- Portada
- volume 13 (2009)
- numéro 2
- Bioactive secondary metabolites from the endophytic fungus Chaetomium sp. isolated from Salvia officinalis growing in Morocco
Vista(s): 9224 (25 ULiège)
Descargar(s): 423 (1 ULiège)
Bioactive secondary metabolites from the endophytic fungus Chaetomium sp. isolated from Salvia officinalis growing in Morocco

Notes de la rédaction
Received on February 4, 2008, accepted on July 2, 2008
Résumé
Métabolites secondaires bioactifs du champignon endophytique Chaetomium sp. isolé de Salvia officinalis qui pousse au Maroc. Cette étude rapporte l'élucidation de la structure et de la cytotoxicité des métabolites secondaires produits par le champignon endophytique Chaetomium sp. isolé de Salvia officinalis qui pousse au Maroc. Cette plante collectée dans les montagnes de Beni-Mellal au Maroc appartient à la famille des Lamiaceae et est nommée au Maroc par le nom vernaculaire “Salmia”. Le champignon endophytique Chaetomium sp. a été isolé des tissus de la tige de cette plante. Ce champignon a été identifié par PCR. L'extrait brut du champignon isolé est très actif sur les cellules cancéreuses L5178Y du lymphome de la souris. L'investigation chimique des métabolites secondaires a montré que le cochliodinol est le composé majoritaire, à coté de l'isocochliodinol. Les structures des composés isolés ont été déterminées par des analyses spectroscopiques, à savoir la RMN (1H, 13C, COSY et HMBC) et la spectrométrie de masse en utilisant le ESI (Electron Spray Ionisation) comme source.
Abstract
This study reports the chemical investigation and cytotoxic activity of the secondary metabolites produced by the endophytic fungus Chaetomium sp. isolated from Salvia officinalis growing in Morocco. This plant was collected from the Beni-Mellal Mountain in Morocco and belongs to the Lamiaceae family and is named in Morocco “Salmia”. The endophytic fungus Chaetomium sp. was isolated from the tissues of the stem of this plant. The fungal strain was identified by PCR. The crude organic extract of the fungal strain was proven to be active when tested for cytotoxicity against L5178Y mouse lymphoma cells. Chemical investigation of the secondary metabolites showed that cochliodinol is the main component beside isocochliodinol. The structures of the isolated compounds were determined on the basis of NMR analysis (1H, 13C, COSY and HMBC) as well as by mass spectrometry using ESI (Electron Spray Ionisation) as source.
Tabla de contenidos
1. Introduction
1Endophytes are microbes that colonize living, internal tissues of plants without causing any immediate negative effects (Bacon et al., 2000). They reside inside the tissues of nearly all healthy plants. The relationship that they establish with the plant varies from symbiotic to bordering on pathogenic (Strobel, 2002a). They are synergistic to their host and at least some of them are thought to be useful to the plant by producing special substances, such as secondary metabolites, that prevent the host from being attacked successfully by fungi and pests (Thongchai et al., 2005). Via what appears to be their contribution to the host plant, the endophytes may produce a plethora of substances of potential use to modern medicine, agriculture, and pharmaceutical industry. The potential prospects of finding new drugs that may be effective candidates for treating newly developing diseases in humans, plants, and animals are great (Strobel et al., 2003).
2The purpose of the present study was to extract, explore and characterize natural products produced by the endophytic fungus Chaetomium sp. isolated from stems of Salvia officinalis growing wild in Morocco, and to evaluate their cytotoxic activity. S. officinalis (Lamiaceae) is a perennial woody sub-shrub native to the Mediterranean area, used in the food-processing industry but also in the area of human health. Historically, it is well known for its fungistatic, virustatic and tannin-based anti-microbial properties. Anti-inflammatory activities were reported for some constituents of the plant such as triterpenes, oleanolic and ursolic acids, or the diterpene carnosol (Baricevic et al., 2001).
2. Material and methods
2.1. General experimental procedures
31D and 2D NMR spectra were recorded on ARX 500 or AVANCE DMX 600 NMR spectrometers. ESI-MS was performed on a Finnigan LCQDeca mass spectrometer. HPLC analysis was carried out using a Dionex P580 HPLC system coupled to a photodiode array detector (UVD340S). Routine detection was at 235, 254, 280 and 340 nm. The separation column (125 x 4 mm, L x ID) was prefilled with Eurospher-10 C18 (Knauer, Germany). The following gradient was used (MeOH, 0.02% H3PO4 in H2O): 0 min, 10% MeOH; 5 min, 10% MeOH; 35 min, 100% MeOH; 45 min, 100% MeOH.
2.2. Plant material and fungal isolation
4Fresh healthy stems of S. officinalis were collected in January 2006 from the mountain of Beni-Mellal, Morocco. Voucher specimens have been deposited in the Laboratory of Natural Substances and Thermolysis Flash, University Mohammed V Agdal, Faculty of Sciences, Rabat, Morocco. Stems were rinsed in sterilized distilled water twice. Surface sterilization was done by immersing the stems in 70% ethanol for 2 min (twice) followed by rinsing again twice in sterilized distilled water. Then, the stems were cleaved aseptically into small segments (≈ 1 cm in length). The material was placed on a Petri dish (malt agar medium) containing an antibiotic to suppress bacterial growth (medium composition: 15 g.l-1 malt extract, 15 g.l-1 agar and 0.2 g.l-1 chloramphenicol in distilled water, pH 7.4-7.8) and incubated at room temperature (25°C).
5After several days hyphae growing from the plant material were transferred to other plates, incubated again for 10 days, and periodically checked for culture purity.
2.3. Taxonomic identification of the fungus
6A section (0.5 cm2) of fungal hyphae was removed from the Petri dish and lyophilized in a sample tube (2 ml) closed with a hydrophobic membrane (LidBac, Eppendorf, Hamburg, Germany). The lyophilized sample was powdered in a MixerMill MM300 (Retsch, Haan, Germany) after adding a tungsten carbide bead (Qiagen, Hilden, Germany). DNA isolation was performed using the DNeasy plant mini kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. The procedure includes cell lysis, digestion of RNA by RNase A, removal of precipitates and cell debris, DNA shearing, DNA precipitation, and purification. The DNA obtained was dissolved in 50 μl of the elution buffer supplied by the manufacturer. PCR was then performed using Hot Star Taq master mix (Qiagen, Hilden, Germany) Taq polymerase and the primer pair ITS1 and ITS4 (Invitrogen, Karlsruhe, Germany) in an iCycler (Bio-Rad, Hercules, CA) thermal cycler according to the following protocol:
7– initial denaturation 95°C 15 min,
8– denaturation 95°C 1 min,
9– annealing 56°C 0.5 min,
10– extension 72°C 1 min,
11– final extension 72°C 10 min.
12Steps 2-4 were repeated 35 times. Each sample consisted of 25 μl of Taq polymerase master mix, 3 μl of primer mix (10 pmol.μl-1 each), 3 μl of template DNA, and 19 μl of water. From this, 20 μl was loaded onto an agarose gel (2% agarose in 1 × TBE, 5 μl of ethidium bromide 1% m/V solution per 100 ml of gel). After electrophoresis at 70 V for 60 min, the band due to the PCR product (approximate size 550 bp) was isolated from the silice gel using the PerfectPrep gel cleanup kit (Eppendorf, Hamburg, Germany) according to the manufacturer's protocol. The PCR product was then submitted for sequencing (BMFZ, Düsseldorf, Germany) with the primer ITS1. The sequence data have been submitted to and deposited at GenBank (accession n°bankit823609, DQ854987). BLAST search of the FASTA sequence was performed with the option “nr”, including GenBank, RefSeq Nucleotides, EMBL, DDBJ, and PDB sequences on the BLAST homepage, NCBI, Bethesda, MD.
2.4. Rice culture of isolated fungus
13Two Erlenmeyer flasks (1 l each) containing 100 g of rice and 100 ml of distilled water were autoclaved. A small part of the medium from a Petri dish containing the purified fungus was transferred under sterile conditions to the rice medium. The fungus strain was grown on solid rice medium at room temperature for 40 days.
2.5. Extraction and fractionation
14The culture was extracted with 300 ml ethyl acetate (twice). The ethyl acetate extract was dried and partitioned between n-hexane and 90% MeOH. Evaporation of the 90% MeOH fraction gave a yield of 220 mg of extract which was chromatographed over a Sephadex LH-20 column with 100% MeOH as solvent. Based on detection by TLC (SiO gel F254, Merck, Darmstadt, Germany) using MeOH: DCM (5:95) as a solvent system, collected fractions were combined, and subjected to semi-preparative HPLC (Merck, Hitachi L-7100) using an Eurosphere 100-10 C18 column (300 × 8 mm, ID) with the following gradient: (acetonitrile and H2O): 0 min, 10% acetonitrile; 5 min, 10% acetonitrile; 35 min, 100% acetonitrile; 45 min, 100% acetonitrile.
2.6. Cytotoxicity assay
15Antiproliferative activity was examined against L5178Y mouse lymphoma cell line using the microculture tetrazolium (MTT) assay (Carmichael et al., 1987). From the test samples, stock solutions in ethanol 96% (v/v) were prepared. Exponentially growing cells were harvested, counted and diluted appropriately. Of the cell suspension, 50 μl containing 3,750 cells were pipetted into 96-well microtiter plates. Subsequently, 50 μl of a solution of the test samples containing the appropriate concentration was added to each well. The concentration range was 3 and 10 μg.ml-1. The small amount of ethanol present in the wells did not affect the experiments. The test plates were incubated at 37°C with 5% CO2 for 72 h. A solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was prepared at 5 mg.ml-1 in phosphate buffered saline (PBS; 1.5 mM KH2PO4, 6.5 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl; pH 7.4) and from this solution, 20 μl was pipetted into each well. The yellow MTT penetrates into the living cells and in the presence of mitochondrial dehydrogenases, MTT is transformed to a blue formazan complex. After an incubation period of 3 h 45 min at 37°C in a humidified incubator with 5% CO2, the medium was centrifuged (15 min, 20°C, 210 x g) and upon addition of 200 μl DMSO, the cells were lysed to liberate the produced formazan. After thorough mixing, the absorbance was measured at 520 nm using a scanning microtiter-well spectrophotometer. The colour intensity is correlated with the number of healthy living cells.
16Cell survival was calculated using the formula:

17All experiments were carried out in triplicates and repeated 3 times. As controls, media with 0.1% EGMME/DMSO were included in the experiments.
3. Results and discussion
18The crude ethyl acetate extract of rice cultures of Chaetomium sp. was subjected to solvent fractionation between 90% MeOH and n-Hexane. Figure 1 shows the HPLC-UV (235 nm) chromatogram of the 90% MeOH fraction that indicates that there is one major component (compound (1)). Column chromatography of the 90% MeOH fraction on sephadex LH-20 yielded compound (1) at a Dionex P580 HPLC-UV (235 nm), 100% pure. Compound (2) was isolated and purified (98%) by semi-preparative RP-HPLC using water and acetonitrile as solvent system.
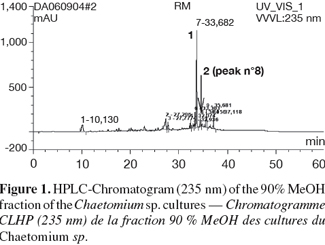
19Compound (1) was isolated as purple crystals (21 mg). The UV spectrum showed λmax (MeOH) at 227.7, 279.8 and 471.4 nm, indicative of an indole chromophore.
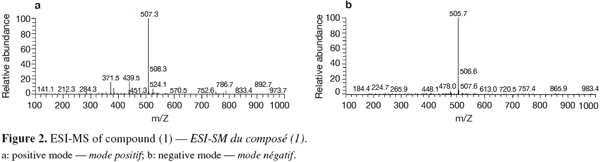
20Positive and negative ESI-MS showed molecular ion peaks at m/z 507.3 [M+H]+ (base peak) and m/z 505.7 [M-H]- (base peak), respectively, indicating a molecular weight of 506 g.mol-1 (Figure 2).
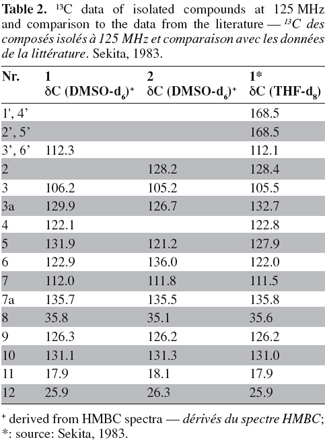
21The results obtained by NMR for compounds (1) and (2) (Figure 3) are listed in table 1 (1H, COSY and HMBC) and table 2 (13C).
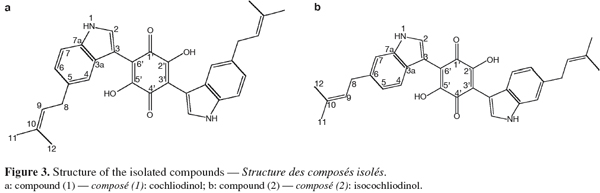
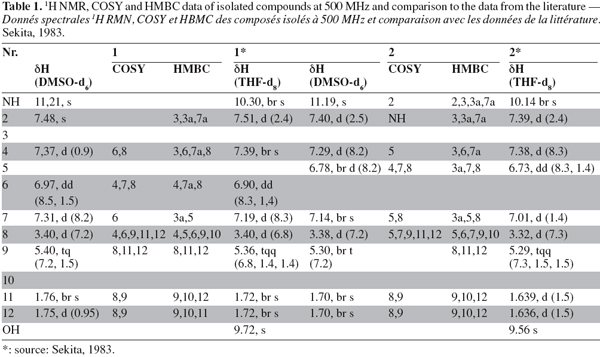
22By combining the results obtained by the different spectrometric methods (UV, mass and NMR) C32H30N2O4 was proposed as the molecular formula for compound (1). In the 1H NMR spectrum, all resonances originate from pairs of chemically equivalent groups due to symmetry in the molecule. Furthermore, aromatic proton and carbon resonances had chemical shifts and multiplicities consistent with the presence of a disubstituted indole residue. In addition, the 1H NMR spectrum indicated that the olefinic proton (H-9) at δ H 5.37 was allylically coupled to the two groups of methyl protons (CH3-11 and -12) at δ H 1.74 and 1.73, and vicinally coupled to the methylene group (CH2-8) at δ H 3.40, thus establishing the presence of a 2-methyl-but-2-enyl (= isoprenyl) substituent. The position of the attachment of the isoprenyl side chain to the aromatic side of the indole substructure was evident by the long range correlations of CH2-8 to both H-4 and H-6 in the COSY spectrum. Accordingly, correlations of H-2 to both C-3a and C-7a indicated that a further substituent had to reside at C-3 in the heteroaromatic ring of the indole moiety. Most diagnostic for unambiguously deducing the position of the isoprenyl side chain proved a correlation of H-4 to C-3 in the HMBC spectrum, the corresponding proton signal was the one exhibiting the meta-coupling, thus representing the proton immediately adjacent to the substituent.
23The identification of compound (1) was confirmed by comparison of the UV, 1H NMR and mass spectral data with published data for cochliodinol (Jerram et al., 1975; Sekita, 1983). Cochliodinol was previously reported from several Chaetomium species (Jerram et al., 1975; Sekita et al., 1981; Sekita, 1983; Brewer et al., 1984).
24Compound (2) was isolated as purple crystals (4.2 mg). Its UV spectrum showed λmax (MeOH) at 226.3, 282.7 and 474.2 nm, with very high similarity to that of (1). Positive and negative ESI-MS showed molecular ion peaks at m/z 507.3 [M+H]+ (base peak) and m/z 505.7 [M-H]- (base peak), respectively, indicating a molecular weight of 506 g.mol-1 and thus identical to that of cochliodinol (1).
25The 1H NMR spectrum disclosed identical spin systems as described above for (1), with the mass spectrum also supporting the presence of a 2-methyl-but-2-enyl group. Thus, (2) had to represent a symmetrical isomer of cochliodinol (1), leaving a positional isomer with the prenyl group attached to C-6 as the most probable alternative. As in the case of cochliodinol, the COSY spectrum confirmed that the isoprenyl group was attached to the aromatic side of the indole substructure since CH2-8 exhibited long range correlations of to both H-7 and H-5. In addition, in the HMBC spectrum also a correlation of H-4 to C-3 was detected. However, in the case of compound (2), this proton signal was the one exhibiting the ortho-coupling, proving that the side chain was situated meta with regard to H-4 and thus resided at C-6. Based on these observations, compound (2) was identified as the known isocochliodinol which was confirmed by comparison of its UV, 1H NMR and mass spectral data with published data (Sekita, 1983). The compound was previously obtained from several Chaetomium sp. (Sekita et al., 1981; Sekita, 1983; Brewer et al., 1984).
26The isolated compounds were subjected to microculture tetrazolium (MTT) assay aimed at determining their cytotoxicity against L5178Y mouse lymphoma cell line. First, the percentage of cell survival was determined and results were compared to those of untreated controls. Less than 10% cell survival indicated cytotoxic activity and EC50 values were estimated for the corresponding compounds. Cochliodinol proved to be highly active against the cancer cell line with an EC50 of 7.0 μg.ml-1, whereas a weak activity was observed for isocochliodinol (EC50 71.5 μg.ml-1). Thus, it may be concluded that cytotoxic activity was affected by the position of prenyl substituents at the indole rings. These results indicate that cochliodinol exhibits potential cytotoxic activity as evaluated by the in vitro screening test. However, in our continuing effort to search for more effective cancer treatment, further studies are needed to evaluate the in vivo activity and investigate pharmacokinetic properties of cochliodinol.
27This study supports the growing evidence that bioactive substances produced by microbial endophytes may not only be involved in the host-endophyte relationship, but may also ultimately have applicability in medicine, agriculture and industry (Strobel, 2002b). Accordingly, because of their role in conferring plants the ability to adapt to stress conditions, and because they are proven or perceived sources of secondary metabolites with pharmaceutical importance, the study of fungal endophytes is expected to become an important component of fungal biology (Maheshwari, 2006). Furthermore, the rapidly evolving recognition that a significant number of natural product drugs/leads are actually produced by microbes and/or microbial interactions with their hosts makes this area of natural product research of special interest (Newman et al., 2007). Taking into consideration that despite competition from other drug discovery methods, natural products are still providing their fair share of new clinical candidates and drugs (Butler, 2004) we intend to continue our specific search for bioactive substances with pharmaceutical potential as well as contribute to the question of the ecological function of secondary metabolites produced by endophytic fungi aiming to a better understanding of this interesting group of organisms.
28Acknowledgements
29A. Debbab wishes to thank the DAAD (German Academic Exchange Service) for a scholarship.
Bibliographie
Bacon C.W. & White J.F., 2000. Microbial endophytes. New York, USA: Marcel Dekker, 4-5.
Baricevic D. et al., 2001. Topical anti-inflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid. J. Ethnopharmacology, 75(2-3), 125-132.
Brewer D., Jen W.C., Jones G.A. & Taylor A., 1984. The antibacterial activity of some naturally occurring 2,5-dihydroxy-1,4-benzoquinones. Can. J. Microbiol., 30(8), 1068-1072.
Butler M.S., 2004. The role of natural product chemistry in drug discovery. J. Nat. Prod., 67(12), 2141-2153.
Carmichael J. et al., 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res., 47(4), 943-946.
Jerram W.A. et al., 1975. The chemistry of cochliodinol, a metabolite of Chaetomium spp. Can. J. Chem., 53(5), 727-737.
Maheshwari R., 2006. What is an endophytic fungus? Curr. Sci., 90(10), 1309.
Newman D.J. & Cragg G.M., 2007. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod., 70(3), 461-477.
Sekita S. et al., 1981. Mycotoxin production by Chaetomium spp. and related fungi. Can. J. Microbiol., 27(8), 766-772.
Sekita S., 1983. Isocochliodinol and neocochliodinol, bis (3-indolyl)- benzoquinones from Chaetomium spp. Chem. Pharm. Bull., 31(9), 2998-3001.
Strobel G.A., 2002a. Rainforest endophytes and bioactive products. Crit. Rev. Biotechnol., 22(4), 315-333.
Strobel G.A., 2002b. Microbial gifts from rain forests. Can. J. Plant Pathol., 24(1), 14-20.
Strobel G. & Daisy B., 2003. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev., 67(4), 491-502.
Thongchai T., Chunhua L. & Yuemao S., 2005. Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc 130 and their antifungal activity. J. Microbiol., 151, 1691-1695.
Para citar este artículo
Acerca de: Abdessamad Debbab
Heinrich-Heine-Universität. Institut für Pharmazeutische Biologie und Biotechnologie. Universitätsstr. 1. Gebäude 26.23. D-40225 Düsseldorf (Germany). E-mail: abdessamad.debbab@uni-duesseldorf.de – Université Mohammed V Agdal. Faculté des Sciences. Département de Chimie. Avenue Ibn Battouta. BP 1014. MA- Rabat (Maroc).
Acerca de: Amal Hassan Aly
Heinrich-Heine-Universität. Institut für Pharmazeutische Biologie und Biotechnologie. Universitätsstr. 1. Gebäude 26.23. D-40225 Düsseldorf (Germany).
Acerca de: Ru Angelie Edrada-Ebel
Heinrich-Heine-Universität. Institut für Pharmazeutische Biologie und Biotechnologie. Universitätsstr. 1. Gebäude 26.23. D-40225 Düsseldorf (Germany).
Acerca de: Werner E.G. Müller
Johannes-Gutenberg-Universität. Institut für Physiologische Chemie und Pathobiochemie. Duesbergweg 6. D-55128 Mainz (Germany).
Acerca de: Mahjouba Mosaddak
Université Mohammed V Agdal. Faculté des Sciences. Département de Chimie. Avenue Ibn Battouta. BP 1014. MA- Rabat (Maroc).
Acerca de: Abdelhak Hakiki
Université Mohammed V Agdal. Faculté des Sciences. Département de Chimie. Avenue Ibn Battouta. BP 1014. MA- Rabat (Maroc).
Acerca de: Rainer Ebel
University of Aberdeen. Department of Chemistry. Meston Building. Meston Walk. SC0-AB243UE Aberdeen (Scotland).
Acerca de: Peter Proksch
Heinrich-Heine-Universität. Institut für Pharmazeutische Biologie und Biotechnologie. Universitätsstr. 1. Gebäude 26.23. D-40225 Düsseldorf (Germany).






