- Portada
- Volume 14 (2010)
- numéro 1
- Intraguild interactions implicating invasive species: Harmonia axyridis as a model species
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
Intraguild interactions implicating invasive species: Harmonia axyridis as a model species

Notes de la rédaction
Received on 20 November 2008; accepted on 11 September 2009
Résumé
Interactions intraguildes impliquant les espèces invasives : Harmonia axyridis en tant qu’espèce modèle. Comprendre les mécanismes conduisant à la réussite des espèces exotiques contribuera à prédire des invasions futures et à gérer les systèmes envahis. Les espèces animales exotiques, qu'elles soient introduites accidentellement ou délibérément, peuvent affecter les communautés des espèces natives à travers des compétitions pour la ressource, des interactions trophiques ou des interactions indirectes. En tant que prédateur généraliste de pucerons et d’autres ravageurs à corps mou, la coccinelle arlequin Harmonia axyridis Pallas est un agent effectif de la lutte biologique. Cette espèce a été délibérément introduite dans plusieurs pays pour la lutte biologique de différents ravageurs arthropodes, mais elle a également été introduite accidentellement dans plusieurs autres pays. Elle-même est devenue une espèce envahissante, affectant la dynamique et la composition de plusieurs guildes à travers des interactions directes et indirectes. Dans cet article, nous passerons particulièrement en revue les données existantes sur les mécanismes d’interactions intraguildes, au sein de guildes exotiques, conduisant à la réussite de H. axyridis en tant qu'espèce invasive. Nous nous servirons de ces études pour interpréter les déclins observés dans la diversité des prédateurs au champ et pour prédire les espèces en danger dans les régions non encore envahies. Enfin, nous examinerons les données disponibles sur l'impact des interactions intraguildes impliquant H. axyridis dans la lutte biologique contre les ravageurs.
Abstract
Understanding the mechanisms that result in the success of exotic species will contribute to predicting future invasions and managing invaded systems. Exotic animal species, whether introduced accidentally or deliberately, may impact communities of native species through different intraguild interactions. As an effective generalist predator of aphids and other soft-body pests the harlequin ladybird Harmonia axyridis Pallas has been a successful biological control agent. This species was deliberately introduced into several countries for biological control of different arthropods pests, but it was also introduced accidentally into several other countries. It became an invasive species, affecting the dynamic and composition of several guilds through direct or indirect interactions. In this paper we will specifically review the existing data on mechanisms of intraguild interactions, within exotic guilds, that result in the success of H. axyridis as an invasive alien.. We will use these studies to interpret the observed population declines in predator diversity in the field, and predict species at risk in regions not yet invaded. Finally, we will review the available data on the impact of intraguild interactions implicating H. axyridis on pest biocontrol.
Tabla de contenidos
1. Introduction
1Terrestrial ecosystems support a diversity of insect species that are directly and indirectly linked to each other within food webs that span multiple trophic levels. Natural enemies contribute to the population regulation of species in both the same and lower trophic levels (top-down pressure) and in this way influence the structure of the community as a whole. In the case of aphids these natural enemies, namely aphidophagous, include specialist and generalist predators, parasitoids and pathogens (Völkl et al., 2007). Together they represent a "guild", i.e. a community of species that share the same host/prey resource (Polis et al., 1989; Rosenheim et al., 1995). As aphids are often pests in managed ecosystems these natural enemies provide a valuable pest management ecosystem service that can be manipulated within biological control strategies (Powell et al., 2007; Pell, 2008). However, aphid individuals represent limiting resources for the development of natural enemies, and provide ample opportunity for competition among these beneficials (Mills, 1999). Thus, when beneficial species share a pest species as their common prey, different intraguild interactions (IG) between them may take place. Following Mills (1999), consumers that share a prey may be subject to intraguild predation/parasitism, in which one species eats its competitor (Polis et al., 1989; Rosenheim et al., 1995), exploitative competition, in which competitors interact through the consumption of a diminishing supply of an essential resource (Grover, 1997), interference competition, in which the activity of one species reduces the access of a competitor to a limited but essential resource (Ridenour et al., 2001) or apparent competition, in which competitors share a common natural enemy (Abrams, 1998). Among these mechanisms of intraguild interactions, intraguild predation is the most common interaction between H. axyridis and other natural enemies.
2The rapid increase in the introduction of exotic species throughout the world, and the potential of these species to become invasive, is a subject of much concern (Mack et al., 2000). The concept of biological invasion is, generally, used to refer to the arrival or introduction, establishment, geographical expansion and integration of a species into a region where it has never been before (Williamson, 1996; Shigesada et al., 1997). Biological invasions usually occur in three successive stages:
3– transport of the invader to the target area,
4– establishment and growth of invasive populations,
5– dispersion of the invader into adjacent areas (Shea et al., 2002).
6The complexity of trophic interactions in the recipient ecosystem is one of the main opposition factors of the ecosystem to the invasion. This is because it is more difficult to get established in a complex food web than in a simpler one (Hewitt et al., 2002; Shea et al., 2002). Based on the availability of plant resources and the limited number of trophic links, agro-ecosystems are highly susceptible targets for biological invasions.
7The impact of some invaders is unquestionably negative and as such they are designated as invasive alien species. The harlequin ladybird Harmonia axyridis Pallas (Coleoptera: Coccinellidae), “the most invasive ladybird on Earth”, is undoubtedly one such species (Roy et al., 2006). While the invading species may interact directly or indirectly with indigenous species, these invaders may affect the dynamics and composition of indigenous species guilds (Lucas et al., 2002; Evans, 2004). Following Reitz et al. (2002), if a superior competitor invades the habitat of an inferior species, the inferior species will be displaced (i.e. competitive displacement occurs). Introductions of exotic species for biological control have generated increasing threats for the conservation of native species, with major implications for biodiversity worldwide in both natural and managed (e.g. agricultural) habitats. Ecologists have long sought to identify key factors that determine why some exotic species turn invasive and damaging to native species while others fail to do so (Williamson, 1999). These topics have recently become of great interest in regards to the establishment and spread of introduced aphidophagous ladybirds (Coleoptera: Coccinellidae). Once introduced species become established, they have the potential to interact with indigenous species in a number of different ways, competition and intraguild predation (IGP) for example. Thus, recent species invasions, such as Coccinella septempunctata L. and H. axyridis into the North American continent (Alyokhin et al., 2004; Harmon et al., 2007), and H. axyridis into the Europe continent (Pell et al., 2008), leading to replacements of indigenous species have occurred across diverse taxa (Woodward et al., 2002). Major factors hypothesized to be important to the success of these two invaders are their tendency to engage in IGP with indigenous ladybirds (Michaud, 2002b; Snyder et al., 2004), and release from natural enemies (Torchin et al., 2003). Following Williamson (2006), the knowledge of processes and factors explaining the invasion success is still, however, rudimentary.
8As an effective generalist predator of aphids and other soft-body pests, H. axyridis has been widely and repeatedly augmented or introduced for biological control worldwide. As such, it has contributed significantly to pest suppression in a wide variety of managed ecosystems including apple, alfalfa, cotton, wheat and soybean (e.g. Majerus et al., 2006; Roy et al., 2006). However, the very functional traits that have made H. axyridis an effective biological control agent, also implicate it as an IG predator that poses significant risk to the diversity of native natural enemies and their ecosystem services. Although natural enemy diversity in the native range of H. axyridis remains relatively stable in the presence of H. axyridis (Kuznetsov, 1997), wherever it has been established after introduction as an exotic species, it has been associated with declines in indigenous natural enemies, particularly ladybirds (Majerus et al., 2006; Roy et al., 2006). These declines have been attributed to direct interspecific competition for resources with less competitive/fecund natural enemies (Michaud, 2002b) but are also likely to be strongly influenced by the role of H. axyridis as an IG predator.
9In fact, most studies investigated on beneficial and negative impacts of H. axyridis in its exotic range were in USA and Europe. H. axyridis ‘‘the most common invader among the ladybird community’’ is known to have strong dispersal capacities (Koch, 2003; Roy et al., 2006) and studies in North America have shown that it can rapidly colonize large areas as an invader species (e.g. Michaud, 2002b; Cottrell, 2005). In Europe, it has spread very rapidly, particularly since 2002, and the species now exists as feral populations in 13 European countries involving Belgium, in which the expansion rate is decreasing in this later because it now colonized the whole country (Adriaens et al., 2008; Brown et al., 2008). In a risk assessment of 31 exotic natural enemies of pest species used in biological control in Europe, H. axyridis had a high environmental risk index. This was based on its wide host range (i.e. multiple prey species), ability to establish and disperse, and direct and indirect effects on non-target species (van Lenteren et al., 2003).
10We will review, in this paper, the key mechanisms of interspecific competition that made the harlequin ladybird H. axyridis as the most invasive ladybird on Earth. Subsequently, we will review the existing data on the competitive displacement following H. axyridis invasion within exotic guilds where it has been established as an invasive alien. We will use these studies to interpret the observed population declines in natural enemy diversity in the field, predict species at risk in regions not yet invaded. Finally, we will review the available data on the impact of intraguild interactions implicating H. axyridis on pest biocontrol.
2. Mechanisms of interspecific competition (IG interactions) implicating H. axyridis
2.1. Exploitative competition
11Exploitative competition is the most common mechanism of competition among arthropod insects in nature. It occurs among all natural enemies that share a common and limiting host, where the consumption of a limiting resource by one species makes that resource unavailable for consumption by another, and is characterized by an absence of direct interaction between competitors (Grover, 1997). This type of competition may occur intraspecifically (within species) or interspecifically (between species). For interactions between predators, more particularly in case of H. axyridis presence, it has proved difficult to separate the effects of exploitative competition from those of IGP. It appears that IGP among coccinellids has a stronger influence in the context of short-term laboratory experiments (Yasuda et al., 2004). Moreover, there is some evidence that a disparity in body size among coccinellid species may increase the likelihood of IGP (Yasuda et al., 2004). The strength of exploitative competition following H. axyridis presence, as a top predator, is often decreased by eating potential competitors (Polis et al., 1989).
12Following Lucas et al. (2007), upon review of 24 studies on the impact of invader H. axyridis on competitors, 15 demonstrated a negative impact by exploitative competition or IGP. A superior ability (foraging efficiency) to acquire resources can strongly affect the survival and fecundity of the invader H. axyridis, and may confer a large competitive advantage vs other natural enemies in the invaded range (Koch, 2003). H. axyridis larvae and adults have been shown to have a higher predation and foraging efficiency than indigenous species, such as C. septempunctata (Yasuda et al., 2001), Coleomegilla maculata DeGeer (Labrie et al., 2006), Hippodamia variegata Goeze and Adalia bipunctata L. (Lanzoni et al., 2004), on which this competitive advantage could increase its population density vs these ladybird species.
2.2. Interference competition
13Interference competition results from the direct interaction of competitors or from the modification of a resource by one competitor such that it becomes unusable by another. If we assume that ladybirds expend some time and/or matter and/or energy on competition or the avoidance of competition, then there are less of those resources available for maintenance and reproduction (Krebs, 1989). In the simplest case, interference among natural enemy species represents a contest between competitors where the victim is excluded from access to the host through direct fighting or conditioning of the host as a resource. Thus success in competition contest is often a specific characteristic of the biology of a natural enemy species although it can also be influenced by the relative timing of attack (Boivin et al., 2006). In addition, as with exploitative competition, interference competition can occur within species as well as between species. However, when interference occurs among predatory species (Rosenheim, 1998) or between a facultative hyperparasitoid and a primary parasitoid (Hunter et al., 2001) the contest frequently leads to IGP, a more complex case, in which the IG predator gains resources directly from its competitor (e.g. Janssen et al., 2006; Rosenheim et al., 2006).
14Recent studies of the magnitude, direction and symmetry of IGP between the different developmental stages of H. axyridis and Coccinella undecimpunctata L. revealed that H. axyridis was most often the IG predator (Felix et al., 2004). Although there is no IGP between adults of the two species, they may interact negatively by interfering with each other’s foraging and oviposition (Lucas et al., 2002; Agarwala et al., 2003). Even when the resource is not in short supply, results of Soares et al. (2007) suggested that H. axyridis had a negative impact on C. undecimpunctata fitness, since fecundity was significantly affected in the presence of a heterospecific and predation of eggs occurred (an average of 21.6% of the eggs laid were eaten by H. axyridis adults). Interference competition was also detected between H. axyridis and the hoverfly Episyrphus balteatus DeGeer; results obtained by Alhmedi et al. (2009) suggested that the ladybird had a negative impact on E. balteatus oviposition, since ovipositional behavior was significantly affected in the presence of a heterospecific and predation of eggs occurred (an average of 79.1% of the eggs laid were eaten by H. axyridis larvae). Interference competition may cause ladybirds to fall off a plant, to switch from intensive to extensive search or to cease feeding (Ferran et al., 1993). It may also result in a decrease in fitness due to IGP (Michaud, 2002b; Felix et al., 2004) and competition for prey (Evans, 1991). It is difficult to speculate on the potential long-term impact of H. axyridis in the field (Lucas et al., 2002) because the other native species may respond differently. For example, A. bipunctata in Siberia avoids some species of plants, mainly Salix sp., where H. axyridis is present (Iablokoff-Khnzorian, 1982).
2.3. Apparent competition
15Apparent competition results from the indirect interaction of two co-existent host species that share a common natural enemy, in which the victor is the host species that is more efficiently exploited by the natural enemy and supports the greatest natural enemy densities (Holt et al., 1993). For example, a more abundant host species can have a strong indirect negative impact on a less abundant host species by supporting a large natural enemy population that can use both hosts. In this way the less abundant host experiences a greater level of predation that it would in the absence of its competitor. Documentation of apparent competition among natural enemies appears to be more frequent among either parasitoid or phytophagous insects than among predators (reviewed by Mills, 1999). In fact, the competitive interaction among predatory communities has been often focused on IGP (Lucas, 2005). However, ladybird species that occur at differential levels of abundance, show differential susceptibility to commonly-occurring shared parasitoids.
16Laboratory and field data already reported low levels of successful parasitism of H. axyridis adults by Dinocampus coccinellae Schrank (Hymenoptera: Braconidae) in North America (Hoogendoorn et al., 2002; Firlej et al., 2005) or in Italy (Burgio et al., 2008). In this area, the indirect interactions recorded between H. axyridis and C. maculata may influence their populations in the field (Hoogendoorn et al., 2002). While both species are parasitized by the braconid wasp D. coccinellae, C. maculata was a more suitable host than H. axyridis for this parasitoid (Hoogendoorn et al., 2002), and this apparent competition increases the opportunities for H. axyridis to invade the native range of C. maculata.
17The apparent competition is also greatly expected between the two co-existent species H. axyridis and C. septempunctata, where the later species is a preferential host of the parasitoid D. coccinellae (Geoghegan et al., 1998). In Japan, this wasp uses both Coccinella septempunctata brucki Mulsant and H. axyridis as hosts, but successfully parasitizes a higher proportion of the former species (Kawauchi, 1984; Koyama et al., 2008). This wasp usually prefers larger coccinellid species (Richerson et al., 1972), with recorded prevalence (proportion parasitized) being highest in C. maculata and Hippodamia convergens Guérin in North America (Obrycki, 1989), C. septempunctata in continental Europe (Iperti, 1964), and Coccinella transversalis Mulsant in Australia (Anderson et al., 1986). Several field and laboratory studies reported that the entomopathogen Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) infects H. axyridis, C. septempunctata and A. bipunctata; however, the two later species were more suitable hosts for B. bassiana than H. axyridis (Roy et al., 2008a). This apparent competition may, as a consequence, increase the opportunities for H. axyridis to invade the native range of these ladybird species.
2.4. Intraguild predation
18Intraguild predation (IGP) has become a major research topic in biological control and conservation ecology. It is assumed to be a widespread interaction within many communities of biocontrol agents (Rosenheim et al., 1995; Holt et al., 1997). IGP occurs when two predator species in a guild compete for the same prey (the extraguild prey), often limiting, and one (IG predator) of them also feeds upon its competitor (the IG prey) within the guild. IGP can be a strong force structuring beneficial insect communities (Polis et al., 1989). Its impact on community structure and diversity can be extremely variable, complex and difficult to predict (Snyder et al., 2006; Straub et al., 2008). IG predator mainly operates from the fourth trophic level suppressing both IG and extraguild preys. IG prey, by definition, is an intermediate predator or parasitoid that functions from the third trophic level suppressing herbivores, whereas the extraguild prey is the herbivore, aphids for example, that acts from the second trophic level.
19Many studies have assessed the prevalence of IGP in aphidophagous guild (e.g. Lucas, 2005; Pell, 2008) and reported it to be a widespread phenomenon with implications for both predator diversity within the guild and the pest management ecosystem services that the guild delivers. IGP may be omnivorous or coincidental. Coincidental IGP occurs when a parasitoid or pathogen is consumed by IG predators, while still developing within its herbivore host and, in this case, the herbivore and the IG prey are directly linked (Polis et al., 1989). However, omnivorous IGP is defined in the food web theory as the act of feeding by one species on resources at different trophic levels (Pimm et al., 1978). One of the simplest conceivable examples of omnivory is a constellation of three species: a predator (top), a consumer (middle), and a resource (bottom) that is common to both consumer and predator. Thus, IGP is a combination of exploitative competition and predation interactions; it reduces however potential exploitative competition (Polis et al., 1989; 1992). Within a guild, omnivorous IGP can be asymmetric when one species (IG predator) is always the predator on the competitive species (IG prey), or symmetric when mutual predation occurs between both species (Polis et al., 1989; Rosenheim et al., 1995). The IG predator benefits not only from the nutritive value of the meal, but also from the removal of a competitor.
20There are a number of functional traits that determine the nature, symmetry and outcome of IGP including: relative size (incidence of mortality is often inversely correlated with size) (Majerus, 1994; Evans, 2000), aggressive strategies and mandibular structure (Yasuda et al., 2001), degree of feeding and habitat specificity, mobility (sessile stages are particularly vulnerable), defence strategies and abundance of extraguild prey (Roy et al., 2006; Straub et al., 2008). We will briefly outline the current knowledge on IGP implicating H. axyridis.
21IGP is the most common interaction between H. axyridis and other natural enemies in comparison with other mechanisms of interspecific competition. Laboratory and field studies reviewed by Pell et al. (2008) indicate that H. axyridis engaged in IGP with many natural enemies (Table 1), and was predicted to contribute to declines in many of these species in the field. Coccinellids are common within aphidophagous guild and most studies considering the role of H. axyridis as an IG predator have focused on interactions within the Coccinellidae. However, H. axyridis interacts with many other non-coccinellid predatory insects. The relatively large size of H. axyridis throughout its life cycle undoubtedly contributes to its success as an IG predator. In predatory interactions between ladybird larvae it is generally the larger that eats the smaller, assuming both are mobile (Majerus, 1994). In fact, the relative size and mobility of the IG predator and prey are known to influence the outcome of IGP, both showing an inverse correlation with the incidence of mortality (Rosenheim et al., 1995; Lucas et al., 1998). Consequently, the immature stages of predators are more vulnerable to IGP than adults, and eggs are particularly threatened (Sato et al., 2004; Cottrell, 2007). Following these last authors, predation of ladybird eggs is most often associated with larval stages and, for some species, may be affected negatively by the relative abundance of extraguild prey. Recent studies concluded that H. axyridis larvae were more likely to engage in IGP of eggs of many predators than for H. axyridis eggs to be the IG prey of the larvae of these species (Phoofolo et al., 1998; Cottrell, 2007).
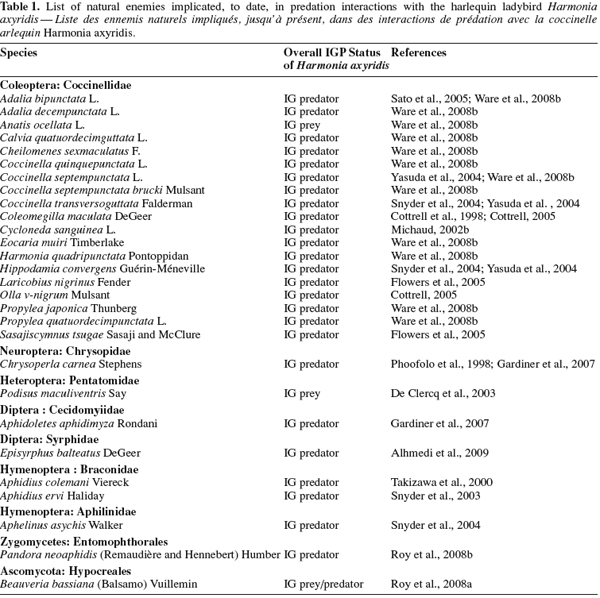
22The chemical defences of coccinellid eggs have been implicated as central to the observed resistance to cannibalism (Hemptinne et al., 2000; Magro et al., 2007) or predation by other aphidophagous insects such as A. bipunctata, C. septempunctata brucki (Sato et al., 2004). Ware et al. (2008b) have recently discussed the role of surface deterrents on eggs of both the European species Calvia quatuordecimguttata L. and the Japanese species Eocaria muiri Timberlake as a defensive mechanism against IGP by H. axyridis. These last authors demonstrated the importance of chemical defence of H. axyridis larvae as a means of preventing counterattacks, where larvae are known to produce similar defensive alkaloids to those present within conspecific eggs and released by adults (King et al., 1996).
23Morphological traits such as the relatively large body size of H. axyridis beetle compared to other aphidophagous species (Hodek, 1973; Michaud, 2002a), and the presence of spines on the back of third and fourth larval instars (superior physical defences) could provide a protection from IGP by other species (Ware et al., 2008b). Moreover, size, strength of the integument and distastefulness of its pupae make this stage less vulnerable to predation (Felix et al., 2004). In the field, coccinellid larvae tend to disperse from a plant when prey abundance is low (Sato, 2001) and this reduces the incidence of cannibalism and IGP by other predatory larvae and adults (Sato et al., 2003). However, this is not absolute safe strategy for an immature coccinellid, where there is always a risk of cannibalism or IGP. H. axyridis commonly co-occurs with C. septempunctata and Propylea japonica Thunberg in their native Japanese range (Sato, 2001). In a field study of these three coccinellid species co-occurring on shrubs, both C. septempunctata and H. axyridis larvae emigrated in response to low prey density whereas P. japonica larvae did not (Sato, 2001). In further studies it was confirmed that the early emigration of C. septempunctata larvae enabled them to escape from IGP by H. axyridis larvae and that the late emigration of P. japonica larvae accounted for the high incidence of IGP by H. axyridis larvae (Sato et al., 2003). P. japonica is also a smaller species than either H. axyridis or C. septempunctata and so this further supports the hypothesis that "size matters" in IGP. Ware et al. (2008a) also reported P. japonica larvae as highly palatable IG prey with little physical defence from attack by H. axyridis.
24Adult coccinellids are generally less susceptible to predation than immature stages due to their protective elytra, mobility and aposematic colour patterns (Majerus, 1994). However, they are exposed to a particularly vulnerable period just after emergence, when their elytra are still soft. Fourth instar H. axyridis larvae were observed to attack and consume new adults of Adalia decempunctata L., Anatis ocellata L., C. quatuordecimguttata, C. septempunctata and E. muiri, when no other food was available (Ware et al., 2008b). In fact, the consumption of newly emerged H. axyridis adults was rare, and only performed by conspecific larvae and larvae of the congeneric species Harmonia quadripunctata Pontoppidan. This supports the speculation that the defensive chemistry of H. axyridis adults may make them unpalatable to other ladybirds (Hough-Goldstein et al., 1996). The ovoid shape of adults as well as bright color may also confer a protection from predators and competitors (De Clercq et al., 2003).
25Regarding the predation interaction between H. axyridis and non-coccinellid predators, a further study examined the interactions between H. axyridis adults, the lacewing Chrysoperla carnea Stephens larvae and the gall midge Aphidoletes aphidimyza Rondani larvae in the presence of the soybean aphid Aphis glycines Matsumara (Homoptera: Aphididae) in microcosms and field cages (Gardiner et al., 2007); H. axyridis engaged in asymmetrical IGP with both C. carnea and A. aphidimyza. However, Phoofolo et al. (1998) then Fremlin (2007) found that C. carnea larvae attack H. axyridis eggs and pupae; therefore, the interaction between them can be considered to be (weakly) symmetrical.
26The hoverfly Episyrphus balteatus DeGeer is known as aphidophagous predator at larva stadium. It is the most frequently encountered hoverfly species at aphid-infested sites in temperate regions, e.g. in central Europe, and one of the most efficient aphid-specific predators (Colignon et al., 2001). H. axyridis larvae engaged in an asymmetric IGP with immature stages (larvae and eggs) of E. balteatus even when aphids are available on host plant (Alhmedi et al., 2009). The heteropteran bug Podisus maculiventris Say is native to North America and has a broad prey range including over 100 species of insect, including larvae of Coleoptera and Lepidoptera, but also aphids (Herrick et al., 2004). A further study demonstrated that interactions between P. maculiventris and H. axyridis in the presence or absence of extraguild prey, Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae) or Myzus persicae Sulzer (Hemiptera: Aphididae), were asymmetric in favor of the bug (De Clercq et al., 2003). Podisus maculiventris fed on H. axyridis eggs and larvae but rarely on adults. As with interactions amongst coccinellids, this interaction was dependent on the life stage of the bug; fourth instar nymphs and adults were more aggressive in their interactions than second instars nymphs. In contrast, H. axyridis rarely attacked P. maculiventris. There was a slight difference in the survival to adulthood of pentatomid nymphs fed on H. axyridis compared to S. littoralis and no nymphs reached adulthood when fed on aphids only (De Clercq et al., 2003).
27Regarding the IGP between predators and parasitoids, it is often asymmetrical in favor of predators and can be described as both coincidental and omnivorous (Polis et al., 1989). In fact, there is very little information in the literature on predation interactions between H. axyridis and parasitoids. Previous studies have reported the consumption of parasitized aphids by coccinellids (Ferguson et al., 1996) and that the presence of predatory coccinellids within an aphid colony can reduce the oviposition rate of aphid parasitoids (Taylor et al., 1998). Takizawa et al. (2000) assessed whether the aphid Aphis craccivora Koch (Hemiptera: Aphididae), parasitized by the bracond wasp Aphidius colemani Viereck, were suitable prey for three coccinellid species: C. septempunctata, P. japonica and H. axyridis. The parasitoid was used at two life stages: 3-day-old larvae within living aphids (coincidental IGP) and sessile aphid "mummies" containing pupae (omnivorous IGP). Consumption of parasitized aphids containing 3-day-old larvae did not reduce survival nor increase development time of any of the coccinellids. In contrast, consumption of aphid "mummies" increased the development time of all three species and reduced survival to adulthood of C. septempunctata by 70% but did not affect the survival of H. axyridis and P. japonica. It is likely that aphid "mummies" are unsuitable prey for these coccinellids. Snyder et al. (2003) found that H. axyridis selectively preyed on pea aphids, Acyrthosiphon pisum, rather than pea aphid "mummies" parasitized by Aphidius ervi Haliday. Snyder et al. (2004) found that although adult H. axyridis did not show discrimination between aphid "mummies" of Aphelinus asychis Walker and aphids, their larvae preferred aphids in feeding trials.
28Entomopathogenic fungi are common pathogens of aphids and can be involved in both coincidental and omnivorous intraguild interactions (asymmetrical or symmetrical) (Völkl et al., 2007). Asymmetrical IGP engaged by H. axyridis was recorded in case of the fungus Pandora neoaphidis (Remaudière and Hennebert) Humber (Zygomycetes: Entomophthorales), this last infects aphids only (Roy et al., 2008b). Whereas, symmetrical IG interactions founded between H. axyridis and the fungus B. bassiana, this last infects both aphids and coccinellids (Roy et al., 2008a).
3. Mechanisms of interspecific competition leading to invasion/competitive displacement
29Negative impacts of introduced species include competitive suppression or displacement of native natural enemies and suppression or extinction of non-target prey species, some of which may be beneficial (Alyokhin et al., 2004). Competitive displacement is the most severe outcome of interspecific competition following H. axyridis invasion, in which competition for resources may lead to the competitive exclusion of one or more co-existent native species. The aggressive behavior of H. axyridis larvae possibly accounts for the replacements recorded particularly in ladybird communities (Yasuda et al., 2001; Agarwala et al., 2003). Based on the studies described above, it has proved difficult to separate the effects of either exploitative or interference competition from those of IGP on the natural enemy diversity following H. axyridis presence, where it appears that IGP among coccinellids has a stronger influence in the context of short-term laboratory experiments (Yasuda et al., 2004). There is clear evidence for declines in diversity of coccinellids in North America and increasingly in Europe following H. axyridis invasion. H. axyridis has certainly become abundant and widely distributed as an adventive species throughout North America and Europe and field data from the USA has reported negative impacts on native coccinellid species in these regions (Michaud, 2002b; Koch, 2003; Brown et al., 2008).
30In addition to its intrinsic competitive advantages, as described above, over many other natural enemies, H. axyridis has other exceptional capacities allowing it to be successful in any new environment as invader, on which they may lead to the competitive displacement of one or more indigenous species (Soares et al., 2008). H. axyridis is a highly polymorphic species (Soares et al., 2001) and this could also help the species to be an efficient invader. The relative frequency of phenotypes seems to be related to geographical and seasonal factors (Iablokoff-Khnzorian, 1982; Osawa et al., 1992), suggesting that some phenotypes may be favorably selected in different parts of the ecosystem or at different times. Thus the genetic polymorphism in H. axyridis seems to be the strategy adopted for facing different habitats at different times.
31Physiological characteristics, such as development, fecundity and low susceptibility to pathogens could allow its successful invasion in a new environment. A key factor in the invasion process is juvenile growth, as safe conditions in these vulnerable stages can ensure high population growth in the new environment (Marco et al., 2002). A shorter development time of younger larval instars of H. axyridis compared to the indigenous ladybird Coleomegilla maculata lengi Timberlake in Canada was observed in North America (Labrie et al., 2006). According to several studies, fecundity of H. axyridis is higher than other species, with between 703 and 3,800 eggs laid by a single female in laboratory studies (Iablokoff-Khnozorian, 1982; Stathas et al., 2001; Mignault et al., 2006). For example, fecundity of H. axyridis (2,008 eggs per female) reared on soybean aphids A. glycines was significantly higher than the invasive P. quatuordecimpunctata (593 eggs per female) or the indigenous C. maculata (390) in Québec, Canada (Mignault et al., 2006).
32Reitz et al. (2002) documented, in their review, 42 cases of interspecific competition leading to competitive displacement, and of these 14 involved natural enemies used in the biological control of insect pests. Recent introductions of coccinellids into the North American environment have also led to competition with native coccinellids, causing a reduction in the abundance and habitat usage of the native species. The widespread release and establishment of C. septempunctata for aphid control has been associated with displacement of native Coccinellidae across North America, such as Coccinella novemnotata Herbst., A. bipunctata, Coccinella transversoguttata richardsoni Brown and Hippodamia tredecimpunctata L., where the relative abundance of the introduced C. septempunctata increased from 6% in 1980 to 100% in 1994 (Alyokhin et al., 2004; Harmon et al., 2007). The subsequent invasion by H. axyridis resulted in the displacement of C. septempunctata from its dominant position through IGP (Brown et al., 1998; Turnock et al., 2003). Evidence is now accumulating to suggest that the abundance of some other coccinellid species, including Brachiacantha ursine F., Chilocorus stigma Say, and Cycloneda munda Say, is declining concurrently in many ecosystems in USA (e.g. arboreal habitats and apple orchards) in the presence of dominated species H. axyridis (Brown et al., 1998).
33Alyokhin et al. (2004) noted through their data set obtained from 1971-2001 that there was a significant decline in the abundance of aphids in the potato plots both in 1980 when C. septempunctata appeared and again after H. axyridis arrived in 1993-1995. These declines suggest that the exotic coccinellids may have been better exploiters of the aphids present on this exotic crop, and that exploitative competition may have played a role in the habitat displacement of native species by the exotic competitors (Evans, 2004). It is possible also that IGP advantage of exotic species may have contributed also in the competitive displacement of native species (Yasuda et al., 2004).
34The ladybird C. maculata is a native species in Québec, Canada (Mignault et al., 2006); it was less affected by the invasion of H. axyridis (Cottrell et al., 1998). Like H. axyridis, C. maculata is a polyphagous predator that feeds mainly on aphids, but also on insect eggs, pollen, and other prey (Hodek et al., 1996). The apparently low food requirements of C. maculata and its avoidance of interactions with H. axyridis may help this species in maintaining its population levels after the invasion of H. axyridis (Schellhorn et al., 2005). As reported by Musser et al. (2003), asynchronies in the temporal and spatial distributions of both species in the field are also likely to limit the effects of H. axyridis on C. maculata populations. On the other hand, C. maculata may also facilitate the invasion by H. axyridis. Shea et al. (2002) discussed invasions in relation to resource opportunities. Resource opportunities arise not only when resources are supplied at a high rate, but also when resident species that compete for these resources with the invader do not greatly reduce the resource densities. The low food requirements of C. maculata may help to create an environment that is easy for H. axyridis to invade.
35The appearance of H. axyridis in Florida dates back to the early 1990s (M. Thomas, personal communication). Before 1996, H. axyridis was relatively rare in Florida citrus, possibly due in part to its inability to produce eggs on a diet of the spirea aphid, Aphis spiraecola Patch (Hemiptera: Aphididae), the dominant aphid prey in citrus before the invasion of the brown citrus aphid, Toxoptera citricida Kirkaldy (Hemiptera: Aphididae) (Michaud, 2000a). Following Michaud (2002b), the ladybird C. sanguinea was one of the most abundant coccinellids in various Florida agro-ecosystems including citrus ecosystem. The subsequent invasion by H. axyridis resulted in the competitive displacement of C. sanguinea from citrus ecosystems in Florida, USA (Michaud, 2002b). One potential mechanism of displacement is resource (exploitative) competition. In citrus groves, Michaud (2002b) showed that H. axyridis was a more voracious predator and had higher fecundity and fertility than C. sanguinea. A comparison of basic biology and life history parameters suggests that H. axyridis has many intrinsic advantages over C. sanguinea. Adult H. axyridis average two to three times the body weight of adult C. sanguinea when reared on aphids, A. spiraecola or T. citricida (Michaud, 2000b). In IGP experiments, Michaud (2002b) showed also that adults and larvae of H. axyridis had a greater tendency to prey on various life stages of C. sanguinea than vice versa. Following this author, it is notable that C. sanguinea larvae possess smooth dorsal surfaces lacking any defensive spines or waxy filaments such as those present in larvae of other coccinellid genera, whereas the dorsal surfaces of H. axyridis larvae are covered with short spines thought to provide some defense against predation (Dixon, 2000). The results presented by Michaud (2002b) indicate also that H. axyridis can complete development on a sole diet of C. sanguinea larvae with only a slight cost in terms of delayed development, whereas C. sanguinea could not develop successfully on H. axyridis larvae. Following this author, when sources of prey are exhausted in the field, the ability of H. axyridis to complete development on an exclusive diet of either heterospecific or conspecific larvae should translate into a substantial competitive advantage over species such as C. sanguinea that lack such capabilities. H. axyridis is also more resistant than C. sanguinea to fungicides (Michaud, 2001), acaricides (Michaud, 2002c) and insecticides (Michaud, 2002d) and more resistant than most native ladybirds to attacks by the red imported fire ant Solenopsis invicta Buren (Dutcher et al., 1999), a species that has the potential to disrupt biological control in citrus (Michaud et al., 1999).
36Regarding the IG interactions with non-coccinellid predators, Gardiner et al. (2007) were found that a diverse guild of insect predators feeds on soybean aphid A. glycines in Michigan (USA) including the exotic coccinellid H. axyridis, the native gall midge A. aphidimyza and the native lacewing C. carnea. In laboratory and field studies, this adventive ladybird engaged in IGP with both predatory species (Gardiner et al., 2007). Moreover, in a laboratory study, Phoofolo et al. (1998) demonstrated that there was no difference in the development time or survival of H. axyridis fed on a diet of pea aphid A. pisum or eggs of the lacewing C. carnea; the last was unable to develop successfully when fed on H. axyridis eggs. These studies indicate that H. axyridis may contribute to local declines in these predatory species in the field (Gardiner et al., 2007).
37H. axyridis has been documented to feed on various stages of many predatory species, and it can also complete development on sole diets of some of them (Yasuda, 1999; Gardiner et al., 2007). Phoofolo et al. (1998) showed that H. axyridis could develop on an exclusive diet of C. carnea eggs, whereas C. septempunctata could not. Yasuda (1999) showed also that H. axyridis could develop on a diet of larvae of C. septempunctata, whereas the reverse was not possible.
38Similar evidence of declines in native coccinellid species after establishment of H. axyridis is accumulating in Europe. For example, evidence suggests that in London numbers of some native coccinellids (particularly A. bipunctata) have declined significantly since the arrival of H. axyridis in 2004 (Majerus, unpublished data). This is almost certainly due to its superior competitive ability and status as an IG predator. However, with consideration of the wider ecologies of H. axyridis and British ladybirds, it is predicted that aphidophagous habitat generalists such as C. septempunctata, A. bipunctata and P. quatuordecimpunctata will be most at risk from the establishment of H. axyridis in the UK, followed by aphidophagous habitat specialists such as Myzia oblongoguttata L., Myrrha octodecimguttata L., C. quinquepunctata and Anisosticta novemdecimpunctata L. (Ware et al., 2005; 2008b; Pell et al., 2008). The only species that may be relatively unaffected by the invader H. axyridis is A. ocellata, and indeed H. axyridis could actually be at risk from detrimental interactions with this species where they co-inhabit coniferous woodland (Ware et al., 2008b). Following Ware et al. (2008b), coccidophagous ladybird species such as Exochomus quadripustulatus L. and Chilocorus renipustulatus Scriba, Chilocorus bipustulatus L. and Coccinella hieroglyphica L. are thought to be less at risk, because H. axyridis has yet to be recorded from their specific habitats. Mycophagous, phytophagous and myrmecophilous ladybirds, such as Thea vigintiduopunctata L., Subcoccinella vigintiquatuorpunctata L. and Coccinella magnifica Redtenbacher respectively, are likely to be the least threatened by the establishment of H. axyridis in Britain. In Belgium, modifications in ladybird communities have been reported through detailed monitoring studies in Brussels (San Martin et al., 2005). Following this author, prior to the invasion of H. axyridis, the native species A. bipunctata was the dominant ladybird on lime (Tilia sp.) and maple (Acer sp.), and was co-dominant with the native congeneric ladybird Harmonia quadripunctata Pontoppidan on Austrian pine (Pinus nigra Arn.). Only two years after its establishment, H. axyridis quickly became the predominant ladybird species in all habitats monitored. In addition, a significant decline in the abundance of two native ladybird species A. bipunctata and A. decempunctata was recorded between 2003 and 2005, while a simultaneous increase of the H. axyridis population was observed (San Martin et al., 2005). Due to its voracity and wide trophic niche, it was believed that H. axyridis would harm indigenous aphidophagous guild. It therefore seems likely that H. axyridis could disrupt aphidophagous/coccidophagous community structure leading to declines in other species in the guild where it establishes as an adventive species.
4. Intraguild interactions implicating H. axyridis: biological control contest
39As predators are essential parts of the functional biodiversity for sustainable pest management in managed ecosystems, a central question in biological control has been, thus, how potential declines in guild diversity following H. axyridis arrival affect the suppression of pest populations. In fact, a single prey type, or even species, can provide multiple feeding niches that a diversity of predators can use if there is niche complementarity rather than redundancy and this is achieved by resource partitioning and facilitation amongst predators. If their is complete complementarity, then predator diversity should increase pest suppression (Casula et al., 2006). There is significant evidence for resource partitioning and facilitation within the aphid/predator system (Pell, 2008). For example variation between aphid species, or within a species, provides an opportunity for preference amongst predators. The coccinellid C. septempunctata exploited red morphs of the pea aphid more than green morphs whereas the parasitoid A. ervi only attacked green morphs, effectively partitioning the resource between them (Losey et al., 1997). Coccinellid species differ in their response to prey density, some being more effective at low densities and others at high densities, which effectively separates them into complementary niches (Schellhorn et al., 2005). A further example of complementarity can be seen for the interactions between C. septempunctata, a foliar aphid predator, and the carabid beetle Harpalus pennsylvanicus DeGeer (Coleoptera: Carabidae), which is restricted to foraging on the soil surface. As the coccinellid forages, it dislodges aphids that are then consumed by the ground predator, enhancing overall aphid suppression (Losey et al., 1998). Coccinellid predators and parasitoids also facilitate transmission and dispersal of the beneficial aphid pathogen P. neoaphidis (Baverstock et al., 2005). Avoidance behavior of parasitoids and coccinellids in relation to other intraguild predators is common (Meyling et al., 2006). Coccinellid species can be phenologically separated, thereby partitioning resources temporally and avoiding competition (Sato, 2001; Flowers et al., 2005) or they can have different patch-leaving times (Sato et al., 2003). As there are numerous examples of complementarity amongst aphidophagous species, it follows that the more species there are in the guild, the greater pest suppression will be. This confirms the hypothesis that declines in guild diversity as a result of introduction of H. axyridis could reduce effective pest suppression in the long-term (antagonistic effect).
40However, the presence of H. axyridis in a system does not necessarily result in reduced pest suppression, particularly in a short-term (Lucas et al., 2002; Gardiner et al., 2007). While the competitive advantages of H. axyridis lead to a decline in many of native natural enemies, the high predation rate of H. axyridis on the pest appears to compensate for the resulting reduction in the abundance of other natural enemies (Gardiner et al., 2007). Losey et al. (1998) found synergistic (facilitation) effect when they studied the interactions between ladybirds and ground beetles both preying on pea aphids; by dropping from the plant in response to an attack by the ladybirds, aphids were more susceptible to predation by ground beetles. Additive effect was found also when predators engage in IGP with either parasitoids or pathogens, where the herbivore and the parasitoid/pathogen are directly linked (coincidental) and IGP will simultaneously result in predation of the herbivore (Rosenheim et al., 1995). For instance, Snyder et al. (2004) found, through different experimental conditions, that IG interactions between H. axyridis and the aphid parasitoid A. asychis did not disrupt aphid control. Gardiner et al. (2007) found also that despite the high asymmetric IGP engaging by H. axyridis against A. aphidimyza and C. carnea, the presence of these species together improved A. glycines aphid control on soybean. Following these last authors, biological control of soybean aphid would not likely be improved by removing H. axyridis from the system.
5. Conclusion
41Based on the studies described above, interspecific competition was considered as a fundamental mechanism in structuring communities. There is a broad agreement that H. axyridis is a unparalleled top predator as it is predominantly a strong asymmetrical intraguild predator of other guild members (Sato et al., 2005; Cottrell, 2007; Ware et al., 2008b; Roy et al., 2008b) and as such can dominate in aggressive intraguild interactions and lead to a decline in guild diversity. In fact, several studies have stressed the functional traits that make H. axyridis a strong invader, among them, its aggressiveness (as an intraguild predator), polyphagy, voracity, fecundity and the fact that it is a less habitat and niche-specific coccinellid than native species. Key aphid mortality factors involving parasitoids and predators demonstrate that natural enemy diversity is necessary for resilient aphid suppression in agro-ecosystems (Straub et al., 2008). The decline recorded in natural enemy diversity following to H. axyridis invasion seems to be compensating by the high predation rate of this ladybird on the pest aphid for the declining of other natural enemies (Gardiner et al., 2007). However, its effectiveness as generalist predators in biological control may be reduced if increased availability of alternative prey causes individual predators to decrease their consumption of the target species. Pest management strategies aimed at maintaining beneficial insect diversity through natural and managed habitat manipulation (the diversification of our agricultural landscapes) could help counter declines particularly in native predator diversity associated with the arrival of H. axyridis and improve as a consequence pest biocontrol (Pell, 2008). To achieve this we need robust research data to fill the gaps in knowledge identified in this paper and develop efficient strategies.
42Acknowledgements
43We deeply thank the anonymous reviewers for their valuable comments on this manuscript. We also gratefully acknowledge François Verheggen and Jacques Mignon for their helpful comments.
Bibliographie
Abrams P.A., 1998. High competition with low similarity and low competition with high similarity: exploitative and apparent competition in consumer-resource systems. Am. Nat., 152, 114-128.
Adriaens T., San Martin y Gomez G. & Maes D., 2008. Invasion history, habitat preferences and phenology of the invasive ladybird Harmonia axyridis in Belgium. BioControl, 53(1), 69-88.
Agarwala B.K., Yasuda H. & Kajita Y., 2003. Effect of conspecific and heterospecific feces on foraging and oviposition of two predatory ladybirds: role of fecal cues in predator avoidance. J. Chem. Ecol., 29, 357-376.
Alhmedi A., Haubruge E. & Francis F., 2009. Intraguild interactions and aphid predators: biological efficiency of Harmonia axyridis and Episyrphus balteatus. J. Appl. Entomol., 134(1), 34-44.
Alyokhin A. & Sewell G., 2004. Changes in a lady beetle community following the establishment of three alien species. Biol. Invasions, 6, 463-471.
Anderson J.M.E., Hales D.F. & van Brunschot K.A., 1986. Parasitisation of coccinellids in Australia. In: Hodek I., ed. Ecology of aphidophaga. Prague: Academia; Dordrecht, The Netherlands: Dr. W. Junk NV Publishers.
Baverstock J., Alderson P.G. & Pell J.K., 2005. Influence of the aphid pathogen Pandora neoaphidis on the foraging behavior of the aphid parasitoid Aphidius ervi. Ecol. Entomol., 30, 665-672.
Boivin G. & Brodeur J., 2006. Intra- and interspecific interactions among parasitoids: mechanisms, outcomes and biological control. In: Brodeur J. & Boivin G., eds. Trophic and guild interactions in biological control. New York, USA: Springer, 123-144.
Brown M.W. & Miller S.S., 1998. Coccinellidae (Coleoptera) in apple orchards of Eastern West Virginia and the impact of invasion by Harmonia axyridis. Entomol. News, 109, 143-151.
Brown P.M.J. et al., 2008. Harmonia axyridis in Europe: spread and distribution of a non-native coccinellid. BioControl, 53, 5-21.
Burgio G., Lanzoni A., Accinelli G. & Maini S., 2008. Estimation of mortality by entomophages on exotic Harmonia axyridis versus native Adalia bipunctata in semi-field conditions in northern Italy. BioControl, 53, 277-287.
Casula P., Wilby A. & Thomas M.B., 2006. Understanding biodiversity effects on prey in multi-enemy systems. Ecol. Lett., 9, 995-1004.
Colignon P., Hastir P., Gaspar C. & Francis F., 2001. Effet de l’environnement proche sur la biodiversité entomologique en cultures maraichères de plein champ. Parasitica, 56, 59-70.
Cottrell T.E., 2005. Predation and cannibalism of lady beetle eggs by adult lady beetles. Biol. Control, 34, 159-164.
Cottrell T.E., 2007. Predation by adult and larval lady beetles (Coleoptera: Coccinellidae) on initial contact with lady beetles eggs. Environ. Entomol., 36, 390-401.
Cottrell T.E. & Yeargan K.V., 1998. Intraguild predation between an introduced lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae) and a native lady beetle, Coleomegilla maculata (Coleoptera: Coccinellidae). J. Kansas Entomol. Soc., 71, 159-163
De Clercq P., Peeters I., Vergauwe G. & Thas O., 2003. Interaction between Podisus maculiventris and Harmonia axyridis, two predators used in augmentative biological control in greenhouse crops. BioControl, 48, 39-55.
Dixon A.F.G., 2000. Insect predator-prey dynamics: ladybird beetles and biological control. Cambridge, UK: Cambridge University Press.
Dutcher J.D., Estes P.M. & Dutcher M.J., 1999. Interactions in entomology: aphids, aphidophaga and ants in pecan orchards. J. Entomol. Sci., 34, 40-56.
Evans E.W., 1991. Intra versus interspecific interactions of ladybeetles (Coleoptera: Coccinellidae) attacking aphids. Oecologia, 87, 401-408.
Evans E.W., 2000. Morphology of invasion: body size patterns associated with establishment of Coccinella septempunctata (Coleoptera: Coccinellidae) in western North America. Eur. J. Entomol., 97, 469-474.
Evans E.W., 2004. Habitat displacement of North American ladybirds by an introduced species. Ecology, 85, 637-647.
Felix S. & Soares A.O., 2004. Intraguild predation between the aphidophagous ladybird beetles Harmonia axyridis and Coccinella undecimpunctata (Coleoptera: Coccinellidae): the role of body weight. Eur. J. Entomol., 101, 237-242.
Ferguson K.I. & Stiling P., 1996. Non-additive effects of multiple natural enemies on aphid populations. Oecologia, 108, 375-379.
Ferran A. & Dixon A.F.G., 1993. Foraging behavior of ladybird larvae (Coleoptera: Coccinellidae). Eur. J. Entomol., 90, 383-402.
Firlej A., Boivin G., Lucas E. & Coderre D., 2005. First report of Harmonia axyridis Pallas being attacked by Dinocampus coccinellae Schrank in Canada. Biol. Invasions, 7, 553-556.
Flowers R.W., Salom S.M. & Kok L.T., 2005. Competitive interactions among two specialist predators and a generalist predator of hemlock woolly adelgid, Adelges tsugae (Homoptera: Adelgidae), in the laboratory. Environ. Entomol., 34, 664-675.
Fremlin M., 2007. Intraguild predation of harlequin ladybird larvae by lacewing larvae. Bull. Amat. Entomol. Soc., 66, 110-116.
Gardiner M.M. & Landis D.A., 2007. Impact of intraguild predation by adult Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Hemiptera: Aphididae) biological control in cage studies. Biol. Control, 40, 386-395.
Geoghegan I.E., Majerus T.M.O. & Majerus M.E.N., 1998. Differential parasitisation of adult and pre-imaginal Coccinella septempunctata (Coleoptera: Coccinellidae) by Dinocampus coccinellae (Hymenoptera: Braconidae). Eur. J. Entomol., 95, 571-579.
Grover J.P., 1997. Resource competition. London: Chapman & Hall.
Harmon J.P., Stephens E. & Losey J., 2007. The decline of native ladybirds (Coleoptera: Coccinellidae) in the United States and Canada. J. Insect Conserv., 11, 85-94.
Hemptinne J.-L. & Dixon A.F.G., 2000. Defence, oviposition and sex: semiochemical parsimony in two species of ladybird beetles (Coleoptera: Coccinellidae)? A short review. Eur. J. Entomol., 97, 443-447.
Herrick N.J. & Reitz S.R., 2004. Temporal occurrence of Podisus maculiventris (Hemiptera: Heteroptera: Pentatomidae) in North Florida. Florida Entomol., 87, 587-590.
Hewitt C.L. & Huxel G.R., 2002. Invasion success and community resistance in single and multiple species invasion models: do the models support the conclusions? Biol. Invasions, 4, 263-271.
Hodek I., 1973. Biology of Coccinellidae. The Hague; Prague: Dr. W. Junk NV Publishers.
Hodek I. & Honck A., 1996. Ecology of Coccinellidae. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Holt R.D. & Lawton J. H., 1993. Apparent competition and enemy-free space in insect host parasitoid communities. Am. Nat., 142, 623-645.
Holt R.D. & Polis G.A., 1997. A theoretical framework for intraguild predation. Am. Nat., 149, 745-764.
Hoogendoorn M. & Heimpel G.E., 2002. Indirect interactions between an introduced and a native ladybird beetle species mediated by a shared parasitoid. Biol. Control, 25, 224-230.
Hough-Goldstein J., Cox J. & Armstrong A., 1996. Podisus maculiventris (Hempitera: Pentatomidae) predation on ladybird beetles (Coleoptera: Coccinellidae). Florida Entomol., 79, 64-68.
Hunter M.S. & Woolley J.B., 2001. Evolution and behavioral ecology of heteronomous aphelinid parasitoids. Annu. Rev. Entomol., 46, 251-290.
Iablokoff-Khnzorian S.M., 1982. Les coccinelles. Coléoptères-Coccinellidae. Paris : Société Nouvelle des Éditions Boubée.
Iperti G., 1964. Les parasites des coccinelles aphidiphages dans les Alpes-Maritimes et les Basses-Alpes. Entomophaga, 9, 153-180.
Janssen A. et al., 2006. Intraguild predation usually does not disrupt biological control. In: Brodeur J. & Boivin G., eds. Trophic and guild interactions in biological control. Dordrecht, The Netherlands: Springer, 21-44.
Kawauchi S., 1984. Ecological studies on the natural enemies of Coccinella septempunctata brucki, Propylea japonica, and Scymnus (Neopullus) hoffmanni (Coleoptera: Coccinellidae). Kurume Univ. J., 33, 63-67.
King A. & Meinwald J., 1996. Review of the defensive chemistry of coccinellids. Chem. Rev., 96, 1105-1122.
Koch R.L., 2003. The multicoloured Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control and non-target impacts. J. Insect Sci., 3, 32-47.
Koyama S. & Majerus M.E.N., 2008. Interactions between the parasitoid wasp Dinocampus coccinellae and two species of coccinellid from Japan and Britain. BioControl, 53, 253-264.
Krebs C.J., 1989. Ecological methodology. New York, USA: HarperCollins Publishers.
Kuznetsov V.N., 1997. Lady beetles of the Russian far east. Gainesville, FL, USA: Center for Systematic Entomology.
Labrie G., Lucas E. & Coderre D., 2006. Can developmental and behavioral characteristics of the multicolored Asian lady beetle Harmonia axyridis explain its invasive success? Biol. Invasions, 8, 743-754.
Lanzoni A., Accinelli G., Bazzocchi G.G. & Burgio G., 2004. Biological traits and life table of the exotic Harmonia axyridis compared with Hippodamia variegata, and Adalia bipunctata (Col., Coccinellidae). J. Appl. Entomol., 128, 298-306.
Losey J.E. et al., 1997. A polymorphism maintained by opposite patterns of parasitism and predation. Nature, 388, 269-272.
Losey J.E. & Denno R.F., 1998. Positive predator-prey interactions: enhanced predation rates and synergistic suppression of aphid populations. Ecology, 79, 2143-2152.
Lucas E., 2005. Intraguild predation among aphidophagous predators. Eur. J. Entomol., 102, 351-364.
Lucas E., Coderre D. & Brodeur J., 1998. Intraguild predation among aphid predators: characterization and influence of extraguild prey density. Ecology, 79, 1084-1092.
Lucas E., Gagné I. & Coderre D., 2002. Impact of the arrival of Harmonia axyridis on adults of Coccinella septempunctata and Coleomegilla maculata (Coleoptera: Coccinellidae). Eur. J. Entomol., 99, 457-463.
Lucas E., Labrie G., Vincent C. & Kovach J., 2007. The multicolored Asian ladybeetle, Harmonia axyridis, beneficial or nuisance organism? In: Vincent C., Goettel M. & Lazarovitz G., eds. Biological control: a global perspective. Wallingford, UK: CABI Publishing.
Mack R.N. et al., 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl., 10, 689-710.
Magro A. et al., 2007. Assessment of patch quality by ladybirds: relative response to conspecific and heterospecific larval tracks a consequence of habitat similarity? Chemoecology, 17, 37-45.
Majerus M.E.N., 1994. Ladybirds. London: HarperCollins.
Majerus M.E.N., Strawson V. & Roy H.E., 2006. The potential impacts of the arrival of the harlequin ladybird, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), in Britain. Ecol. Entomol., 31, 207-215.
Marco D.A., Páez S.A. & Cannas S.A., 2002. Species invasiveness in biological invasions: a modelling approach. Biol. Invasions, 4, 193-205.
Meyling N.V. & Pell J.K., 2006. Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecol. Entomol., 31, 162-171.
Michaud J.P., 2000a. Sources of mortality in colonies of the brown citrus aphid, Toxoptera citricida. BioControl, 44, 347-367.
Michaud J.P., 2000b. Development and reproduction of ladybeetles, (Coleoptera: Coccinellidae), on the citrus aphids Aphis spiraecola Patch and Toxoptera citricida (Kirkaldy) (Homoptera: Aphididae). Biol. Control, 18, 287-297.
Michaud J.P., 2001. Responses of two ladybeetles to eight fungicides used in Florida citrus: implications for biological control. J. Insect Sci., 1, 1-6.
Michaud J.P., 2002a. Biological control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae) in Florida: a preliminary report. Entomol. News, 113, 216-222.
Michaud J.P., 2002b. Invasion of the Florida citrus ecosystem by Harmonia axyridis (Coleoptera: Coccinellidae) and asymmetric competition with a native species, Cycloneda sanguinea. Environ. Entomol., 31, 827-835.
Michaud J.P., 2002c. Non-target impacts of acaricides on ladybeetles in citrus: a laboratory study. Florida Entomol., 85, 191-196.
Michaud J.P., 2002d. Relative toxicity of six insecticides to Cycloneda sanguinea and Harmonia axyridis (Coleoptera: Coccinellidae). J. Entomol. Sci., 37, 83-93.
Michaud J.P. & Browning H.W., 1999. Seasonal abundance of the brown citrus aphid, Toxoptera citricida, (Homoptera: Aphididae) and its natural enemies in Puerto Rico. Florida Entomol., 82, 424-447.
Mignault M.-P., Roy M. & Brodeur J., 2006. Soybean aphid predators in Quebec and the suitability of Aphis glycines as prey for three Coccinellidae. BioControl, 51, 89-106.
Mills N.J., 1999. Interspecific competition in insects. In: Huffaker C.B. & Gutierrez A.P., eds. Ecological Entomology. 2nd ed. New York, USA: Wiley, 355-387.
Musser F.R. & Shelton A.M., 2003. Bt sweet corn and selective insecticides: impacts on pests and predators. J. Econ. Entomol., 96, 71-80.
Obrycki J.J., 1989. Parasitization of native and exotic coccinellids by Dinocampus coccinellae (Schrank) (Hymenoptera: Braconidae). J. Kansas Entomol. Soc., 62, 211-218.
Osawa N. & Nishida T., 1992. Seasonal variation in elytral color polymorphism in Harmonia axyridis (the ladybird beetle): the role of non-random mating. Heredity, 69, 297-307.
Pell J.K., 2008. Ecological approaches to pest management using entomopathogenic fungi; concepts, theory, practice and opportunities. In: Ekesi S. & Maniania N., eds. Use of entomopathogenic fungi in biological pest management. Kerala, India: Research Signpost
Pell J.K. et al., 2008. Intraguild predation involving Harmonia axyridis: a review of current knowledge and future perspectives. BioControl, 53, 147-168.
Phoofolo M.W. & Obrycki J.J., 1998. Potential for intraguild predation and competition among predatory Coccinellidae and Chrysopidae. Entomol. Exp. Appl., 89, 47-55.
Pimm S.L. & Lawton J.H., 1978. On feeding on more than one trophic level. Nature, 275, 542-544.
Polis G.A., Myers C.A. & Holt R.D., 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. Ann. Rev. Ecol. Syst., 20, 297-330.
Polis G.A. & Holt R.D., 1992. Intraguild predation–the dynamics of complex trophic interactions. Tree, 7, 151-154.
Powell W. & Pell J.K., 2007. Biological control. In: Harrington R. & van Emden H., eds. Aphids as crop pests. Wallingford, UK: CABI International, 469-513.
Reitz S.R. & Trumble J.T., 2002. Competitive displacement among insects and arachnids. Annu. Rev. Entomol., 47, 435-465.
Richerson J.V. & DeLoach C.J., 1972. Some aspects of host selection by Perilitus coccinellae. Ann. Entomol. Soc. Am., 65, 834-839.
Ridenour W.M. & Callaway R.M., 2001. The relative importance of allelopathy in interference: the effects of an invasive weed on a native bunchgrass. Oecologia, 126, 444-450.
Rosenheim J.A., 1998. Higher-order predators and the regulation of insect herbivore populations. Annu. Rev. Entomol., 43, 421-447.
Rosenheim J.A. et al., 1995. Intraguild predation among biological-control agents: theory and evidence. Biol. Control, 5, 303-335.
Rosenheim J.A. & Harmon J.P., 2006. The influence of intraguild predation on the suppression of a shared prey population: an empirical assessment. In: Brodeur J. & Boivin G., eds. Trophic and guild interactions in biological control. Dordrecht, The Netherlands: Springer, 1-20.
Roy H.E., Brown P. & Majerus M.E.N., 2006. Harmonia axyridis: a successful biocontrol agent or an invasive threat? In: Eilenberg J. & Hokkanen H., eds. An ecological and societal approach to biological control. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Roy H.E. et al., 2008a. Interactions between the fungal pathogen Beauveria bassiana and three species of Coccinellid: Harmonia axyridis, Coccinella septempunctata and Adalia bipunctata. BioControl, 53, 265-276.
Roy H.E. et al., 2008b. Intraguild predation of the aphid pathogenic fungus Pandora neoaphidis by the invasive coccinellid Harmonia axyridis. Ecol. Entomol., 33, 175-182.
San Martin G., Adriaens T., Hautier L. & Ottart N., 2005. La coccinelle asiatique Harmonia axyridis. Insectes, 136(1), 7-11.
Sato S., 2001. Ecology of ladybirds: factors influencing their survival. PhD Thesis: University of East Anglia, Norwich (UK).
Sato S., Dixon A.F.G. & Hironori Y., 2003. Effect of emigration on cannibalism and intraguild predation in aphidophagous ladybirds. Ecol. Entomol., 28, 628-633.
Sato S. & Dixon A.F.G., 2004. Effect of intraguild predation on the survival and development of three species of aphidophagous ladybirds: consequences for invasive species. Agric. Forest Entomol., 6, 21-24.
Sato S., Yasuda H. & Evans E.W., 2005. Dropping behaviour of larvae of aphidophagous ladybirds and its effects on incidence of intraguild predation: interactions between the intraguild prey, Adalia bipunctata (L.) and Coccinella septempunctata (L.), and the intraguild predator, Harmonia axyridis Pallas. Ecol. Entomol., 30, 220-224.
Schellhorn N.A. & Andow D., 2005. Response of coccinellids to their aphid prey at different spatial scales. Popul. Ecol., 47, 281-288.
Shea K. & Chesson P., 2002. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol., 17, 170-176.
Shigesada N. & Kawasaki K., 1997. Biological invasions: theory and practice. Oxford, UK: Oxford University Press.
Snyder W.E. & Ives A.R., 2003. Interactions between specialist and generalist natural enemies: parasitoids, predators and pea aphid biocontrol. Ecology, 84, 91-107.
Snyder W.E., Clevenger G.M. & Eigenbrode S.D., 2004. Intraguild predation and successful invasion by introduced ladybird beetles. Oecologia, 140, 559-565.
Snyder W.E. & Evans E.W., 2006. Ecological effects of invasive arthropod generalist predators. Annu. Rev. Ecol. Evol. Syst., 37, 95-122.
Soares A.O., Coderre D. & Schanderl H., 2001. Influence of phenotype on fitness parameters of Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Eur. J. Entomol., 98, 287-293.
Soares A.O. & Serpa A., 2007. Interference competition between ladybird beetle adults (Coleoptera: Coccinellidae): effects on the growth and reproductive capacity. Popul. Ecol., 49, 37-43.
Soares A.O. et al., 2008. Harmonia axyridis: what will stop the invader? BioControl, 53, 127-145.
Stathas G.J., Eliopoulos P.A., Kontodimas D.C. & Giannopapas J., 2001. Parameters of reproductive activity in females of Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol., 98, 547-549.
Straub C.S., Finke D.L. & Snyder W.E., 2008. Are the conservation of natural enemy biodiversity and biological control compatible goals? Biol. Control, 45, 225-237.
Takizawa T., Yasuda H. & Agarwala B.K., 2000. Effect of three species of predatory ladybirds on oviposition of aphid parasitoids. Entomol. Sci., 3, 465-469.
Taylor A.J., Müller C.B. & Godfray H.C.J., 1998. Effect of aphid predators on oviposition behaviour of aphid parasitoids. J. Insect Behav., 11, 297-302.
Torchin M.E. et al., 2003. Introduced species and their missing parasites. Nature, 421, 628-630.
Turnock W.J., Wise I.L. & Matheson F.O., 2003. Abundance of some native coccinellines (Coleoptera: Coccinellidae) before and after the appearance of Coccinella septempunctata. Can. Entomol., 135, 391-404.
van Lenteren J.C. et al., 2003. Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl, 48, 3-38.
Völkl W., Mackauer M., Pell J.K. & Brodeur J., 2007. Predators, parasitoids and fungal pathogens. In: Harrington R. & van Emden H., eds. Aphids as crop pests. Wallingford, UK: CABI International, 187-233.
Ware R.L., Majerus M.E.N., Roy H.E. & Symington F., 2005. The harlequin ladybird arrives in Britain: a threat to our native species? Bull. Amat. Entomol. Soc., 64, 175-186.
Ware R.L. & Majerus M.E.N., 2008a. Intraguild predation of immature stages of British and Japanese coccinellids by the invasive ladybird Harmonia axyridis. BioControl, 53, 169-188.
Ware R.L. et al., 2008b. Chemical protection of Calvia quatuordecimguttata eggs against intraguild predation by the invasive ladybird Harmonia axyridis. BioControl, 53, 189-200.
Williamson M., 1996. Biological invasions. London: Chapman & Hall.
Williamson M., 1999. Invasions. Ecography, 22, 5-12.
Williamson M., 2006. Explaining and predicting the success of invading species at different stages of invasion. Biol. Invasions, 8, 1561-1568.
Woodward G. & Hildrew A.G., 2002. Body-size determinants of niche overlap and intraguild predation within a complex food web. J. Anim. Ecol., 71, 1063-1074.
Yasuda H., 1999. Effect of cannibalism and predation on the larval performance of two ladybird beetles. Entomol. Exp. Appl., 93, 63-67.
Yasuda H., Kikuchi T., Kindlmann P. & Sato S., 2001. Relationships between attacks and escape rates, cannibalism, and intraguild predation in larvae of two predatory ladybirds. J. Insect Behav., 14, 373-384.
Yasuda H. et al., 2004. Asymmetric larval interactions between introduced and indigenous ladybirds in North America. Oecologia, 141, 722-731.
Para citar este artículo
Acerca de: Ammar Alhmedi
ULg - Gembloux Agro-Bio Tech. Functional and Evolutionary Entomology. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: Ammar.Alhmedi@student.ulg.ac.be.
Acerca de: Éric Haubruge
ULg - Gembloux Agro-Bio Tech. Functional and Evolutionary Entomology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Frédéric Francis
ULg - Gembloux Agro-Bio Tech. Functional and Evolutionary Entomology. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






