- Portada
- Volume 14 (2010)
- numéro 4
- From biological membranes to biomimetic model membranes
Vista(s): 0 (0 ULiège)
Descargar(s): 0 (0 ULiège)
From biological membranes to biomimetic model membranes

Notes de la rédaction
Received on June 4, 2009; accepted on December 9, 2009
Résumé
Des membranes biologiques aux modèles membranaires biomimétiques. Les membranes biologiques jouent un rôle essentiel dans la protection cellulaire ainsi que dans le contrôle et le transport des éléments nutritifs. Elles sont le lieu de nombreux mécanismes biologiques tels que la reconnaissance moléculaire, la catalyse enzymatique, l’adhésion cellulaire ou encore la fusion membranaire. En 1972, Singer et al. ont proposé un modèle membranaire, appelé modèle de la mosaïque fluide. Selon ce modèle, les membranes biologiques consistent en des bicouches lipidiques dynamiques renfermant des protéines et des glycoprotéines. Les protéines membranaires forment des icebergs globulaires dans une mer homogène de lipides à l’état fluide. Depuis sa conception, le modèle de la mosaïque fluide a fortement évolué notamment suite aux travaux de Simons et al. (1997) et de Brown et al. (1997). Ces chercheurs ont montré que les lipides membranaires sont en réalité organisés en microdomaines (rafts lipidiques) dont la composition et la dynamique moléculaire sont bien spécifiques et différentes de celles de la phase liquide cristalline formée par les phospholipides environnants. Une telle découverte a entrainé un intérêt sans cesse croissant pour l’étude des membranes biologiques et a provoqué par la même occasion une émergence de nouvelles technologies de pointe pour améliorer nos connaissances sur ces systèmes biologiques extrêmement complexes. Aussi, en raison de la grande complexité des membranes biologiques, la plupart des études ciblant les mécanismes biologiques qui se déroulent à la surface des membranes ou au sein de leur bicouche lipidique sont réalisées en utilisant des modèles membranaires biomimétiques. Cette synthèse a donc pour principaux objectifs de revisiter les propriétés de base des membranes biologiques en termes de composition membranaire, de dynamique membranaire et d’organisation moléculaire, ainsi que de décrire les modèles membranaires les plus couramment utilisés pour étudier des mécanismes biologiques tels que la fusion membranaire, le transport membranaire, la formation de pores membranaires, ainsi que les interactions membranaires à l’échelle moléculaire.
Abstract
Biological membranes play an essential role in the cellular protection as well as in the control and the transport of nutrients. Many mechanisms such as molecular recognition, enzymatic catalysis, cellular adhesion and membrane fusion take place into the biological membranes. In 1972, Singer et al. provided a membrane model, called fluid mosaic model, in which each leaflet of the bilayer is formed by a homogeneous environment of lipids in a fluid state including globular assembling of proteins and glycoproteins. Since its conception in 1972, many developments were brought to this model in terms of composition and molecular organization. The main development of the fluid mosaic model was made by Simons et al. (1997) and Brown et al. (1997) who suggested that membrane lipids are organized into lateral microdomains (or lipid rafts) with a specific composition and a molecular dynamic that are different to the composition and the dynamic of the surrounding liquid crystalline phase. The discovery of a phase separation in the plane of the membrane has induced an explosion in the research efforts related to the biology of cell membranes but also in the development of new technologies for the study of these biological systems. Due to the high complexity of biological membranes and in order to investigate the biological processes that occur on the membrane surface or within the membrane lipid bilayer, a large number of studies are performed using biomimicking model membranes. This paper aims at revisiting the fundamental properties of biological membranes in terms of membrane composition, membrane dynamic and molecular organization, as well as at describing the most common biomimicking models that are frequently used for investigating biological processes such as membrane fusion, membrane trafficking, pore formation as well as membrane interactions at a molecular level.
Tabla de contenidos
1. Introduction
1Biological membranes play an essential role in the cellular protection as well as in the control and the transport of nutrients. Many mechanisms such as molecular recognition, enzymatic catalysis, cellular adhesion and membrane fusion take place into the biological membranes. The detailed organization of these membranes at a molecular level is currently not yet fully determined even if many experiments were conducted in the last century to achieve such a goal.
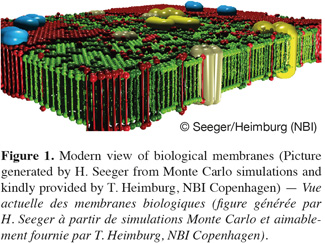
2The concept that biological membranes are composed of two opposite layers of lipids was already found out in 1925 by Gorter et al. who observed using the Langmuir trough technique that the molecular area of lipids extracted from red blood cells was two times the area of the red blood cells measured by microscopy. The first membrane model including proteins dates from 1935 and was proposed by Danielli et al. These researchers postulated that a protein layer is tightly associated to the polar heads of lipids composing the cell membranes. It was forced to wait more than thirty years to find out that proteins may also span through membranes. Such discovery led to the so-called fluid mosaic model proposed by Singer et al. in 1972. According to this model, each leaflet of the bilayer is formed by a homogeneous environment of lipids in a fluid state incorporating globular assembling of proteins and glycoproteins. Singer et al. also assumed that the lipid composition within the bilayers is most likely asymmetric. Since its conception in 1972, some developments and refinements were brought to the fluid mosaic model especially in terms of composition and molecular organization. The most important evolution of this model was obtained in 1997 with the works of Simons et al. and of Brown et al. These authors showed that biological membranes do not form a homogeneous fluid lipid phase as predicted by Singer et al. In contrast, they suggested that membrane lipids are organized into phase-separated microdomains, called lipid rafts, with both a specific composition and a molecular dynamic that are different to the ones of the surrounding liquid crystalline phase. The discovery of such phase separation in the plane of the membrane has induced in the last decade an explosion in the research efforts related to the biology of the cell membrane as well as in the development of new technologies for detecting lateral heterogeneities in biological membranes. Nowadays, while there is no doubt about the presence of phase separation in the plane of the membrane, the existence of lipid rafts, which are believed to be enriched in sphingolipids and cholesterol, to present a high mobility in the plane of the membrane and to be involved in many biological processes such as signal transduction, membrane transport and protein sorting (Simons et al., 1997) is still controversial. A more detailed discussion about this hot and controversial issue is given later in this paper (see “Membrane complexity at the nanometre scale”). Figure 1 depicts the actual view of biological membranes, which exhibit lateral heterogeneities, cluster and domain formation within the membrane plane.
2. Lipid composition of membranes
3Biological membranes display a very complex composition in terms of lipids and proteins. Membrane lipids are amphiphilic, i.e. they are constituted of a hydrophilic head group and a hydrophobic region. The latter one is principally composed of aliphatic chains, aromatic groups or polycyclic structures (Helenius et al., 1975; Lichtenberg et al., 1983). Due to their amphipathicity and to their geometric constraints, membrane lipids self-associate into bilayers in aqueous medium. Membrane lipids are classified into three main groups, namely phospholipids, glycolipids and sterols.
4The main phospholipids found in biological membranes are glycerophospholipids (40-60 mol % of the total lipid fraction) (Figure 2A). These compounds are composed of a glycerol backbone on which two fatty acid chains are esterified in position sn-1 and sn-2, respectively. The third carbon atom of the glycerol backbone (position sn-3) supports the phospholipid polar head group, which is composed of an alcohol molecule (choline, ethanolamine, serine, glycerol or inositol) linked to a negatively charged phosphate group. The phospholipid polar head group can be zwitterionic or negatively charged. The fatty acid chain in position sn-1 is generally saturated and composed of 16 or 18 carbon atoms while the fatty acid chain in position sn-2 is longer and usually unsaturated (one or several double bonds in cis configuration) (McElhaney et al., 1971).
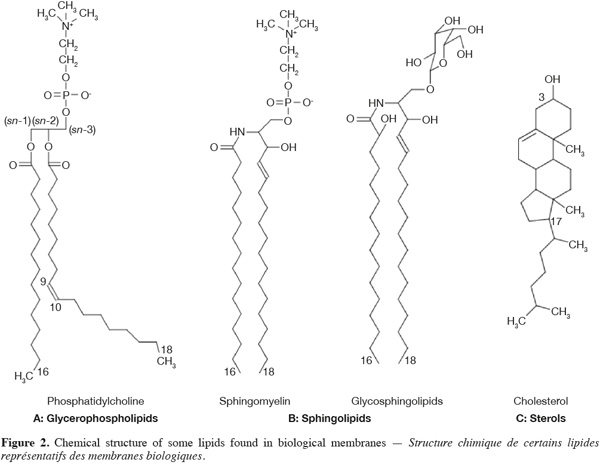
5Sphingolipids are another important class of membrane lipids and are believed to be involved in the formation of lateral microdomains in biological membranes. These lipids are composed of a sphingosine (or phytosphingosine) base on which is linked a relatively long (up to 24 carbon atoms) saturated fatty acid chain. Acylated sphingosines are referred to as ceramides. Sphingomyelin and glycosphingolipids (Figure 2B) result from the attachment of a choline molecule and an oligosaccharide to the hydroxyl group of ceramides, respectively.
6Sterols are a particular class of membrane lipids. While the hydrophobic moiety of most of membrane lipids is constituted of relatively long aliphatic chains, the one of sterols is composed of polycyclic structures. The most abundant sterol in mammal is cholesterol (Figure 2C). This compound is very abundant in erythrocyte membranes, other plasma membranes and various sub-cellular compartments in eukaryotes (30-50 mol % of the total lipid fraction). It comprises four fused cycles in trans configuration, a hydroxyl group in position 3, a double bond between the carbon 5 and 6, as well as an iso-octyl lateral chain in position 17. The hydroxyl group is responsible for the amphiphilic nature of cholesterol and consequently for its orientation in biological membranes (Tanford, 1980). Ergosterol and lanosterol are two other representatives of the sterol class. These compounds exhibit a similar structure to the one of cholesterol. Ergosterol is found in the membranes of fungi, yeasts and protozoans, (Brennan et al., 1974) while lanosterol is the sterol of prokaryotes and the chemical precursor of both cholesterol and ergosterol (Henriksen et al., 2006).
7The shape of a membrane lipid depends on the effective area of its polar head group compared to the dimension of its hydrophobic moiety (Cullis et al., 1979; Chernomordik, 1996). Membrane lipids display a cylindrical shape (e.g. phosphatidylcholine, phosphatidylserine), a conical shape (e.g. phosphatidylethanolamine) or an inverted conical shape (e.g. lysophosphatidylcholine). Such a polymorphism influences the localization of lipid molecules within the biological membranes. The lipid composition of biological membranes is qualified as asymmetric (Bretscher, 1973; Op den Kamp, 1979), i.e. the lipid composition is different within the two leaflets of the same membrane. Phosphatidylethanolamines and phosphatidylserines are mainly found in the inner leaflet of the plasma membrane, while phosphatidylcholines and sphingomyelins are essentially located in the outer leaflet (Rothman et al., 1977). Due to its ability to undergo a fast flip-flop between the outer and inner leaflets of the lipid bilayers (Muller et al., 2002), cholesterol is assumed to be equally distributed on the two leaflets of biological membranes. The lipid asymmetry across the membranes is responsible for membrane curvature, which is essential for biological processes such as vesicle budding and membrane fusion (Zimmerberg et al., 1999), and contributes also to membrane potential, which is a key player in many membrane-mediated phenomena such as binding of drugs or proteins to membrane surface, insertion of integral proteins, and membrane transport (McLaughlin, 1989). While it is generally accepted that the transmembrane potential drop arises from a charge imbalance of salt ions across the plasma membrane, it has been recently shown by Gurtovenko et al. (2007; 2008; 2009) that the electrostatic transmembrane potential can be nonzero even in the absence of salt ions, provided that the lipid distribution is asymmetric. These authors pointed out that the observed potential originates from a difference in the dipole moments of the two leaflets of the asymmetric membrane and is not related to the transmembrane potential arising from concentration differences of ionic substances across the membrane.
8Lipid polymorphism not only induces lipid asymmetry between the two leaflets of membranes, but it is also responsible for phase separation within one monolayer leaflet. For example, it is assumed that lipid polymorphism is involved in the formation of lipid rafts, which are enriched in sphingolipids and cholesterol (Simons et al., 1997). Due to its conical shape, cholesterol may play the role of molecular spacer to fulfil the free space between sphingolipid molecules, which exhibit an inverted conical shape.
3. Molecular dynamic of membranes
9Biological membranes are highly dynamic structures (Figure 3). Both the position (i.e. lateral order) and the orientation (i.e. rotational order) of a lipid within the membrane bilayers are continuously changing with time. Moreover, conformational changes (such as trans-gauche isomerisation) within the hydrocarbon lipid chains may also occur (over time scales of a few picoseconds) and affect the conformational order of lipid molecules. Different diffusion coefficients are used to characterize the lipid dynamic within the membranes. The lateral diffusion coefficient (CD), ranging typically from 10-7 to 10-10 cm2.s-1, determines the ability of a lipid molecule to laterally exchange with one of its neighbours (this phenomenon occurs over time scales less than a minute), while the rotational diffusion coefficient defines the angular rotation of a lipid molecule around its axis perpendicular to the plane of the bilayer (this motion takes place over times scale of nanoseconds). The transfer of one lipid molecule from one leaflet of the bilayer to the other one is a special case of molecular dynamic. Such process, called transversal diffusion or flip-flop, involves the rotation of the lipid molecule in the plane of the bilayer followed by its translation perpendicularly to the plane of the bilayer. The transversal diffusion is a very slow process (of the order of hours, possibly days) and is energetically unfavorable as it forces the passage of the polar lipid head group through the hydrophobic core of the lipid bilayer. However, some lipid molecules such as cholesterol are able to undergo a fast flip-flop (< 1.s-1) between the two leaflets of the lipid bilayer (Muller et al., 2002; Steck et al., 2002). Such property most likely arises from the very small effective area of the polar head group of cholesterol, which is limited to one hydroxyl group.
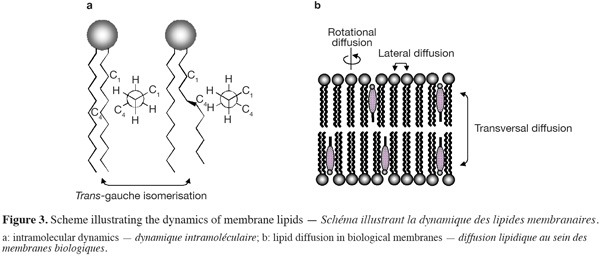
10Proteins may also diffuse laterally within the biological membranes, but their diffusion rate is typically a hundred times slower than lipid diffusion. However, proteins are not able to diffuse transversally between the two leaflets of the lipid bilayers.
4. Thermotropic phase behavior of membranes
11In aqueous medium, lipid bilayer constituting the biological membranes can exist in different physical states, which are characterized by the lateral organization, the molecular order as well as the mobility of the lipid molecules within the bilayer (Figure 4). Consequently, physicochemical parameters such as temperature, pH, ionic strength and other factors such as the chemical structure of the lipid constituents and the presence of cholesterol strongly influence the nature of the lamellar phase.
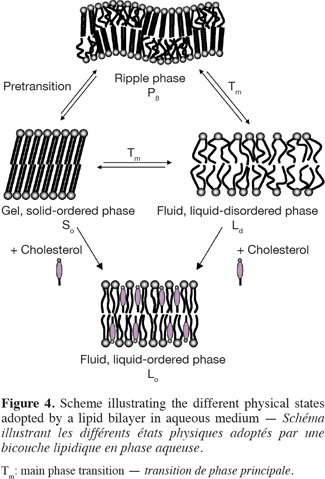
12The two extreme lipid phases that occur in biological membranes are the so-called gel and fluid phases (Figure 4). In the gel phase (Lβ’ or Lβ), also called solid-ordered (So) phase, the lipids are arranged on a two-dimensional triangular lattice in the plane of the membrane (Janiak et al., 1979). The hydrocarbon lipid chains display an all-trans configuration and are elongated at the maximum, giving rise to an extremely compact lipid network. Consequently, the lateral diffusion of lipids is strongly reduced (CD ~ 10-11 cm2.s-1). Note that, as a function of the hydration level, the hydrocarbon chains of lipids in the gel phase may be tilted (Lβ’) or not tilted (Lβ) with respect to the membrane normal, the angle of tilt increasing with the increase of water content (Tardieu et al., 1973). As a result, the thickness of a lipid bilayer in the gel state decreases as the amount of water increases. Other parameters such as the nature of the polar head group and the presence of counterions, which affect the head group conformation, may also influence the tilt of the lipid alkyl chains in the gel phase (McIntosh, 1980). For example, while the hydrocarbon chains of hydrated PC are tilted with respect to the bilayers, the alkyl chains of hydrated PE are approximately normal to the plane of the bilayers. Such a difference in the degree of tilt arises from the smaller head group of PE compared to PC and from the fact that hydrated PE bilayers do not contain as much water as hydrated PC bilayers (McIntosh, 1980).
13In the fluid phase, also called liquid-disordered (Lα or Ld) phase, trans-gauche isomerisation occurs giving rise to much less extended lipid chains. Moreover, the two-dimensional triangular lattice is completely lost. As a result, both the lateral diffusion (CD ~ 10-8 cm2.s-1) and the rotational diffusion of lipids are favored in fluid lipid bilayers.
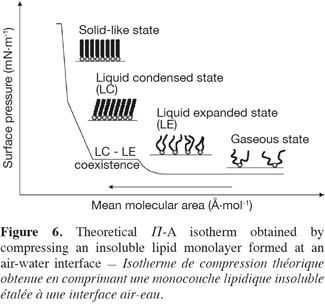
14The transition between the gel and fluid phases occurs at a specific temperature called thermotropic phase transition (Tm). The phase transition temperature of a membrane lipid, i.e. the temperature that is required for inducing the lipid melting from a solid-ordered to a liquid-disordered phase, is depending on the nature of its hydrophobic moiety and can be determined by using the differential scanning calorimetry technique.
15For some membrane lipids, such as phosphatidylcholines, the lipid disordering occurs in two steps when increasing temperature. A first transition is observed a few degrees below the main transition Tm. This pretransition may be due to changes in the vicinity of the polar head group such an increase of the interaction of the lipid head groups with the solvent (Heimburg, 2000). For example, phosphatidylethanolamines that differ from phosphatidylcholines by the nature of the polar head group do not display a pretransition (McIntosh, 1980). According to Heimburg (2000; 2007), pretransition and main transition are both part of the chain melting transition with the splitting into two transitions being the consequence of simultaneous changes in the lipid order and membrane curvature. Consequently, for the lipids that exhibit a pretransition temperature, an additional lamellar phase exists. This phase, called the ripple phase (Pβ), is characterized by periodic one-dimensional undulations on the surface of the lipid bilayer (Janiak et al., 1979) (Figure 4). As this phase appears prior to the main chain melting, it must correspond to a partially disordered lipid phase. For this reason, it has been supposed that the undulations observed on the top of the lipid bilayers arise from periodic arrangements of linear ordered and disordered lipid domains (Heimburg, 2000; 2007; de Vries et al., 2005).
16In presence of cholesterol, lipid bilayers can adopt an extra lamellar phase, called the liquid-ordered (Lo) phase, which shares the characteristics of both gel and fluid phases (Figures 4 and 5) (Ipsen et al., 1987). In other words, this phase resembles to the gel phase with less lateral packing order and at the same time to the fluid phase with more packing order. The incorporation of cholesterol into a solid-ordered lamellar phase disturbs the lateral triangular lattice and consequently reduces the ordering of the lipid chains. At the opposite, in a liquid-disordered lamellar phase, the rigid hydrophobic moiety of cholesterol is intercalated between the lipid chains and favors a trans chain conformation (Sankaram et al., 1990b). Consequently, the liquid-ordered phase displays both a lateral and a rotational diffusion that are close to the ones of the liquid-disordered phase (Almeida et al., 1993; Filippov et al., 2003), but a conformational order similar to the one of the solid-ordered phase (Gally et al., 1976).
17As shown in figure 5, liquid-disordered and liquid-ordered phases as well as liquid-ordered and solid-ordered phases can coexist in a same lipid bilayer (Vist et al., 1990). For example, a phase-coexistence between a cholesterol-poor liquid-disordered phase and a cholesterol-rich liquid-ordered phase has been experimentally observed for lipid bilayers composed of phosphatidylcholine/cholesterol (Sankaram et al., 1991) and sphingomyelin/cholesterol (Ahmed et al., 1997) mixtures.
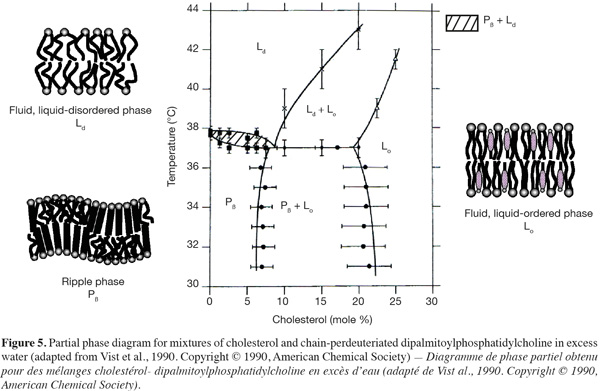
18The preferential partitioning of membrane lipids into a liquid-disordered or a liquid-ordered phase is strongly depending on their chemical structure. Most of glycerophospholipids found in biological membranes are composed of an unsaturated fatty acid chain in position sn-2 of the glycerol backbone. The presence of double bonds in configuration cis induces a kink in the hydrocarbon chain and hampers a very compact assembling of the lipids. Consequently, this class of membrane lipids has very little affinity for highly ordered lipid domains. At the opposite, sphingolipids display long saturated alkyl chains and segregate together via van der Waals and hydrophobic interactions. Moreover, hydrogen bonds between the hydroxyl groups of sphingomyelin polar heads (Ramstedt et al., 2002) or between the oligosaccharidic head groups of glycosphingolipids (Rock et al., 1990) may also accentuate the auto-assembling of these lipids. Therefore, sphingolipids have a high tendency to form ordered lipid phases (Wang et al., 2000).
19The lateral organization of membrane lipids is also influenced by the nature of their polar head group. Membrane lipids displaying a relatively small polar head group such as phosphatidylethanolamines allow a more compact lipid assembling due to a reduced steric hindrance (Brown et al., 2002; Rappolt et al., 2004). Furthermore, cholesterol differently interacts with glycerophospholipids as a function of their polar head group. For example, cholesterol exhibits a higher affinity for negatively charged phospholipids compared to their zwitterionic analogues (Sankaram et al., 1990a).
20The existence of phase-separated zones in the lipid bilayers affects also the lateral organization of membrane proteins. Proteins comprising one or several saturated aliphatic chains display a higher tendency to segregate into ordered lipid phases. It is notably the case for glycosyl-phosphatidylinositol (GPI)-anchored proteins (Brown et al., 1992; Schroeder et al., 1994) and other acylated proteins such as the Src kinases (Shenoy-Scaria et al., 1994).
5. Membrane complexity at the nanometre scale
21The assumption of Singer et al. (1972) that plasma membranes and organelle membranes in eukaryotes are composed of a unique liquid-disordered lamellar phase in which the lipids are randomly distributed and allow the spanning of membrane proteins is not fully correct. Experimental and theoretical data obtained in the last ten years in the field of membrane biophysics are all in favor of the existence of a phase separation in the plane of the membrane.
22At the end of the nineties, it has been postulated that membranes are constituted of small, heterogeneous, and highly dynamic domains which are believed to be enriched in sphingolipids and sterols and to be involved in many biological processes (Brown et al., 1997; Simons et al., 1997; Rietveld et al., 1998). These membrane microdomains, better known as lipid rafts, exhibit the physical properties of a relatively ordered liquid crystalline lamellar phase and coexist within a liquid-disordered environment. They are supposed to be responsible for the lateral distribution of proteins and the concentration of membrane constituents in small compartments facilitating their interaction.
23The existence of lipid rafts in membranes is however still under debate. The raft hypothesis is originally based on the detergent extraction of membrane lipids. As the lipids involved in putative membrane rafts form liquid-ordered (Lo) phases, they present a lower solubility in non-ionic detergents (e.g. Triton X-100 and Brij 58) at low temperature (4°C) than lipids from the surrounding liquid-disordered phase. The use of such an extraction procedure for investigating the presence of lipid rafts in membranes is today questionable. Indeed, the extraction of lipid constituents at very low temperature may affect the lipid organization of the native membrane and induce a lateral aggregation, which would not occur in physiological conditions (de Almeida et al., 2003). Depending on the nature and the concentration of the non-ionic detergent as well as on the extraction parameters (temperature, duration), changes in terms of lipid/protein composition and distribution may also take place within the two leaflets of lipid bilayers (Schuck et al., 2003; Shogomori et al., 2003). In addition, Triton X-100 has been shown to induce the formation of liquid-ordered domains in model membranes by decreasing the proportions of sphingolipids and cholesterol in the liquid-disordered phase (Heerklotz, 2002). This detergent could be also responsible for the fusion of rafts entities in the membrane and the formation of large interconnected membrane aggregates (Giocondi et al., 2000; Simons et al., 2004). As a consequence, it is very unlikely that membrane compartments that cannot be solubilised in non-ionic detergent reflect both the native composition and organization of lipids within membrane rafts (Lichtenberg et al., 2005).
24The fact that putative rafts are thought to be highly dynamic structures makes also their characterization extremely difficult. It has been postulated that lipid rafts exist in biological membranes only if they are small entities with a short lifetime (Subczynski et al., 2003). It is generally accepted that these lipid microdomains display a size distribution (10-200 nm) that is inferior to the resolution of the conventional optical microscopy (Simons et al., 1997; Bagatolli et al., 1999; Jacobson et al., 1999; Simons et al., 2000; Pike, 2003). The size of lipid rafts seems to be influenced by the local lipid composition, the incorporation of external molecules that can act as nucleation sites for the formation of larger membrane domains (Brown et al., 1998b; Radhakrishnan et al., 2000; Anderson et al., 2002), as well as by the protein conformation, which can perturb the lipid assembling within these domains (Heerklotz, 2002). Lipid rafts may also auto-associate within the membrane leaflets to form larger lipid platforms that become detectable by optical microscopy (Subczynski et al., 2003; Simons et al., 2004). The aggregation of these small entities may arise from protein-lipid interaction, protein oligomerisation or from the binding of proteins to specific antibodies at the cell surface (Friedrichson et al., 1998; Harder et al., 1998).
25Alternative theories are nowadays proposed for explaining the submicron lateral heterogeneities in cell plasma membranes. The presence of submicron lipid-rich entities in the plane of biological membranes could arise from dynamic submicron critical fluctuations, inhomogeneous lipid mixing, 2-D microemulsions, or small-scale structure within a single gel (So) phase (Veatch et al., 2005; Honerkamp-Smith et al., 2009). Simple ternary lipid mixtures constituted of a sterol and two other lipid components (one with a high chain melting temperature Tm and one with a low chain melting temperature) are good model systems for investigating the lateral organization in biological membranes as these lipid mixtures phase-separate and form micron-scale liquid domains as a function of temperature (Veatch et al., 2005). By measuring the miscibility transition temperature as a function of the lipid composition, thermodynamic phase diagrams that are specific of the ternary lipid mixtures of interest can be mapped (Goñi et al., 2008). Using the combination of fluorescence microscopy and deuterium NMR, it has been observed that dynamic submicron liquid domains exhibiting a large distribution of sizes, compositions and lifetimes are created in the vicinity of miscibility critical points (Veatch, 2007; Veatch et al., 2007). Critical fluctuations have been also found in giant plasma membrane vesicles, which are spherical vesicles isolated directly from the plasma membranes of living cells, near their transition temperature (Veatch et al., 2008). Such a manifestation of submicron critical fluctuations in model lipid systems could explain some of the nanometre scale membrane heterogeneity attributed to putative lipid rafts in biological membranes (Veatch et al., 2008).
26The coexistence of liquid-ordered and liquid-disordered phases in the plane of the membrane and the lateral distribution of proteins play certainly an important role in many biological processes. It is widely accepted that such phase segregation is involved in the sorting and the transport of both membrane proteins and lipids during endocytosis and exocytosis phenomena, in cascade signalling as well as in other cellular processes such as apoptosis, membrane fusion, cell adhesion and migration (Brown et al., 1998a; Simons et al., 2000).
27It has been assumed that ordered lipid entities may also be preferential attack sites for cellular invasion by pathogens or toxins (van der Goot et al., 2001; Duncan et al., 2002; Manes et al., 2003). They could indeed concentrate cellular receptors that are necessary for the binding of pathogens to the plasma membrane of target cells or for the oligomerisation of toxins favoring by this way their entry in the cell. Ordered lipid domains within the membrane may also provide preferential platforms for the assembling and the budding of viral particles (such as Ebola, influenza, and human immunodeficiency-1 viruses) as well as for the formation of pathological forms of the prion protein and of the β-amyloid peptide, which is associated with Alzheimer’s disease (Campbell et al., 2001; Fantini et al., 2002).
28Nowadays, while there is no doubt about the presence of phase separation between liquid-ordered and liquid-disordered phases in the plane of the membrane (Swamy et al., 2006; Sengupta et al., 2007), additional research involving both cell membranes and biomimetic model membranes is still required to further investigate the nanoscale lateral organization of lipids in both intracellular and extracellular membrane leaflets as well as to better understand the biological functions associated to these phase-separated lipid domains.
6. Biomimetic model membranes
29As biological membranes are very complex systems, many model membranes have been developed over the last century for studying membrane properties, structure and processes as well as for investigating the membrane activity of diverse natural or synthetic compounds such as surfactants, peptides, and drugs. The most well-known and common biomimetic systems used for such purposes are lipid monolayers, lipid vesicles and supported lipid bilayers. While each of these systems exhibits advantages and disadvantages, they all mimic the lipid arrangement of natural cell membranes.
6.1. Lipid monolayers
30Lipid monolayers provide a simple model for mimicking biological membranes and for evaluating membrane insertion of amphipathic compounds (Brockman, 1999; Maget-Dana, 1999). These monomolecular insoluble films, also referred to as Langmuir monolayers, are formed by spreading amphiphilic molecules at the surface of a liquid and can be considered as half the bilayer of biological membranes. These two-dimensional systems display many advantages compared to the other model membranes. Parameters such as the nature and the packing of the spread molecules, the composition of the subphase (pH, ionic strength) and temperature can be varied in a controlled way and without limitation.
31Lipid monolayers are very useful to characterize drug-lipid or lipid-lipid interactions at a molecular level. Such a characterization can be deduced from compression isotherms, which are obtained by measuring the surface pressure (Π) of the interfacial film as a function of the mean molecular area (A) of the compounds spread at an air-water interface (Figure 6). Under compression, a two-dimensional insoluble monolayer adopts different physical states (typically gaseous, liquid expanded, liquid condensed and solid-like states), which are related to the level of conformational order of the molecules at the interface and to the presence of intermolecular interactions within the monolayer.
32During its compression, an insoluble monolayer is also characterized by changes in terms of two-dimensional compressibility (Cs). This parameter corresponds to the slope of the compression isotherm and can be determined for each point of the Π-A curve using the following equation (Equation 1):

33Molecular interactions occurring at an air-water interface between molecules of different nature can be evaluated by performing a simple thermodynamic analysis. From the Π-A isotherm of pure and of mixed monolayers, information about the mixing behavior of spread molecules can be obtained. When the mean molecular area of a mixed monolayer (Am) at a defined surface pressure corresponds to the relative sum of the molecular areas of the separated components (A1 and A2) at the same surface pressure, the mixing behavior is defined as ideal (Equation 2) (Gaines, 1966). In other words, the components are either totally immiscible or ideally miscible at the interface. Any deviation from the ideal behavior can be attributed to specific interactions between the two compounds (Maget-Dana, 1999).

34A more detailed analysis of the thermodynamics of the system, by calculating the excess free energy of mixing ∆ Gex (Equation 3) developed by Goodrich (1957), can provide further information about the miscibility process and the possible specific interactions between the interfacial components.
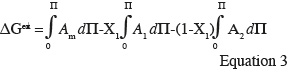
35Positive values of ∆Gex signify that mutual interactions between the two components are weaker than interactions between the pure compounds themselves and suggest phase separation between the components at the interface. Negative values of ∆Gex indicate the presence of strong mutual interactions at the interface and are in favor of complex formation between the monolayer constituents (Maget-Dana, 1999).
36Lipid monolayers are also excellent model membranes for evaluating the insertion of amphipathic compounds such as antimicrobial peptides, biosurfactants and drugs into the membrane of target cells (Maget-Dana, 1999). For this specific purpose, an insoluble monolayer mimicking the lipid composition of the biological membrane of interest is formed at the air-water interface of a Langmuir trough (Figure 7A). After stabilization of the lipid monolayer at a defined initial surface pressure (Πi), the active compound, solubilised in an appropriate solvent, is injected into the water subphase. At this point, the increase of the surface pressure resulting from the interaction of the active compound with the lipid monolayer is recorded (Figure 7B).
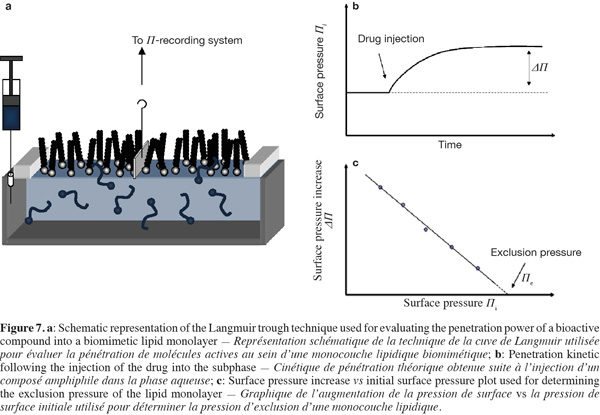
37By plotting the maximum surface pressure increase (ΔΠ) observed as a function of the initial surface pressure of the lipid monolayer, an exclusion surface pressure (Πe) is determined (Figure 7C). This parameter corresponds to the initial surface pressure of the lipid monolayer above which no more active compound can penetrate the lipid film and increase the surface pressure. In other words, this parameter reflects the penetration power of the active compound of interest into a well-defined two-dimensional model membrane.
38In order to visualize the interfacial organization of lipid constituents of a monolayer or the changes in the interfacial behavior resulting from the insertion of a compound of interest into the monolayer, the Langmuir trough technique can be easily combined with fluorescence or Brewster angle microscopy. The latter technique presents the advantage of not using a fluorescent probe that may result in domain instability for highly compressed monolayers (McConlongue et al., 1997). Fluorescence and Brewster angle microscopy offer a lateral resolution in the micrometre range and are thus not suited to visualize the phase properties of lipid monolayers at high resolution. For such purpose, atomic force microscopy (AFM) is an excellent alternative probing technique since it allows the visualization of lipid domains in phase-separated films with a nanometre scale resolution (Dufrêne et al., 1997; Reviakine et al., 2000; Milhiet et al., 2001). However, the use of AFM for imaging monolayers implies the transfer of the interfacial film onto a solid support.
39The most common technique used to transfer a monolayer is the Langmuir-Blodgett (LB) technique (Motschmann et al., 2001). The solid support can be either hydrophilic or hydrophobic. When using a hydrophilic support, the lipid polar heads are facing towards the support, whereas the transfer onto a hydrophobic support is obtained via hydrophobic interactions with the lipid hydrocarbon chains. The transfer of an interfacial film onto a solid support is performed at constant surface pressure and is monitored via the so-called transfer ratio. In order to maintain a constant surface pressure during the transfer process, the interfacial film is continuously compressed resulting in a decrease of the monolayer surface area. The transfer ratio is thus defined as the ratio of the decrease of the monolayer surface area to the area of the solid support which has been covered by the constituents of the interfacial film. A transfer ratio close to one indicates that the deposition process has been successful, i.e. the supported monolayer is representative of the spread monolayer at the air-water interface. However, it has to be kept in mind that, depending on both the nature of the constituents of the interfacial film and the surface pressure at which the monolayer has been transferred onto the solid support, changes in the molecular organization at the support surface may occur.
40The Langmuir trough technique can also be combined with spectroscopic (e.g. polarized infrared spectroscopy), reflection and scattering (e.g. ellipsometry and grazing-incidence X-ray) techniques in order to obtain direct structural information (i.e. conformation and orientation of the monolayer constituents) in phase-separated two-dimensional systems.
6.2. Lipid vesicles
41Lipid vesicles or liposomes are versatile biomimetic model membranes commonly used for studying membrane phase behavior and membrane processes such as membrane fusion, molecular recognition, cell adhesion, and membrane trafficking. These lipid assemblies enclose a small aqueous compartment and are produced from the aqueous dispersion of membrane lipids (single lipid component or mixture of different types of lipids). Whereas lipid monolayers are constituted of only one lipid leaflet and therefore do not reflect the complexity of biological membrane structure, lipid vesicles are composed of two lipid leaflets, which are arranged in a way that is similar to that of biological membranes.
42According to the method of preparation, different types of bilayer structures can be obtained (Gregoriadis, 1991; Mui et al., 2003; Lorin et al., 2004; Uhumwangho et al., 2005). When a dried lipid film is vigorously hydrated at temperatures above the lipid phase transition, multilamellar lipid vesicles (MLV) are formed. These vesicles display a size range of 0.5-10 µm and are characterized by several concentric lipid bilayers, which are separated by water molecules. The size of these MLVs can be reduced and homogenized by performing several freeze-thaw cycles. The extrusion of MLVs through a porous membrane gives rise to the formation of large unilamellar vesicles (LUV) while small unilamellar vesicles (SUV) are formed by sonicating the MLVs in a classical bath-type sonicator or using a probe sonicator. LUVs and SUVs are both characterized by a single lipid bilayer. SUVs usually exhibit a mean diameter inferior to 50 nm whereas the size of LUVs varies from 100 to 500 nm. Giant unilamellar vesicles (GUV) are liposomes having a size range of 5-100 µm. These model membranes can be obtained by hydrating a dried lipid film at temperatures above the lipid phase transition either for a long period of time (up to 36 h) (i.e. gentle hydration method) or in presence of an external electrical field (i.e. electroformation method) (Bagatolli et al., 2000; Rodriguez et al., 2005; Wesolowska et al., 2009). The size of these giant vesicles allows their visualization by optical microscopy such as fluorescence or confocal microscopy, as well as the micromanipulation of individual vesicles. Although these techniques have a lower lateral resolution than AFM, they allow the investigation of molecular interactions with lipid vesicles in a bulk solution whereas AFM requires the fusion of lipid vesicles onto a solid support.
43The main disadvantages of using lipid vesicles as biomimetic model membranes are that the lipid asymmetry found in native biological membranes cannot be mimicked and that the final lipid composition of the vesicles may be relatively different from the initial lipid mixture used for vesicle formation. As demonstrated by phase diagrams of complex lipid mixtures, small differences in composition may strongly affect the phase behavior of lipid systems (Goñi et al., 2008). Consequently, an appropriate control of the final lipid composition needs to be performed before using the model for studying membrane properties and/or processes. In the case of GUVs, it has been demonstrated that the lipid composition of the vesicles is closer to the one of the initial lipid mixture when preparing the vesicles via the gentle hydration method rather than via the electroformation method (Rodriguez et al., 2005). However, the former technique is responsible for a higher percentage of defects in GUVs, which are lipid structures bound to the inner or outer lipid leaflet or encapsulated inside the lipid vesicles (Rodriguez et al., 2005). Consequently, depending on the application of the GUVs, one of the two preparation techniques will be preferred.
44It is worth to note that, as lipid vesicles are usually formed from dilute lamellar dispersions with the input of mechanical (e.g. sonication or extrusion), chemical (e.g. change of solubility conditions, incorporation of external compounds) or electrochemical (e.g. change of pH, ionic strength) energy, they are metastable structures offering poor long-term stability (Lasic, 1990; Madani et al., 1990; Marques, 2000). This means that, upon aging, vesicle dispersion may aggregate (clustering formation), fuse or evolve to the thermodynamically stable two-phase region (consisting of a lamellar phase dispersed in large excess of solvent) from which they were formed. However, depending on the composition and the size of lipid vesicles as well as on the environmental parameters (temperature, pH, ionic strength, presence of external molecules and ions), these thermodynamically nonstable systems can be stable for prolonged periods of time (up to several months) and are then suitable model membranes for investigating membrane properties and biological processes. In particular, they are very interesting model systems for studying cell adhesion and membrane fusion phenomena which are mediated by non-covalent protein-protein and protein-carbohydrate interactions (Voskuhl et al., 2009).
45In addition to be relevant biomimetic model membranes for investigating membrane properties, structure and processes, lipid vesicles have been proved to be suitable transport vehicles for drugs, proteins, enzymes, or DNA. The applications of these lipid systems are abundant in particular in pharmacology and in dermato-cosmetology where these lipid assemblies are used as drug delivery systems and allow the prediction of pharmacokinetic properties of drugs such as their transport, their distribution, their accumulation, and hence their efficacy (El Maghraby et al., 2008; Peetla et al., 2009).
6.3. Supported lipid bilayers
46Supported lipid bilayers (SLBs) are biomimetic model membranes constituted of a flat lipid bilayer supported onto a solid surface such as mica, glass or silicon oxide wafers. In such model system, the polar head groups of the first lipid monolayer are facing towards the support while the hydrocarbon chains of this lipid monolayer are in contact with the lipid chains of the second monolayer. SLBs offer many advantages over lipid vesicles (Loose et al., 2009). These model membranes can be prepared quite easily and are much more stable than lipid vesicles. Besides, both the overall composition and the lipid asymmetry of SLBs can be controlled while it is not the case when using vesicular model systems. In addition, as these membrane assemblies are confined to the surface of a solid support, they can be characterized much easier than free-floating vesicles using a large variety of surface sensitive techniques such as AFM (Lin et al., 2007; Mingeot-Leclercq et al., 2008; Goksu et al., 2009), secondary ion mass spectrometry (SIMS) (Chan et al., 2007), fluorescence microscopy (Crane et al., 2007), optical ellipsometry (Puu et al., 1997), quartz-crystal microbalance (Keller et al., 1998), X-ray reflectivity (Miller et al., 2006) and neutron reflectivity (Vacklin et al., 2007).
47Different techniques are commonly used to prepare SLBs. The first one is the LB technique. After the transfer of a lipid monolayer spread at the air-water interface of a Langmuir trough onto a solid support, the same support is immersed a second time through the interface in order to obtain a supported lipid bilayer (Figure 8A).
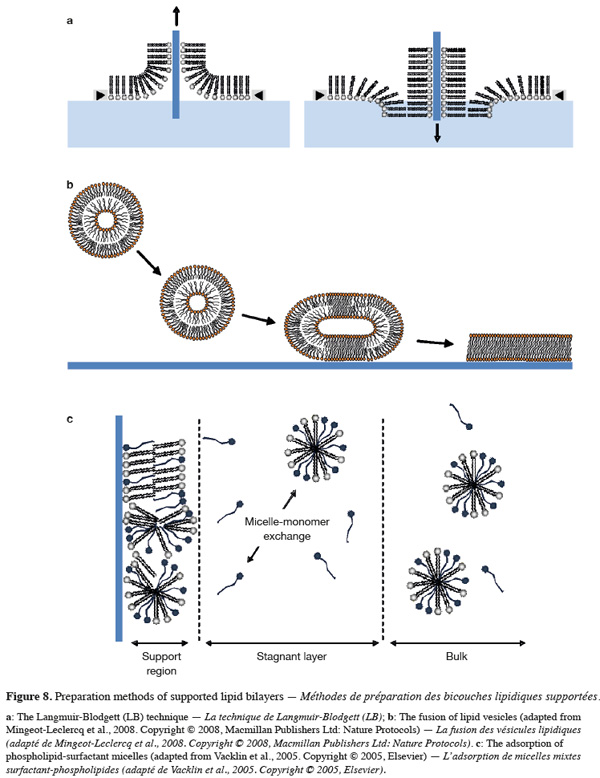
48A second method for preparing SLBs is the fusion of lipid vesicles onto a solid support. This method is relatively simple and can be completed in few hours. The detailed protocol to achieve the fusion of lipid vesicles has been recently reviewed in the literature (Mingeot-Leclercq et al., 2008). Briefly, the fusion is obtained by heating a SUV suspension in contact with the support at temperatures above the lipid phase transition. The fusion process is not yet fully understood but involves the adsorption of the lipid vesicles on the surface, followed by their deformation, their flattening and their rupture. The fusion of the edges of the bilayer patches through hydrophobic interactions gives rise in final to a continuous supported lipid bilayer (Figure 8B) (Jass et al., 2000: Reviakine et al., 2000; Richter et al., 2005; Anderson et al., 2009). As SLBs prepared from the fusion of lipid vesicles requires temperatures above the lipid phase transition, this technique is not appropriate when temperature-sensitive membrane components such as proteins has to be incorporated in SLBs. However, it is currently the most frequently used method for preparing SLBs.
49Supported model membranes can be also obtained from micellar solutions composed of a mixture of surfactants and phospholipids (Tiberg et al., 2000; Vacklin et al., 2005; Lee et al., 2009). In this method, the surfactant (e.g. non-ionic β-D-dodecyl maltoside) is used as a lipid solubilising agent and acts as a transporter to drive the water-insoluble lipid to the surface. When mixed surfactant-phospholipid micelles adsorb at the solid surface, the concentration of mixed micelles at the vicinity of the surface (i.e. within the stagnant layer) is reduced compared to their concentration in the bulk solution (Figure 8C). The formation of phospholipid-enriched supported bilayers is favored by the solubility difference between the phospholipid and the surfactant, and is obtained by repetitively rinsing the adsorbed layer with bulk solutions of decreasing micelle concentration. In doing so, the more soluble surfactant monomers adsorbed to the surface are progressively solubilised while both the solid support and the mixed micelles are gradually enriched in the less soluble component. After each addition of more diluted bulk solutions, a rinsing step is usually performed to remove any excess of the soluble surfactant. As phospholipids exhibit very low water solubility, the progressive solubilisation of the surfactant from the solid support is responsible for the formation of a pure phospholipid bilayer.
50Since their development two decades ago as biomimetic model membranes (Tamm et al., 1985), supported lipid bilayers have been largely used by the biophysical community to predict the phase behavior and the molecular organization of biological membranes. Moreover, over the past ten years, it has been demonstrated that these supported lipid bilayers are also highly relevant model membranes for investigating the molecular interactions of drugs with cell membranes. An overview of the applications of supported lipid bilayers as well as of other biomimetic model membranes for investigating the pharmacokinetic properties of drugs has been recently reported by Peetla et al. (2009).
51One of the main drawbacks of using classical supported lipid bilayers is that the proximity between the lipid bilayer and the solid substrate may affect the membrane properties of the biomimetic system, such as the mobility of membrane components or the incorporation of transmembrane proteins. In order to solve such proximity problem, tethered lipid bilayers made up of a lipid bilayer spaced from the solid surface by spacer molecules or layers have been developed. The different strategies currently available for separating a lipid bilayer from a solid support have been critically reviewed by Rossi et al. (2007). The formation of these tethered bilayers is usually achieved via the addition of a polymer film or the self-assembling of chemically modified lipids on the solid surface, or via the direct fusion of spacer lipids containing vesicles on functionalized surfaces, and involves the LB technique, the fusion of lipid vesicles, or the combination of both techniques.
52New applications of such tethered supported lipid bilayers are constantly discovered (Rossi et al., 2007). As these biomimetic systems allow protein incorporation in non-denaturing conditions, they are very suitable model membranes for investigating membrane-protein interactions in a functional manner. The reconstitution of membrane receptors in such lipid bilayers opens also doors for the design of specific sensors that could be used in a very near future for a variety of medical applications for example as pharmaceutical screening.
7. Limitations of biomimetic model membranes
53Over the last century, model membranes have been proved to play a considerable role in the elucidation of the structure and the properties of biological membranes as well as in the understanding of biological processes that occur at the membrane surface or that are associated with cell membranes.
54However, it has to be kept in mind that these model systems have some limitations as they do not capture the whole complexity of biological membranes. While the simplification of the membrane system is crucial for the analysis of specific molecular interactions at the membrane level, it can also be an obstacle to the accurate understanding of some membrane functions.
55Some of the main limitations associated with the use of model membranes have been highlighted recently by Vestergaard et al. (2008) and are presented in this paper.
56The number of components that can be incorporated in a model system is relatively limited mainly due to experimental constraints and to the capabilities of analysis of the currently available technologies. For example, most of biophysical studies based on model membranes only involve up to three or four different lipid species, while biological membranes enclose more than thousand different lipids (van Meer, 2005).
57Another limitation of these model systems lies on the fact that it is relatively challenging to reconstitute proteins in model membranes (Chan et al., 2007). Consequently, proteins are much less considered in membrane research, while these membrane constituents affect also the membrane structure and contribute to membrane properties and functions.
58Up to now, the remarkable lipid asymmetry between the two leaflets of biological membranes has not been fully achieved in model systems, whereas it is known to play various functional roles in plasma membranes (Manno et al., 2002). Furthermore, model membranes do not contain cytoskeletal components which strongly participate to the lipid and protein diffusion across the cell surface and consequently to the phase behavior of cell membranes.
59Other limitations are also directly associated with the preparation technique of these model systems. While lipid monolayers can be quite easily prepared from each type of membrane lipids, the preparation technique of lipid vesicles is more complex and selective. For example, it is very challenging to reconstitute membrane lipids with a high chain melting temperature (such as ceramides) into vesicles as the vesicle formation requires an aqueous dispersion of lipids at temperatures above their phase transition temperature.
8. Concluding remarks
60To conclude it is worth to note that this article does not have the pretention to present the last developments in membrane research resulting from the use of biomimetic model membranes.
61While also revisiting the fundamental properties of biological membranes in terms of membrane composition, membrane dynamic and molecular organization at the nanometre scale, this article provides a general and consistent description of the main model membranes used in membrane research.
62This article should be then considered as a first reference document for scientists who desire to be initiated into the fascinating world of biological membranes. Afterwards, the reader is referred to more specialized articles and reviews for more in-depth information related to the different topics discussed in the present paper.
Bibliographie
Ahmed S.N., Brown D.A. & London E., 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry, 36, 10944-10953.
Almeida P.F., Vaz W.L. & Thompson T.E., 1993. Percolation and diffusion in three-component lipid bilayers: effect of cholesterol on an equimolar mixture of two phosphatidylcholines. Biophys. J., 64, 399-412.
Anderson R.G.W. & Jacobson K., 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science, 296, 1821-1825.
Anderson T.H. et al., 2009. Formation of supported bilayers on silica substrates. Langmuir, 25, 6997-7005.
Bagatolli L.A. & Gratton E., 1999. Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys. J., 77, 2090-2101.
Bagatolli L.A., Parasassi T. & Gratton E., 2000. Giant phospholipid vesicles: comparison among the whole lipid sample characteristics using different preparation methods. A two photon fluorescence microscopy study. Chem. Phys. Lipids, 105, 135-147.
Brennan P.J., Griffin P.F.S., Losel D.M. & Tyrrell D., 1974. The lipids of fungi. Prog. Chem. Fats Other Lipids, 14, 49-89.
Bretscher M.S., 1973. Membrane structure: some general principles. Science, 181, 622-629.
Brockman H., 1999. Lipid monolayers: why use half a membrane to characterize protein-membrane interactions? Curr. Opin. Struct. Biol., 9, 438-443.
Brown D.A. & Rose J.K., 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell, 68, 533-544.
Brown D.A. & London E., 1997. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun., 240, 1-7.
Brown D.A. & London E., 1998a. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol., 164, 103-114.
Brown D.A. & London E., 1998b. Function of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol., 14, 111-136.
Brown M.F. et al., 2002. Elastic deformation of membrane bilayers probed by deuterium NMR relaxation. J. Am. Chem. Soc., 124, 8471-8484.
Campbell S.M., Crowe S.M. & Mak J., 2001. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J. Clin. Virol., 22, 217-227.
Chan Y.H.M. & Boxer S.G., 2007. Model membrane systems and their applications. Curr. Opin. Chem. Biol., 11, 581-587.
Chernomordik L., 1996. Non-bilayer lipids and biological fusion intermediates. Chem. Phys. Lipids, 81, 203-213.
Crane J.M. & Tamm L.K., 2007. Fluorescence microscopy to study domains in supported lipid bilayers. Methods Mol. Biol., 400, 481-488.
Cullis P.R. & de Kruijff B., 1979. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta, 559, 399-420.
Danielli J.F. & Davson H., 1935. A contribution to the theory of permeability of thin films. J. Cell Comp. Physiol., 5, 495-508.
de Almeida R.F., Fedorov A. & Prieto M., 2003. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J., 85, 2406-2416.
de Vries A.H., Yefimov S., Mark A.E. & Marrink S.J., 2005. Molecular structure of the lecithin ripple phase. Proc. Natl Acad. Sci. USA, 102, 5392-5396.
Dufrêne Y.F., Barger W.R., Green J.-B. D. & Lee G.U., 1997. Nanometer-scale surface properties of mixed phospholipid monolayers and bilayers. Langmuir, 13, 4779-4784.
Duncan M.J., Shin J.S. & Abraham S.N., 2002. Microbial entry through caveolae: variations on a theme. Cell Microbiol., 4, 783-791.
El Maghraby G.M., Barry B.W. & Williams A.C., 2008. Liposomes and skin: from drug delivery to model membranes. Eur. J. Pharm. Sci., 34, 203-222.
Fantini J., Garmy N., Mahfoud R. & Yahi N., 2002. Lipid rafts: structure, function and role in HIV, Alzheimer’s and prion diseases. Exp. Rev. Mol. Med., http://www.expertreviews.org/02005392h.htm, (20/12/02).
Filippov A., Orädd G. & Lindblom G., 2003. The effect of cholesterol on the lateral diffusion of phospholipids in oriented bilayers. Biophys. J., 84, 3079-3086.
Friedrichson T. & Kurzchalia T.V., 1998. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature, 394, 802-805.
Gaines G.L.Jr., 1966. Insoluble monolayers at liquid-gas interfaces. New York, USA: Wiley.
Gally H.U., Seelig A. & Seelig J., 1976. Cholesterol-induced rod-like motion of fatty acyl chains in lipid bilayers: a deuterium magnetic resonance study. Hopp-Seyler's Z. Physiol. Chem., 357, 1447-1450.
Giocondi M.C. et al., 2000. In situ imaging of detergent-resistant membranes by atomic force microscopy. J. Struct. Biol., 131, 38-43.
Goksu E.I. et al., 2009. AFM for structure and dynamics of biomembranes. Biochim. Biophys. Acta, 1788, 254-266.
Goñi F.M. et al., 2008. Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim. Biophys. Acta, 1781, 665-684.
Goodrich F.C., 1957. Molecular interaction in mixed monolayers. In: Schulman J.H., ed. Proceedings of the 2nd International Congress of Surface Activity. London: Butterworth.
Gorter E. & Grendel F., 1925. On bimolecular layers of lipoids on the chromocytes of the blood. J. Exp. Med., 41, 439-443.
Gregoriadis G., 1991. Overview of liposomes. J. Antimicrob. Chemother., 28, 39-48.
Gurtovenko A.A. & Vattulainen I., 2007. Lipid transmembrane asymmetry and intrinsic membrane potential: two sides of the same coin. J. Am. Chem. Soc., 129, 5358-5359.
Gurtovenko A.A. & Vattulainen I., 2008. Membrane potential and electrostatics of phospholipid bilayers with asymmetric transmembrane distribution of anionic lipids. J. Phys. Chem. B, 112, 4629-4634.
Gurtovenko A.A. & Vattulainen I., 2009. Intrinsic potential of cell membranes: opposite effects of lipid transmembrane asymmetry and asymmetric salt ion distribution. J. Phys. Chem. B, 113, 7194-7198.
Harder T., Scheiffele P., Verkade P. & Simons K., 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell. Biol., 141, 929-942.
Heerklotz H., 2002. Triton promotes domain formation in lipid raft mixtures. Biophys. J., 83, 2693-2701.
Heimburg T., 2000. A model for the lipid pretransition: coupling of ripple formation with the chain-melting transition. Biophys. J., 78, 1154-1165.
Heimburg T., 2007. Thermal biophysics of membranes. Weinheim, Germany: Wiley.
Helenius A. & Simons K., 1975. Solubilization of membranes by detergents. Biochim. Biophys. Acta, 415, 29-79.
Henriksen J. et al., 2006. Universal behaviour of membranes with sterols. Biophys. J., 90, 1639-1649.
Honerkamp-Smith A.R., Veatch S.L. & Keller S.L., 2009. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta, 1788, 53-63.
Ipsen J.H. et al., 1987. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta, 905, 162-172.
Jacobson K. & Dietrich C., 1999. Looking at lipid rafts. Trends Cell Biol., 9, 87-91.
Janiak M.J., Small D.M. & Shipley G.G., 1979. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J. Biol. Chem., 254, 6068-6078.
Jass J., Tjämhage T. & Puu G., 2000. From liposomes to supported, planar bilayer structures on hydrophilic and hydrophobic surfaces: an atomic force microscopy study. Biophys. J., 79, 3153-3163.
Keller C.A. & Kasemo B., 1998. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J., 75, 1397-1402.
Lasic D.D., 1990. On the thermodynamic stability of liposomes. J. Colloid Interface Sci., 140, 302-304.
Lee C., Wacklin H. & Bain C.D., 2009. Changes in molecular composition and packing during lipid membrane reconstitution from phospholipid–surfactant micelles. Soft Matter, 5, 568-575.
Lichtenberg D., Robson R.J. & Dennis E.A., 1983. Solubilization of phospholipids by detergents: structural and kinetic aspects. Biochim. Biophys. Acta, 737, 285-304.
Lichtenberg D., Goñi F.M. & Heerklotz H., 2005. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci., 30, 430-436.
Lin W.C., Blanchette C.D., Ratto T.V. & Longo M.L., 2007. Lipid domains in supported lipid bilayer for atomic force microscopy. Methods Mol. Biol., 400, 503-513.
Loose M. & Schwille P., 2009. Biomimetic membrane systems to study cellular organization. J. Struct. Biol., 168, 143-151.
Lorin A., Flore C., Thomas A. & Brasseur R., 2004. Les liposomes : description, fabrication et applications. Biotechnol. Agron. Soc. Environ., 8, 163-176.
Madani H. & Kaler E.W., 1990. Aging and stability of vesicular dispersions. Langmuir, 6, 125-132.
Maget-Dana R., 1999. The monolayer technique: a potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim. Biophys. Acta, 1462, 109-140.
Manes S., del Real G. & Martinez A.C., 2003. Pathogens: raft hijackers. Nat. Rev. Immunol., 3, 557-568.
Manno S., Takakuwa Y. & Mohandas N., 2002. Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl Acad. Sci. USA, 99, 1943-1948.
Marques E.F., 2000. Size and stability of catanionic vesicles: effects of formation path, sonication, and aging. Langmuir, 16, 4798-4807.
McConlongue C.W. & Vanderlick T.K., 1997. A close look at domain formation in DPPC monolayers. Langmuir, 13, 7158-7164.
McElhaney R.N. & Tourtellotte M.E., 1971. The relationship between fatty acid structure and the positional distribution of esterified fatty acids in phosphatidylglycerol from Mycoplasma laidlawii B. Biochim. Biophys. Acta, 202, 120-128.
McIntosh T.J., 1980. Difference in hydrocarbon chain tilt between hydrated phosphatidylethanolamine and phosphatidylcholine bilayers. A molecular packing model. Biophys. J., 29, 237-246.
McLaughlin S., 1989. The electrostatic properties of membranes. Annu. Rev. Biophys. Biophys. Chem., 18, 113-136.
Milhiet P.E. et al., 2001. Domain formation in models of the renal brush border membrane outer leaflet. Biophys. J., 81, 547-555.
Miller C.E., Majewski J. & Kuhl T.L., 2006. Characterization of single biological membranes at the solid-liquid interface by X-ray reflectivity. Colloid Surf. A, 284-285, 434-439.
Mingeot-Leclercq M.-P., Deleu M., Brasseur R. & Dufrêne Y.F., 2008. Atomic force microscopy of supported lipid bilayers. Nat. Protoc., 3, 1654-1659.
Motschmann H. & Möhwald H., 2001. Langmuir-Blodgett films. In: Holmberg K., ed. Handbook of applied surface and colloid chemistry. New York, USA: Wiley-VCH.
Mui B., Chow L. & Hope M.J., 2003. Extrusion technique to generate liposomes of defined size. Methods Enzymol., 367, 3-14.
Muller P. & Herrmann A., 2002. Rapid transbilayer movement of spin-labeled steroids in human erythrocytes and in liposomes. Biophys. J., 82, 1418-1428.
Op den Kamp J.A.F., 1979. Lipid asymmetry in membranes. Annu. Rev. Biochem., 48, 47-71.
Peetla C., Stine A. & Labhasetwar V., 2009. Biophysical interactions with model lipid membranes: applications in drug discovery and drug delivery. Mol. Pharmaceutics, 6, 1264-1276.
Pike L.J., 2003. Lipid rafts: bringing order to chaos. J. Lipid Res., 44, 655-667.
Puu G. & Gustafson I., 1997. Planar lipid bilayers on solid supports from liposomes - factors of importance for kinetics and stability. Biochim. Biophys. Acta, 1327, 149-161.
Radhakrishnan A., Anderson T.G. & McConnell H.M., 2000. Condensed complexes, rafts, and the chemical activity of cholesterol in membranes. Proc. Natl Acad. Sci. USA, 97, 12422-12427.
Ramstedt B. & Slotte J.P., 2002. Membrane properties of sphingomyelins. FEBS Lett., 531, 33-37.
Rappolt M., Laggner P. & Pabst G., 2004. Structure and elasticity of phospholipid bilayers in the Lα phase: a comparison of phosphatiylcholine and phosphatidylethanolamine membranes. Recent Res. Dev. Biophys., 3, 363-394.
Reviakine I. & Brisson A., 2000. Formation of supported phospholipid bilayers from unilamellar vesicles investigated by atomic force microscopy. Langmuir, 16, 1806-1815.
Richter R.P. & Brisson A.R., 2005. Following the formation of supported lipid bilayers on mica: a study combining AFM, QCM-D, and ellipsometry. Biophys. J., 88, 3422-3433.
Rietveld A. & Simons K., 1998. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim. Biophys. Acta, 1376, 467-479.
Rock P. et al., 1990. Organization of glycosphingolipids in phosphatidylcholine bilayers: use of antibody molecules and Fab fragments as morphologic markers. Biochemistry, 29, 8484-8490.
Rodriguez N., Pincet F. & Cribier S., 2005. Giant vesicles formed by gentle hydration and electroformation: a comparison by fluorescence microscopy. Colloid Surf. B, 42, 125-130.
Rossi C. & Chopineau J., 2007. Biomimetic tethered lipid membranes designed for membrane-protein interaction studies. Eur. Biophys. J., 36, 955-965.
Rothman J.E. & Lenard J., 1977. Membrane asymmetry. Science, 195, 743-753.
Sankaram M.B. & Thompson T.E., 1990a. Interaction of cholesterol with various glycerophospholipides and sphingomyelin. Biochemistry, 29, 10670-10675.
Sankaram M.B. & Thompson T.E., 1990b. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry, 29, 10676-10684.
Sankaram M.B. & Thompson T.E., 1991. Cholesterol-induced fluid-phase immiscibility in membranes. Proc. Natl Acad. Sci. USA, 88, 8686-8690.
Schroeder R., London E. & Brown D.A., 1994. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI-) anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl Acad. Sci. USA, 91, 12130-12134.
Schuck S. et al., 2003. Resistance of cell membranes to different detergents. Proc. Natl Acad. Sci. USA, 100, 5795-5800.
Sengupta P., Holowka D. & Baird B., 2007. Fluorescence resonance energy transfer between lipid probes detects nanoscopic heterogeneity in the plasma membrane of live cells. Biophys. J., 92, 3564-3574.
Shenoy-Scaria A.M. et al., 1994. Cysteine3 of Src family protein tyrosine kinases determines palmitoylation and localization in caveolae. J. Cell Biol., 126, 353-364.
Shogomori H. & Brown D.A., 2003. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol. Chem., 384, 1259-1263.
Simons K. & Ikonen E., 1997. Functional rafts in cell membranes. Nature, 397, 569-572.
Simons K. & Toomre D., 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol., 1, 31-41.
Simons K. & Vaz W.L.C., 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct., 33, 269-295.
Singer S.J. & Nicholson G.L., 1972. The fluid mosaic model of the structure of cell membranes. Science, 175, 720-731.
Steck T.L., Ye J. & Lange Y., 2002. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys. J., 83, 2118-2125.
Subczynski W.K. & Kusumi A., 2003. Dynamics of raft molecules in the cell and artificial membranes: approaches by pulse EPR spin labelling and single molecule optical microscopy. Biochim. Biophys. Acta, 1610, 231-243.
Swamy M.J. et al., 2006. Coexisting domains in the plasma membrane of live cells characterized by spin-label ESR spectroscopy. Biophys. J., 90, 4452-4465.
Tamm L. & McConnell H., 1985. Supported phospholipid bilayers. Biophys. J., 47, 105-113.
Tanford C., 1980. The hydrophobic effect: formation of micelles and biological membranes. New York, USA: John Wiley & Sons.
Tardieu A., Luzzati V. & Reman F.C., 1973. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J. Mol. Biol., 75, 711-733.
Tiberg F., Harwigsson I. & Malmsten M., 2000. Formation of model lipid bilayers at the silica-water interface by co-adsorption with non-ionic dodecyl maltoside surfactant. Eur. Biophys. J. Biophys. Lett., 29, 196-203.
Uhumwangho M.U. & Okor R.S., 2005. Current trends in the production and biomedical applications of liposomes: a review. J. Med. Biomed. Res., 4, 9-21.
Vacklin H.P., Tiberg F. & Thomas R.K., 2005. Formation of supported phospholipid bilayers via co-adsorption with β-D-dodecyl maltoside. Biochim. Biophys. Acta, 1668, 17-24.
Vacklin H.P. & Thomas R.K., 2007. Spontaneous formation of asymmetric lipid bilayers by adsorption of vesicles. Langmuir, 23, 7644-7651.
Van der Goot F.G. & Harder T., 2001. Raft membrane domains: from a liquid-ordered membrane phase to a site of pathogen attack. Semin. Immunol., 13, 89-97.
van Meer G., 2005. Cellular lipidomics. EMBO J., 24, 3159-3165.
Veatch S.L., 2007. From small fluctuations to large-scale phase separation: lateral organization in model membranes containing cholesterol. Semin. Cell Dev. Biol., 18, 573-582.
Veatch S.L. & Keller S.L., 2005. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta, 1746, 172-185.
Veatch S.L., Soubias O., Keller S.L. & Gawrisch K., 2007. Critical fluctuations in domain-forming lipid mixtures. Proc. Natl Acad. Sci. USA, 104, 17650-17655.
Veatch S.L. et al., 2008. Critical fluctuations in plasma membrane vesicles. ACS Chem. Biol., 3, 287-293.
Vestergaard M., Hamada T. & Takagi M., 2008. Using model membranes for the study of amyloid beta:lipid interactions and neurotoxicity. Biotechnol. Bioeng., 99, 753-763.
Vist M.E. & Davis J.H., 1990. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry, 29, 451-464.
Voskuhl J. & Ravoo B.J., 2009. Molecular recognition of bilayer vesicles. Chem. Soc. Rev., 38, 495-505.
Wang T.Y., Leventis R. & Silvius J.R., 2000. Fluorescent-based evaluation of the partitioning of lipids and lipidated peptides into liquid-ordered lipid microdomaines: a model for molecular partitioning into lipid rafts. Biophys. J., 79, 919-933.
Wesolowska O., Michalak K., Maniewska J. & Hendrich A.B., 2009. Giant unilamellar vesicles: a perfect tool to visualize phase separation and lipid rafts in model systems. Acta Biochim. Pol., 56, 33-39.
Zimmerberg J. & Chernomordik L., 1999. Membrane fusion. Adv. Drug Delivery Rev., 38, 197-205.
Para citar este artículo
Acerca de: Marc Eeman
Univ. Liege - Gembloux Agro-Bio-Tech. Department of Biological Industrial Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium).
Acerca de: Magali Deleu
Univ. Liege - Gembloux Agro-Bio-Tech. Department of Biological Industrial Chemistry. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: magali.deleu@ulg.ac.be






