction
1Shea tree (Vitellaria paradoxa C.F.Gaertn.) also known as Karité (in French) is an indigenous fruit tree of Sudano-Sahelian Africa. There are two subspecies of V. paradoxa, one of which (subsp. paradoxa) extends from Senegal eastwards to the Central African Republic whilst the other (subsp. nilotica) occurs in southern Sudan and Ethiopia, Uganda and northeast Zaire (Boffa, 1999). In most parts of its natural range, shea is of great economic and ecologic importance, often being the main component of the tree stratum in traditional parkland systems, which are farmlands with scattered trees forming an open permanent over-storey of associated annual crops (Bonkoungou, 1992). As a perennial woody species, that shed its leaves annually, shea tree is thought to play a major role in nutrients recycling through the decay of its leaves and fine roots at the soil surface (De Bie et al., 1998; Bayala et al., 2006). The litter of shea was shown to have lower nutrient content compared with Parkia biglobosa (another common parkland non N2-fixing Leguminous tree), and it was found to decompose at a low rate with time (Bayala et al., 2005), suggesting a more sustainable impact on soil fertility (Bayala et al., 2006).
2Furthermore, shea tree is highly valued by farmers, mostly because of its fat containing kernels which are sold both in local and international markets, thereby considerably contributing to wealth creation. The vegetable fat of shea nut is second in importance only to palm oil in Africa (Hall et al., 1996). The commercialization of shea products represents an important source of income at different parts of the community chain, from community levels, with rural children and women who gather and process nuts, to town dwellers as well as entire countries (Bonkoungou, 1992; Boffa et al., 1996). For instance, shea nut was the third export product of Burkina Faso in the 1980’s (World Bank, 1989). Shea tree also provides fruits, medicine, construction materials, fuel wood and carving wood (Hall et al., 1996).
3Despite its great contribution to the local economies, shea tree remains undomesticated probably because of lack of tradition to plant local tree species. Indeed, shea tree parklands result from naturally occurring individual trees that are protected by farmers when clearing their fields, thus creating parkland systems (Boffa et al., 1996). These parklands have been reported to be degrading steadily resulting in decreasing tree density and vegetation cover as well as reduced soil fertility (Gijsbers et al., 1994; De Bie et al., 1998; Ouédraogo, 2006). This trend suggests the need to use artificial regeneration to promote this species in farmer’s fields.
4Nutrient deficiencies, especially low soil phosphorus (P) and nitrogen (N), constitute the most important soil limiting factors for food crop production throughout the Sudano-Sahelian zone of Africa (Bado et al., 2006; Tittonell et al., 2007), but their effects on sexual tree regeneration is less known, possibly because so far little attention has been paid to the nutrition of the woody plants of arid parklands. Most existing information on the response of wild trees to N and P application on soils of low nutrient status is either through the common use of starter fertilizers to boost initial seedling growth rate, or from labeling experiments mainly in N2-fixing legumes (Karanja et al., 1999; Binkley et al., 2003). Thus, very few studies can be found that have varied nutrients availability to assess their full potential in tree management (Sanginga et al., 1990; Karanja et al., 1999). Consequently, basic information on nutrient requirements of important indigenous tree species is not readily available, leading to lack of practical fertilizer prescription. The practice of using N and P fertilizers separately is seen as one factor contributing to low fertilization efficiency because it always overlooks the advantageous interaction that often occurs between both elements when fertilizers are incorporated in association into the soil (Teng et al., 1996). Therefore, from a domestication perspective, the evaluation of N and P interaction appears to be important as a prerequisite for determining how both fertilizer types can be managed to promote rapid seedling establishment and growth in slow growing tree species on the one hand. On the other hand, crops and tree species native to arid and semi-arid areas are widely reported to rely, to some extent, on arbuscular mycorrhizal (AM) fungi for nutrients acquisition and growth, especially when soils are nutrient-depleted (Ingleby et al., 1997; Guissou et al., 1998; Bâ et al., 2000). By developing an external mycelium that extends beyond the rooting zone, AM fungi enable the host plant to explore a greater volume of soil for increased nutrient absorption, particularly less mobile elements such as P, resulting in improved host growth and survival (Smith et al., 1997). Although the occurrence and efficiency of AM fungi have been widely examined in most valuable undomesticated fruit trees (Bâ et al., 2000; Mathur et al., 2000), little is known of the mycorrhizal status and responsiveness of inherently slow growing species such as shea tree. Here we report the results from the nursery of a fertilization experiment that aimed at examining the interaction between N and P addition on growth and nutrient uptake of shea seedlings. We also examined root symbiosis with arbuscular mycorrhizal fungi, as an important factor that mediates plant P nutrition, by investigating to what extent root colonization and mycorrhizal efficiency in this species could cope with N and P fertilization. We hypothesized that colimitation of N and P could contribute to the slow growth rate of shea species, and that the availability of these nutrients could interact to influence seedling growth performance.
2. Material and methods
2.1. Experiment soil characteristics and its preparation
5The experimental soil was collected from alluvial deposits near Manga (Zoundweogo Province, Burkina Faso). The soil consisted of clay 2.3%, sands 93.1% and silt 1%, and had the following characteristics: pH in water (1:2.5) 6.66, pH KCl 5.49, P total 123 ppm, Olsen-Dabin available P 4 ppm, organic carbon 0.31%, total Kjeldahl nitrogen 0.018%, exchangeable bases 2.2 meq%, Ca 1.76 meq%, Mg 0.30 meq%, Na 0.04 meq%, K 0.10 meq%, and CEC 1.51%. The soil was sieved (2 mm mesh), autoclaved at 120°C for 1 hour, and transferred to 15 l pots (30 cm diameter, 30 cm high) (7 kg.pot-1).
2.2. AM fungal inoculum
6Arbuscular mycorrhizal fungus Glomus intraradices Schenk & Smith (isolate IR14 obtained from IRD, Dakar, Senegal) was propagated using pearl millet (Pennisetum glaucum) as the host crop. For the trapping cultures, 15 cm diameter pots were planted with 40-50 seeds per pot of pearl millet. Cultures were grown in a greenhouse for three months to increase the inoculum. For the AM fungi treatments, inoculation was done at the time of transplanting by adding 10 g of crude stock culture below seedlings that consisted of soil, spores (approximately 110.g-1), hyphae and infected root fragments. Non-mycorrhizal pots received no inoculum or soil washings.
2.3. Plant material and culture
7Seeds of Vitellaria paradoxa were collected in June 2002 in a parkland at Saria, a village located 80 km West of Ouagadougou, the capital city of Burkina Faso. The fruits were transferred to the laboratory in dark plastic bags and kept at room temperature for one week. Seeds were carefully separated from the fleshy pulp under tap water stream and surface-sterilized with 10% sodium hypochlorite for 10 min. They were sown for germination in pots (50 cm diameter, 20 cm high) filled with sterilized soil. The pots were mounted on metallic benches 25-30 cm high in a greenhouse at ambient air conditions. Watering was done daily at field capacity. To allow free draining several 2-3 mm lateral holes were made 2 cm above the base of each pot. After six months single seedlings were transplanted to 15 l pots containing 7 kg of sterile sandy soil. Each seedling was inoculated with 10 g of soil when transplanting. The pots were watered daily with 400 ml, which represent two-third of field capacity of the substrate used in the present experiment. Biweekly, the daily water supply was replaced by similar amount of a nutrient solution which was free of both N and P, and consisted of 2 mol.m-3 MgSO4.7H2O, 0.55 mol.m-3 K2SO4, 1.9 mol.m-3 KCl, 2.8 mol.m-3 CaCl2.2H2O, plus Sequestrene and micronutrients (Mahon, 1977).
2.4. Treatments and experimental design
8Treatments consisted of a 3 x factorial combination of three rates of P (0.7, 3.6 and 6.4 mg.kg-1) as Ca(H2PO4)2 and three levels of N (2, 7 and 12 mg.kg-1) as NH4NO3-N (Table 1). For each treatment, the appropriate quantities of both fertilizers were delivered in the nutrient solution outlined above. Under each pot there was a container collecting the drained solution that was regularly put back in the pot between two solution application events. There were two additional control treatments. In one case, seedlings were raised as described above but without N and P additions (control 1). In the second control treatment (control 2), seedlings were solely irrigated with water, i.e. without either fungal inoculum, nutrient solution addition, nor N and P amendements (Table 1). The levels of K and Ca were adjusted accordingly using KOH and Ca(OH)2, respectively. The experiment was performed as a randomized complete block design with five replicates.
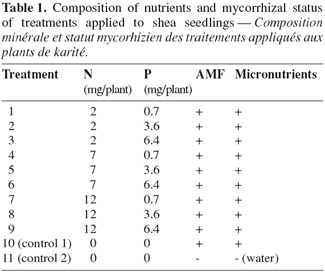
2.5. Data collection
9Shoot height and collar diameter were recorded before plants were depoted six months after transplanting. Roots were washed free of soil, and were separated from shoots. Root sub-samples each of about 1 g (fresh weight) were taken randomly to access mycorrhizal colonization. Root samples were stained with 0.05% Trypan blue (Phillips et al., 1970), and the percentage of root infection was assessed according to Tomlinson et al. (1995). All plant fractions were dried at 65°C for 48 hours and weighed. Shoot tissue was ground for chemical analyses of N, P, K and C in the laboratory of the Institut de l’Environnement et de Recherches Agricoles (INERA). For Ntot, Ptot and Ktot, samples were first digested in a mixture of H2SO4-Se- H2O2 at 450°C for 4 h, following the method of Walinga et al. (1995). An automatic colorimeter (Skalar SANplus Segmented flow analyzer, Model 4000-02, Breda, The Netherlands) was used to determine the Ntot and Ptot contents in the digested solution. Ktot was determined using a flame photometer (Jencons PFP 7, Jenway LTD, Felsted, England). Total organic matter content was determined from mass loss after ignition at 550°C for 5 h and then total carbon was derived from total organic matter.
2.6. Statistical analyses
10All data were subjected to analysis of variance (ANOVA) using the General Linear Model procedure (GLM) (SAS, 1990). For all variables, homogeneity of variance was tested before the ANOVA was calculated. Orthogonal contrasts were performed to test linear and quadratic trends (linear regressions) in seedling responses to N and P fertilization, and to compare means of treatments.
3. Results
3.1. Plant growth patterns and dry matter partitioning
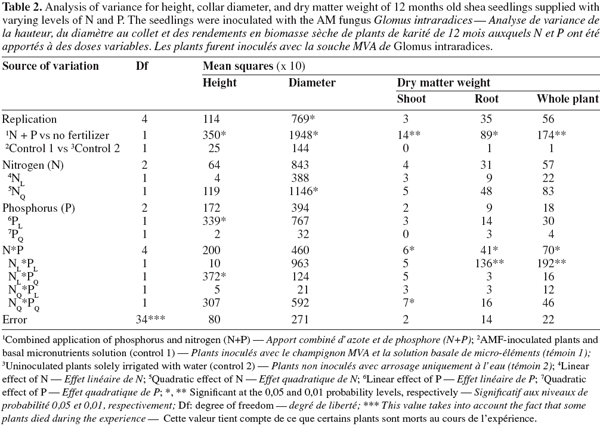
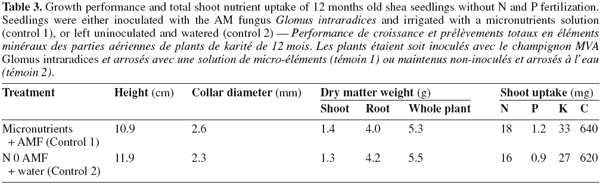
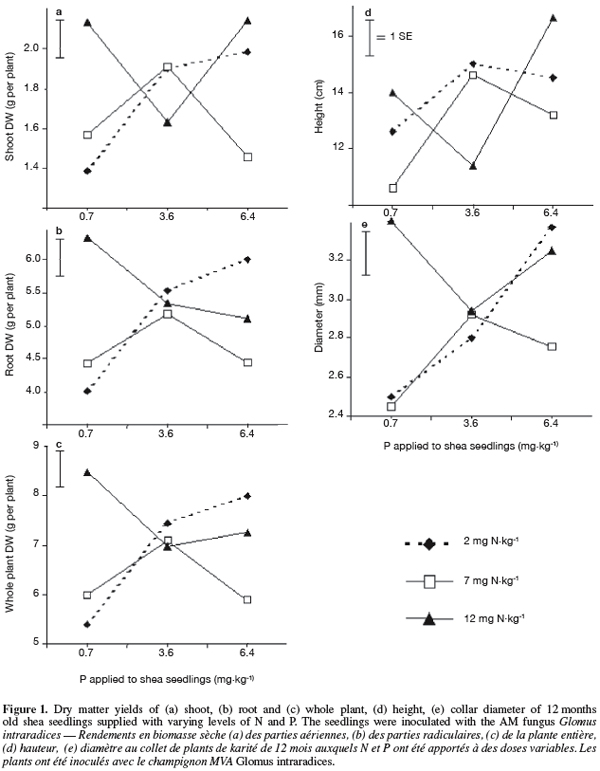
11Seedlings supplied with N and P fertilizers displayed higher collar diameter and height compared with non-fertilized plants (P < 0.05; Tables 2 and 3; Figure 1). Without fertilization, there was no significant difference in these variables between treatments (control treatments), either seedlings were inoculated with AM fungi and received the basal nutrient solution, or were solely irrigated with water (Tables 2 and 3). The combined application of N and P fertilizers improved shoot, root and whole plant dry weights (DWs) compared with the control treatments (P < 0.01 to 0.05; Tables 2 and 3; Figure 1). No significant main effects of N or P were found on any of these growth parameters (P > 0.05, Table 2). A significant N x P interaction was however found for all seedling DWs components (P < 0.05; Table 2; Figure 1). In general, responses to fertilization in shoot, root and whole plant DWs were the lowest in the low N-low P treatment (2 mg N and 0.7 mg P.kg-1). Furthermore, the application of P fertilizer at increasing rates consistently stimulated DWs accumulation in treatments that received the lowest N level. Conversely, there was a trend of severe decrease in seedlings DWs with increasing P addition for treatments that received higher N rates. Overall, on the basis of root and whole plant DWs the greatest responses were associated with two different treatments, i.e. low N-high P and high N-low P (Figure 1).
3.2. Nutrient uptake of shoots
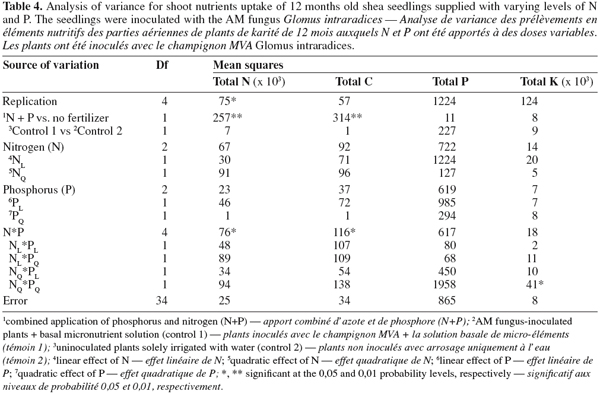
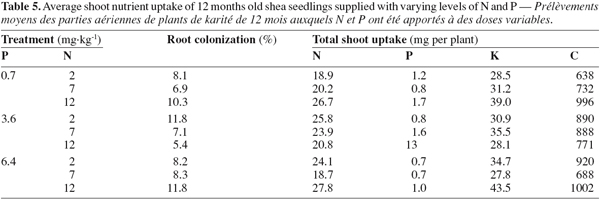
12As for seedling DWs, the main effects of N and P on total shoot nutrients uptake were not significant (P > 0.05, Table 4). There was no significant difference between treatments in total shoot P and K. The fertilized seedlings however had higher total shoot N and C (33% on average) than the control treatments (P < 0.01; Tables 3, 4 and 5). Moreover, there was a significant N x P interaction for total shoot C and N contents which followed similar patterns as DWs (P < 0.05; Tables 4 and 5). At the lowest P rate, total shoot N and C response to N addition occurred, mean values at the highest N rates being 1.4 and 1.6 times than these observed at the lowest N rate, respectively. The corresponding N:P ratio for the solution varied from 3 to 17 while that for shoots increased from 15 to 34. The magnitude of the response was however reduced when fertilizer supply exceeded the lowest rate of 0.7 mg P.kg-1, so that in high P treatments, there was only about 15% difference in shoot N content and less than 10% variation in shoot C uptake between the low N and high N-treated seedlings (Table 5). These treatments resulted in very low N:P ratios for the solution (0 to 3) and inconsistent shoot N:P ratios which varied from 15 to 28.
3.3. AM fungi colonization
13Percentage of root colonization by G. intraradices was generally low, ranging from 5 to 12% across all AM fungi-inoculated treatments. There was no significant effect of N and P addition on percentage of root colonization (P > 0.05). Moreover, similar root colonization was found between NP-fertilized and non-fertilized mycorrhizal seedlings (Table 5).
4. Discussion
14The results of the present study showed that growth of shea seedlings was responsive to N and P fertilization, irrespective of their mycorrhizal status. This is clear from the improved height growth, and increased shoot, root and whole plant DWs in NP-treated seedlings relative to non-fertilized seedlings. At first sight, one important implication of these results is that although shea tree is widely recognized as a slow growing species, its inherent poor growth may be partly due to unfavorable soil nutrient status even though the sandy substrate used in the present study was much less fertile compared to most of the soils in the distribution range of shea.
15The application of N and P fertilizers also caused increases in shoot N and C contents whereas total shoot P and K uptakes were not affected, suggesting that inadequate soil N supply was limiting seedling growth. Thus, although both soil N and P were simultaneously increased by fertilizers application, fertilization mainly acts by overcoming a N limitation to the effective use of assimilates for growth. Nitrogen stress of growth may be more widespread in trees species than P deficiency since similar results have been found in other studies (Elliot et al., 1994; Davidson et al., 2004). Tree growth in the Amazonian forests was found to be responsive to N, but not to P fertilization, though soils had low P availability (Davidson et al., 2004). Elliot et al. (1994) found that P addition had no effect on growth of temperate red pine seedlings whereas N supply affected both total biomass and other biomass components. Baker et al. (2003) also reported the limitation in growth of Celtis mildbraedii due to the relative availability of N in valley soils in semi-deciduous tropical forest of Ghana. In our study, shoot P and K uptake showed no significant difference between NP-amended and control treatments possibly because of sufficient soil supplies of these elements. In particular, similar shoot K content was expected since K fertilization was made similar among all treatments. An alternative explanation for the lack of response in shoot P content may be that the seed source P could have been sufficient to meet growth requirements of this nutrient. Seed length of shea tree as large as 1.7 to 3.7 cm have been reported for several accessions in Ghana (Lovett et al., 2000). This may be associated with large seed P reserves that could have contributed at least a major portion of P allocated to shoot at the early seedling development phase (Mulligan et al., 1988; Kabir et al., 2000).
16Among the fertilized treatments however, responses to increasing N addition in plant parts growth, and shoot N and C uptake were strongly dependent upon P rates, as also reported in other studies (Teng et al., 1996; Davidson et al., 2004). Patterns of responses in shoot nutrient contents were very similar to those in growth characteristics but trends were closer to root DWs. There was a relatively higher variability in shoot than root-related traits because the variation in shoot traits was largely influenced by the fact that some individual seedlings randomly produced resprouts and therefore, had developed multi-stemmed above ground parts (data not shown). On the other hand, the highest magnitude of responses to fertilization was observed in the high N-low P and low N-high P additions, which were the most contrasted treatments studied. The ability of shea seedlings to withstand such a broad spectrum of nutrient concentrations suggests that the species could be tolerant or adapted to a wide range of nutritional conditions. Most NP-treated seedlings however showed reduced responses to fertilization which may be related to differences in N:P ratios between treatments. Elliot et al. (1994) found that N response of red pine decreased with leaf N:P ratios as a result of a relative lack of P for N:P values above 10. In the present study responses to N additions at the lowest P rate were associated with increases in N:P ratios of seedling shoots. This supports the observation that soil N supply was limiting growth of shea seedlings. At the highest growth response, N:P ratios was about 34 for shoot. Nitrogen fertilization alone has been found to increase both foliar N concentration and N:P ratios of hybrid poplars stands growing on soils of low N and P status (Brown et al., 2005). With the exception of the low P treatments, the fertilization resulted in low N:P ratios (less than 4 for the nutrients solution and between 15 and 28 for shoots), which were less related to the plant responses. This suggests that other factors could have been involved in the growth patterns of seedlings in these treatments. For example, toxic levels of some nutrients could have been reached at the highest fertilizer rates. In this regard, symptoms of excessive N supply likely occurred at the highest N rate (12 mg N.kg-1) since seedling responses dropped severely from peaks as P increased, similarly to trends reported by Teng et al. (1996) in white spruce. For P fertilization however, the rates applied to shea seedlings in this study can be considered as in the range of available P (about 0.6 to 5.2 mg.kg-1) recorded for soils in shea tree and Parkia sp. parklands in Burkina Faso (Bayala et al., 2002). According to Ågren (2004), difficulty in analyzing N:P ratios stems from possible uptake above what is immediately required for growth. Thus, N and P requirements for growth are best determined at limiting rates of both nutrients under similar conditions. From the results of the present study, it is clear that to investigate the N need for optimal growth of shea seedlings, N dosage much lower than 12 mg.kg-1 should be used under conditions of P shortage. Nevertheless, the results showed that good growth of shea seedlings can be obtained from different combinations of N and P fertilizers, as revealed by the large extent of responses observed in the two best fertilization treatments.
17Without fertilizer application, the seedlings displayed similar DWs and comparable shoot nutrient uptake, regardless of the mycorrhizal treatment, possibly because the association between shea tree and the AM fungus G. intraradices was ineffective. Guissou et al. (1998) observed that the same strain (G. intraradices) was invasive but not efficient for three Sahelian fruit trees, though root colonization in their study was higher than that observed in the present work. Bâ et al. (2000) also showed relatively low efficiency of this fungal strain using a larger set of fruit trees species from the Sahel area. Responses to AM fungi inoculation may however have been influenced by other factors such as the host (Sylvia et al., 2003) or soil properties (Karanja et al., 1999). Mycorrhizal associations are generally expected to improve P nutrition of susceptible species, especially under conditions of P limitation (Smith et al., 1997). In our study the lack of response to AM fungi inoculation is consistent with the observation that even P fertilization did not affect P nutrition of shea seedlings, confirming that no P stress was evident. The mycorrhizal relationships with shea seedlings were similar to those of several other Sahelian fruit trees that were reported to be mycorrhized but inherently non responsive to AM fungi inoculation (Bâ et al., 2000). Since our results are based on data from a single fungal strain, it appears impossible however to make a general statement on the responsiveness of shea seedlings to mycorrhizal inoculation. Therefore, further investigations using different fungal strains should be carried out to address the potential effects of AM fungi inoculation in this fruit species. Nevertheless, the occurrence of root colonization by the strain G. intraradices provides clear evidence that shea tree can be considered as a susceptible host species.
18Mycorrhizal root infection was found in all AM fungi-inoculated treatments regardless of fertilization rate. It is well recognized that increasing P supply can either suppress root colonization by AM fungi when N is sufficient, or promote it under conditions of N stress (Sylvia et al., 1990). Stimulation of root colonization in N limited Artemisia vulgaris on highly P-rich sites (120 g P.kg-1) (Blanke et al., 2005) further highlighted the importance of soil N status on the formation of mycorrhiza. In the present study, the ability of G. intraradices to colonize roots of shea seedlings at increasing P rates may be explained by N stress.
5. Conclusion
19The results of the present investigation demonstrated that shea tree is susceptible to AM fungi infection. There was no evidence however that mycorrhizal association had any beneficial effects on this species at seedling stage when inoculated with AM fungus G. intraradices, probably because of either sandy nature of the substrate used or the established symbiosis was ineffective. Thus, further studies are needed to address potential advantages of mycorrhizal associations for shea. The results also showed that limited N growth conditions appeared to be the main constraint to biomass accumulation for shea seedlings. This could be partially alleviated by the combined application of N and P fertilizers, but seedling response to fertilization was ultimately dependent on the ratio between both nutrients. Maximum growth improvement was achieved by applying 2 mg N and 6.4 mg P.kg-1, or 12 mg N and 0.7 mg P.kg-1. Additionally, the data provided indications that N requirements for optimal growth of shea seedlings could be much lower than 12 g.kg-1, whereas the need for P remained unresolved. Overall, from the study reported here and the very limited literature on shea nutrition it can be concluded that consideration by growers of external N input relative to P supply, possibly along with soil K concentration, is important to the successful use of mineral fertilizers to promote growth of shea seedlings in nurseries.
20Acknowledgements
21This work was funded by a grant from the International Foundation for Science (IFS) to Mr Dianda (Grant N°D3125-1). The authors are grateful to Mr Bazié for technical assistance.
Bibliographie
Ågren G.I., 2004. The C:N:P stoichiometry of autotrophs. Theory and observations. Ecol. Lett., 7, 185-191.
Bâ A.M. et al., 2000. Functional compatibility of two arbuscular mycorrhizae with thirteen fruit trees in Senegal. Agroforestry Syst., 34, 129-137.
Bado V.B., Bationo A. & Cescas M.P., 2006. Assessment of cowpea and groundnut contributions to soil fertility and succeeding sorghum yields in the Guinean savannah zone of Burkina Faso (West Africa). Biol. Fertil. Soils, 43, 171-176.
Baker T.R., Burslem D.F.R.P. & Swaine M.D., 2003. Association between tree growth, soil fertility and water availability at local and regional scales in Ghanaian tropical rain forest. J. Trop. Ecol., 19, 109-125.
Bayala J., Teklehaimanot Z. & Ouédraogo S.J., 2002. Millet production under pruned tree crowns in a parkland system in Burkina Faso. Agroforestry Syst., 54, 203-214.
Bayala J., Mando A., Teklehaimanot Z. & Ouédraogo S.J., 2005. Nutrient release from decomposing leaf mulches of karité (Vitellaria paradoxa) and néré (Parkia biglobosa) under semi-arid conditions in Burkina Faso, West Africa. Soil Biol. Biochem., 37, 533-539.
Bayala J. et al., 2006. Relative contribution of trees and crops to soil carbon content in a parkland system in Burkina Faso using variations in natural 13C abundance. Nutrient Cycling Agroecosystem, 76, 193-201.
Binkley D., Senock R. & Cromack Jr K., 2003. Phosphorus limitation on nitrogen fixation by Facaltaria seedlings. Forest Ecol. Manage., 186, 171-176.
Blanke V. et al., 2005. Nitrogen supply affects arbuscular mycorrhizal colonization of Artemisia vulgaris in a phosphate-polluted field site. New Phytol., 166, 981-992.
Boffa J.M., 1999. Agroforestry parklands in Sub-Saharan Africa. Forest Conservation Guide 34. Roma: FAO.
Boffa J.M., Yaméogo G., Nikiéma P. & Knudson D.M., 1996. Shea nut (Vitellaria paradoxa) production and collection in agroforestry parklands of Burkina Faso. In: Leakey R.R.B., Temu A.B., Melnyk M. & Vantomme P., eds. Domestication and commercialization of non-timber forest products in agroforestry systems. Non-wood Forest Products 9. Roma: FAO, 110-122.
Bonkoungou E.G., 1992. Sociocultural and economic functions of Acacia albida in West Africa. In: Vandenbeldt R.J., ed. The West African semi-arid tropics: proceedings of a workshop, 22-26 April 1991, Niamey, Niger. Nairobi: International Centre for Research in Agroforestry, 1-6.
Brown K.R. & van den Driessche R., 2005. Effects of nitrogen and phosphorus fertilization on the growth and nutrition of hybrid poplars on Vancouver Island. New Forests, 29, 89-104.
Davidson E.A. et al., 2004. Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest. Ecol. Appl., 14, 150-163.
De Bie S., Ketner P., Paasse M. & Geerling C., 1998. Woody plant phenology in the West Africa savanna. J. Biogeogr., 25, 883-900.
Elliott K.J. & White A.S., 1994. Effects of light, nitrogen, and phosphorus on red pine seedling growth and nutrient use efficiency. Forest Sci., 40, 47-58.
Gijsbers H.J.M., Kessler J.J. & Knevel M.K., 1994. Dynamics and natural regeneration of woody species in farmed parklands in the Sahel region (Province of Passore, Burkina Faso). Forest Ecol. Manage., 64, 1-12.
Guissou T. et al., 1998. Responses of Parkia biglobosa (Jacq.) Benth, Tamarindus indica L. and Ziziphus mauritiana Lam. to arbuscular mycorrhizal fungi in a phosphorus-deficient sandy soil. Biol. Fertil. Soils, 26, 194-198.
Hall J.B. et al., 1996. Vitellaria paradoxa. A monograph. Bangor, UK: University of Wales, School of Agricultural and Forest Sciences.
Ingleby K. et al., 1997. Distribution of roots, arbuscular mycorrhizal colonization and spores around fast growing tree species in Senegal. Forest Ecol. Manage., 90, 19-27.
Kabir Z. & Koide R.T., 2000. The effect of dandelion or a cover crop on mycorrhiza inoculum potential, soil aggregation and yield of maize. Agric. Ecosystem Environ., 78, 167-174.
Karanja N.K., Mwendwa K.A. & Zapata F., 1999. Growth response of Grevillea robusta A. Cunn. seedlings to phosphorus fertilization in acid soils from Kenya. Biotechnol. Agron. Soc. Environ., 3, 57-64.
Lovett P.N. & Haq N., 2000. Diversity of the sheanut tree (Vitellaria paradoxa C.F.Gaertn.) in Ghana. Genet. Resour. Crop Evol., 47, 293-304.
Mahon J.D., 1977. Root and nodule respiration in relation to acetylene reduction in intact nodulated peas. Plant Physiol., 60, 812-816.
Mathur M. & Vyas A., 2000. Influence of arbuscular mycorrhizae on biomass production, nutrient uptake and physiological changes in Ziziphus mauritiana Lam. under water stress. J. Arid Environ., 45, 191-195.
Mulligan D.R. & Sands R., 1988. Dry matter, phosphorus and nitrogen partitioning in three Eucalyptus species grown under a nutrient deficit. New Phytol., 109, 21-28.
Ouédraogo I., 2006. Land use dynamics in Bieha district, Sissili province, southern Burkina Faso, West Africa. Bull. Afr. Afr. Am. Stud. Program., 1(2), 18-34.
Phillips J.M. & Hayman D.S., 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc., 55, 158-160.
Sanginga N., Bowen G.D. & Danso S.K.A., 1990. Intra-specific variation in growth and P accumulation of Leucaena leucocephala and Gliricidia sepium as influenced by soil phosphate status. Plant Soil, 112, 129-135.
SAS, 1990. Sas procedures guide, version 6. 3rd ed. Cary, NC, USA: SAS Institute Inc.
Smith S.E. & Read D.J., 1997. Mycorrhizal symbiosis. 2nd ed. San Diego, CA, USA: Academic Press.
Sylvia D.M. & Neal L.H., 1990. Nitrogen and phosphorus response of VA mycorrhiza. New Phytol., 115, 303-310.
Sylvia D.M., Alagely A.K., Kane M.E. & Philman N.L., 2003. Compatible host/mycorrhizal fungus combinations for micropropagated sea oats. Mycorrhiza, 13, 177-183.
Teng Y. & Timmer V.R., 1996. Modeling nitrogen and phosphorus interactions in intensively managed nursery soil-plant systems. Can. J. Soil Sci., 76, 523-530.
Tittonell P., Shepherd K.D., Vanlauwe B. & Giller K.E., 2007. Unravelling the effects of soil and crop management on maize productivity in smallholder agricultural systems of western Kenya. An application of classification and regression tree analysis. Agric. Ecosystems Environ., DOI:10.1016/j.agee.2007.05.005.
Tomlinson H., Teklehaimanot Z., Traoré A. & Olapade E., 1995. Soil amelioration and root symbioses of Parkia biglobosa (Jacq.) Benth. in West Africa. Agroforestry Syst., 30, 145-159.
Walinga I. et al., 1995. Plant analysis manual. Dordrecht, the Netherlands: Kluwer Academic.
World Bank, 1989. Burkina Faso. In: Trends in developing economies. Washington, DC, USA: World Bank, 45-50.
Para citar este artículo
Acerca de: Mahamadi Dianda
Institut de l’Environnement et de Recherches Agricoles (INERA). Département Productions forestières. 03 PB 7047. BF-Ouagadougou 03 (Burkina Faso). E-mail: dmahamadi@yahoo.fr
Acerca de: Jules Bayala
Institut de l’Environnement et de Recherches Agricoles (INERA). Département Productions forestières. 03 PB 7047. BF-Ouagadougou 03 (Burkina Faso).
Acerca de: Tahir Diop
UCAD. Faculté des Sciences et Techniques. Département de Biologie végétale. BP 5005. SN-Dakar (Sénégal).
Acerca de: Sibiri Jean Ouédraogo
Institut de l’Environnement et de Recherches Agricoles (INERA). Département Productions forestières. 03 PB 7047. BF-Ouagadougou 03 (Burkina Faso).






