- Startpagina tijdschrift
- volume 16 (2012)
- numéro 3
- Present status of the development of mycoherbicides against water hyacinth: successes and challenges. A review
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Present status of the development of mycoherbicides against water hyacinth: successes and challenges. A review

Nota's van de redactie
Received on January 4, 2011; accepted on March 20, 2012
Résumé
État d’avancement de la recherche de mycoherbicides contre la jacinthe d’eau : succès et défis (synthèse bibliographique). Le développement des bioherbicides dans la gestion de l’invasion de la jacinthe d’eau, Eichhornia crassipes (Martius) Solms Laubach, vise à trouver une alternative à l’utilisation des herbicides de synthèse. En effet, les résidus d’herbicide peuvent avoir un impact négatif non seulement sur la santé humaine, mais également sur l'écosystème. En outre, l'une des principales stratégies de cette lutte biologique au moyen des mycoherbicides est de l’intégrer en tant que composante essentielle du plan de gestion intégrée des adventices aquatiques. Ceci dans l’objectif d’obtenir un résultat satisfaisant de contrôle de la prolifération de la jacinthe d’eau tout en réduisant au minimum possible l'application des herbicides. Plusieurs pathogènes fongiques précurseurs de mycoherbicides (Sclerotinia sclerotiorum dans Hyakill et ABG-5003 à base de Cercospora rodmanii) ont été identifiés sur des plants malades de la jacinthe d’eau, même si de nos jours aucun mycoherbicide n’est commercialement disponible. Les contraintes biologiques, technologiques et commerciales en sont la cause. La recherche ayant trouvé la réponse à bon nombre de ces contraintes, il est cependant urgent de mieux comprendre les aspects biochimiques et physiologiques de la pathogenèse des champignons contenus dans ces bioherbicides. Les émulsions d’huiles seraient un moyen important de formulation pour accroître l'efficacité des mycoherbicides.
Abstract
Recent trends in the implementation of bioherbicide use in the control of water hyacinth (Eichhornia crassipes [Martius] Solms Laubach) have depended primarily on several strategies. The use of bioherbicides has been stimulated as part of the search for alternatives to chemical control, as the use of these more environmentally-friendly formulations minimizes hazards resulting from herbicide residue to both human and animal health, and to the ecology. In addition, one of the major strategies in the concept of biological control is the attempt to incorporate biological weed control methods as a component of integrated weed management, in order to achieve satisfactory results while reducing herbicide application to a minimum. Several fungal pathogens with mycoherbicide potential (Sclerotinia sclerotiorum in Hyakill and Cercospora rodmanii, named ABG-5003) have been discovered on diseased water hyacinth plants, but none has become commercially available in the market. Biological, technological, and commercial constraints have hindered progress in this area. Many of these constraints are being addressed, but there is a critical need to better understand the biochemical and physiological data regarding the pathogenesis of these new bioherbicides. Oil emulsions are recognized as a way to increase both efficiency of application and efficacy of biocontrol agents.
Inhoudstafel
1. Introduction
1Water hyacinth (Eichhornia crassipes [Martius] Solms Laubach) from the Pontederiaceae family is one of the world’s most important aquatic weeds. This noxious weed spreads by vegetative reproduction. Its high growth rate and ability to infest a wide range of freshwater habitats have created many ecological problems (Gopal, 1987): hyacinth infestations can reduce the availability of water for irrigation, aquaculture, potable water and navigation infrastructure, as well as obstruct drainage canals worldwide.
2Several millions of US dollars are spent annually to control hyacinth infestation in the USA. From 1980 to 1991, Florida spent more than $43 million US on countering the problem and since then, water hyacinth management has cost that state $3 million each year. In addition, control of this weed costs the California State $500,000 US annually (Mullin et al., 2000). Introduction of water hyacinth into the USA is prohibited (US-EPA, 1988).
3It is difficult to quantify the economic impact of water hyacinth infestation worldwide, particularly in developing countries. In Africa, its spread has caused widespread problems to millions of water bodies and water resource users, and the problem is especially severe in Mali and Egypt, where human activities and livelihoods are closely linked to the water systems. Dagno (2006) reported that mechanical management of the weed costs $80,000–100,000 US per year in Mali. Fayad et al. (2001) demonstrated that 487 km2 of irrigation canals and 151 km2 of lakes were covered by hyacinth plants in many areas of Egypt; this infestation was causing a loss of 3.5 x 1,012 m3 of water per year, corresponding to the quantity of water sufficient to irrigate approximately 432 km2 per year.
4Methods for controlling water hyacinth include conventional control (manual or mechanical removal) and chemical control, but both approaches have proven generally inadequate and very expensive to apply in areas of high infestation. Herbicides have the added disadvantage of causing possible adverse environmental effects, and thus they must be applied carefully and selectively (Bateman, 2001).
5Biological control appears to be an efficient alternative method to control hyacinth in a sustainable management system. The use of bioherbicides to control weeds has gained major prominence (Senthilkumar, 2007). Bioherbicides mainly use endemic pathogens that are destructive to the weed and they are usually applied in massive doses at vulnerable stages of host growth (El-Morsy et al., 2006). There is, however, current interest in the use of plant pathogenic microbes for the biological control of weeds. Inert solid carriers, alginate granules, invert emulsions and oil-in-water emulsions have been considered as vehicles for mycoherbicides, as they reduce or eliminate the dew requirement for fungal colonization.
6A survey of pathogenic fungi on water hyacinth infesting rivers and other water bodies has allowed identification of many pathogens as potential mycoherbicides (Charudattan, 2001; Dagno, 2006). Some pathogens have been shown to induce significant biomass reductions in hyacinth plants (50 to 100%) when used as bioherbicides (Charudattan, 1986; Shabana et al., 1995; El-Morsy, 2004). Several reasons can be considered for using fungi as biocontrol agents (BCAs). This review describes the main biocontrol approaches in sustainable management systems and highlights the challenges involved in developing mycoherbicides for use against water hyacinth.
2. Biocontrol strategy for weeds
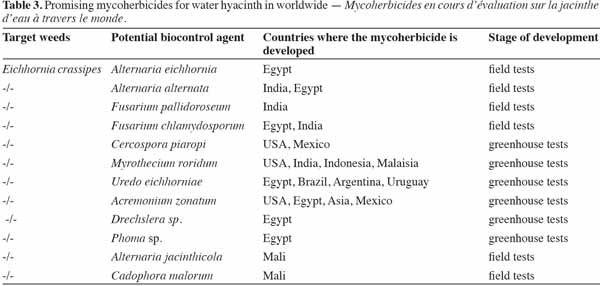
7Weed biocontrol strategies are based on the use of natural enemies to suppress the growth of a weed or to reduce its population (Center et al., 1992; Cruttwell, 2000; Charudattan, 2005). There are two basic strategies for implementing the biological control of weeds by pathogens: the introduction of foreign pathogenic organisms (often called the classical approach), and augmentative bioherbicide strategies, where the pathogenic organisms are already present (native or introduced) and their population is increased by mass rearing (El-Sayed, 2005). In epidemiological terms, these approaches are also often described as the inoculative and the inundative strategy, respectively (Hasan et al., 1990).
8The inoculative, or classical, approach implies the control of invasive weeds by introducing a few populations of control organisms from the weed’s natural habitat. These pathogens are released in only a small part of the total infested area and control is achieved by the gradual spread of the initial population (El-Sayed, 2005).
9The classical method presents some disadvantages. Among these are firstly, the high initial costs; secondly, the fact that the number of natural enemies for each target weed species is limited and thirdly, the fact that the dissemination of a BCA cannot be controlled after its release in nature. In addition, successful weed control strongly depends on favorable conditions promoting an effective increase in the BCA population, and the establishment of epiphytotics to reduce the target weed population (El-Sayed, 2005).
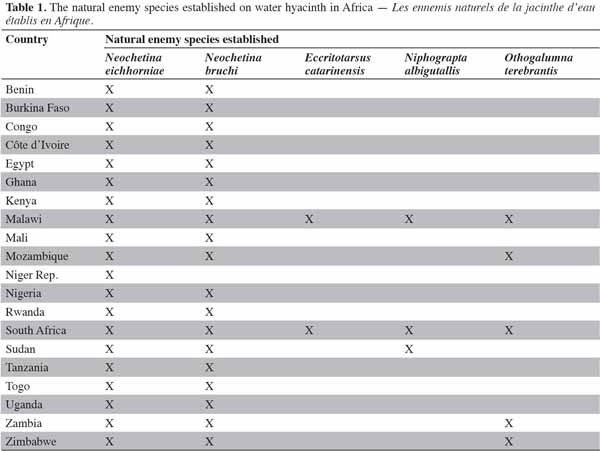
10Several insects have been used in weed management through the inoculative strategy worldwide. Table 1 shows the insects used in the biological control of water hyacinth in Africa (Cilliers et al., 2002).
11Inundative, or bioherbicide, strategies use the periodic release of an overabundant supply of the controlling organism to suppress the entire weed population. Such pathogens or BCAs are generally “manufactured”, formulated, standardized, packed and registered in the same way as chemical herbicides (Auld et al., 1995). One group of such BCAs with promising potential for weed control is mycoherbicides. The use of the inundative approach may offer several advantages in the control of weeds. Indeed, the plant pathogen is mass-produced and applied at a high dose to the target weed in much the same way as a chemical herbicide. Finally, the large number of biological control agents used is intended to immediately suppress the target population.
12This review focuses on the development of a mycoherbicide rather than an inoculative control. A mycoherbicide is preferable because introducing a fungus pathogen which does not exist in a new area after the weed has already become established, as in the inoculative approach, is dangerous compared to a local indigenous fungus multiplied and slackened in the region (Charudattan, 2005).
3. Bioherbicides registered up to 2011
13There is a long history of research on microbial control agents, and it is not always appreciated that obtaining an active isolate is only the beginning of a series of activities necessary for implementing the use of a new mycoherbicide (O’Connell et al., 1996). There are important issues to consider, including: mass production, delivery systems and “laboratory to field” studies, strategies for use, registration and commercialization (Bateman, 2001).
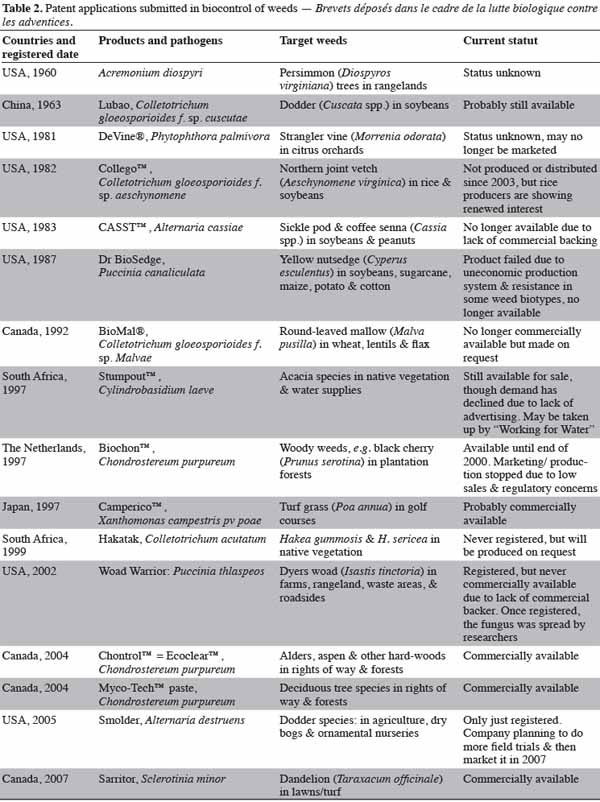
14The number of research reports on bioherbicide research has increased tremendously since the early 1980s. Both the number of weeds targeted for control and the number of candidate pathogens studied has increased. Practical registered or unregistered uses of bioherbicides have also increased worldwide. Likewise, the numbers of US patents issued for the bioherbicidal use of fungi and their technology have increased, perhaps foretelling an increased reliance on bioherbicides in the future (El-Sayed, 2005). Table 2 shows the registered bioherbicides and their current status throughout the world (Landcard Research, 2008).
4. Factors influencing bioherbicide efficacy
15The efficacy of a bioherbicide depends on the establishment of the disease during the primary infection by the formulation and also on the complete control of the weed via its secondary infection.
4.1. Primary infection
16The importance of environmental conditions such as dew and temperature on primary infection has been demonstrated by Walker et al. (1983) and Auld (1993). Environmental conditions vary from field to field and from year to year. Differences in primary infection between years and locations have been observed during experiments with Colletotrichum gloesporioides (Penz) f.sp. malvae (BioMal, Philom Bios) and Alternaria alternata (Babu et al., 2003). Even for Collego, which is used in free moisture, low levels of initial infections were recorded in some years.
17However, low initial infections due to a varying effect of environmental parameters may be increased as a result of the high capacity for dispersal and secondary infections by these pathogens for control efficacy (Boyette et al., 1979; Walker et al., 1983).
4.2. Secondary infection
18Numerous mycoherbicide studies indicate the importance of secondary infection and subsequent dispersal for effective control, both requiring time. Boyette et al. (1979) reported that anthracnose disease caused by Colletotrichum gloeosporioides f.sp. jussiaea, which has an incubation period of 3-5 days, required 28 days to progress from 29% (primary infection) to 94% on the winged water primrose in a rice field. In the same field experiment, the dispersal of fungal disease was evident, because 25% of the plants in untreated plots were infected, even at a distance of 100 m from inoculated plants.
19Some authors have also reported the importance of secondary infection in reaching a high disease level in the weed population. According to Hassan et al. (1990), Stagonospora sp. required three weeks after spraying to develop severe disease symptoms on Calystegia sepium (L.). El Morsy (2004) reported that A. alternata, a potential mycoherbicide for water hyacinth in an aquatic environment, required two months to achieve lethal levels, although the incubation period required to observe first symptoms was 12 days. In Brazil, Helminthosporium sp. required 36 days post-application to defoliate 73% of inoculated wild poinsettia (Euphorbia heterophylla L.) plants in soybean fields. Field studies by Charudattan (1986) and Shabana (1997) have clearly demonstrated that diseases caused by fungal pathogens progressed from 5% to 90% plant mortality in five weeks and two months, respectively, after spraying. The important role of secondary infection in control efficacy has also been demonstrated for commercial mycoherbicides. Experiments with the mycoherbicide BioMal on Malva pusilla L. showed that, at high inoculum concentrations, the level of control increased from 30-50% at 22 days after application to about 90% at crop harvest. Dispersal of inoculum was evident from the severe disease levels in the control plots. Another mycoherbicide, Collego, required up to five weeks for killing Aeschynomene virginica L. (Boyette et al., 1979).
5. Promising bioherbicides
20Only two mycoherbicides have been developed to control water hyacinth with the intention of becoming commercialized. The first mycoherbicide was registered by the US-EPA (United States Environmental Protection Agency) under the patent US4097261 in the USA (Freeman et al., 1984). This product contains Cercospora rodmanii, which is a fungal specific pathogen for Eichhornia crassipes (TeBeest, 1991). Abbott Laboratories in the USA have developed an experimental formulation of C. rodmanii, named ABG-5003, for use against E. crassipes (Praveena et al., 2004).
21The second mycoherbicide that has been developed is called Hyakill, which contains Sclerotinia sclerotiorum. This mycoherbicide was submitted to the European Patent Office in 2003 (de Jong et al., 2003). Sclerotinia sclerotiorum is not a pathogen specific to water hyacinth; it has been recognized as a plant pathogenic fungus on several crops (bean, sunflower, carrot and other dicotyledonous plant families). This disadvantage may be the reason why Hyakill has not obtained commercial authorization use from the European Patent Office.
22Many research groups have identified promising microbial agents that might be used as biopesticides against water hyacinth (Table 3). Fungal species presented in table 2 are evaluated for their host specificity, biocontrol efficacy and formulation efficiency (Babu et al., 2003; El-Morsy et al., 2006). Of these fungal species, Alternaria eichhorniae and Alternaria alternata have been extensively studied for the biocontrol of water hyacinth.
5.1. Alternaria eichhorniae
23Alternaria eichhorniae was reported on water hyacinth in 1984 in Egypt and it appears to be specific to this aquatic weed (Shabana et al., 1995). The fungus has also been discovered on water hyacinth in Australia, Bangladesh, Indonesia, and South Africa (Dagno, 2006). Alternaria eichhorniae is currently being developed as a mycoherbicide for controlling water hyacinth in Egypt (Shabana, 1997). The symptoms of disease are discrete necrotic foliar spots (oblong, 2-4 mm long) surrounded by a bright yellow halo.
24Shabana (1997) reported that fresher mycelial inoculum (4 weeks old) was more virulent than older inoculum (9 or 16 weeks old). In 2005, Shabana showed the biocontrol efficacy of A. eichhorniae in vegetable oil emulsions under low relative humidity in greenhouse trials. Indeed, inoculum concentrations above 10% of mycelium were all found to be equally effective in controlling water hyacinth at the 100% level (weed kill). Alternaria eichhorniae formulated in cottonseed oil emulsion caused 100% control of water hyacinth 7–13 weeks after application in the field (Shabana, 2005).
25However, Babu et al. (2002) reported that Spinacea oleracea, Cucumis sativus, Cucurbita pepo, Helianthus annuus, Ricinus communis, Daucus carota, Allium cepa, Raphanus sativus, Phaseolus vulgaris, Ficus carica and Lycopersicon esculentum were susceptible to A. eichhorniae. This factor of crop susceptibility is unfavorable for the large-scale use of this mycoherbicide.
26Two other fungal species have shown effective control of the development of water hyacinth in a greenhouse: Alternaria jacinthicola, strain MUCL 53159 and Cadophora malorum, strain Mln715. These pathogens have been shown to cause 87 and 93% of leaf diseases, respectively, and to result in plant death six weeks after application (Dagno et al., 2011b).
5.2. Alternaria alternata
27Alternaria alternata is a cosmopolitan fungus, which has been isolated from almost all habitats (Dagno et al., 2011b). The fungus has been described as a pathogen of water hyacinth in Bangladesh, Australia, Egypt and India (Shabana et al., 1995; El-Morsy, 2004; El-Morsy et al., 2006). This fungal species induces spots and lesions, mainly on leaves, and less severely on stolons, and it ultimately leads to complete plant death (Babu et al., 2003).
28El-Morsy (2004) showed that the formulation of spores in an oil emulsion (10% oil in water) enhanced the efficacy of A. alternata in controlling water hyacinth plants. The necrotic leaf area of inoculated plants increased as did the length of exposure to 100% relative humidity (RH). Inoculation of plants with 1 × 106 spores·ml-1 in oil emulsion caused 79% plant tissue death. Water hyacinth was found to be susceptible to the fungus at all stages of growth tested; however, older plants were more susceptible than younger ones. The toxin produced by A. alternata is known to play an important role in the pathogenesis of water hyacinth blight disease (Babu et al., 2003)
29The most probable limitation for mycoherbicides based on the A. alternata model is that the fungus is associated with several leaf blight diseases of cotton and other economic crops (e.g., bean, sunflower, rice, cucumber and peanut) (Bashan et al., 1991).
5.3. Alternaria jacinthicola
30From 2006 to 2007, a survey of fungal species present on water hyacinth was conducted in Mali (Dagno et al., 2011a). Alternaria jacinthicola was isolated from diseased plants. This pathogen, described for the first time in Mali, is specific to the water hyacinth. Indeed, it induces no disease symptoms after inoculation on tomato, rice, sorghum, carrot, onion, pepper or okra crops. Thus A. jacinthicola has emerged as a promising potential biocontrol agent for water hyacinth. The vegetable oil formulation of this pathogen induces leaf blight symptoms on water hyacinth (Dagno et al., 2011b).
5.4. Cadophora malorum
31Cadophora malorum, Mln715 strain was described for the first time as a pathogen on water hyacinth in Mali (Dagno, 2006). The fungus is recognized as being responsible for leaf disease of wheat and rice crops. It is also a fruit rot agent of apples and pears in storage.
32Cadophora malorum, Mln715 strain requires a high relative humidity for its development; a water activity of 0.880 stops growth (Dagno et al., 2011a). This requirement would be a major constraint for its application in the field.
6. Challenges in bioherbicide development for use against water hyacinth
33A number of challenges are encountered in the formulation of promising BCAs isolated from water hyacinth. It is necessary to include good market potential, ease of production and application, adequate product stability and shelf life during transportation as well as in storage, in order to ensure propagule viability and efficacy over the long term (Boyetchko et al., 1999). Some reasons why BCAs have met with limited commercial success are: difficulty of production, sensitivity to UV light and desiccation, a requirement for high humidity for infection, insufficient performance over a wide range of environmental conditions, and lack of appropriate formulation (El-Sayed, 2005).
34Formulation is recognized as a way to increase both efficiency of application and efficacy of the BCA (Evans et al., 2001). Oil emulsion formulations, in particular, may reduce the dew requirement of fungi (Shabana, 1997; El-Morsy et al., 2006) and the number of spores required to ensure BCA efficacy (Egley et al., 1995). Formulations need to be used to improve product stability, bioactivity, and delivery (i.e., the ability to mix and spray the product) as well as to integrate the biopesticide into a pest management system (Charudattan, 2001). Other important characteristics of a successful formulation are convenience of use, compatibility with end-user equipment and practices, and effectiveness at rates consistent with agricultural practices (Boyetchko et al., 1999).
35For foliar BCAs, such as A. eichhorniae and A. alternata, some environmental factors that influence plant infection and disease development are: temperature, free moisture or dew period, and protection against UV irradiation and desiccation (Shabana, 2005; El-Morsy et al., 2006). The inclusion of novel synergists into bioherbicide formulations could take these BCAs past the point of research, and into the development of efficacious, reliable, and economical products for the marketplace (Bateman, 2001). All of these parameters need to be taken into account in developing an appropriate mycoherbicide for the control of water hyacinth, such as in the production and formulation of fungal mycoherbicides.
7. Conclusion
36Several fungal candidates exist for the control of water hyacinth infestation, and preliminary research into the biological characterization of these fungi has been conducted for several decades. The literature is replete with reviews on the subject. Despite all of this research and expense poured into development of BCAs, few such products have been successful and fewer still have persisted in the marketplace. Several BCAs have failed, and often for one of a number of common reasons: production problems, lack of adequate shelf life of formulations under warehouse temperatures, lack of an economically viable delivery system, or loss of virulence of the product before reaching the target. Therefore, there is a critical need to better understand the mode of action of bioherbicides involved in host-pathogen interaction, an interaction that consequently leads to an enhanced virulence of the pathogen and/or suppresses the defense reactions of the water hyacinth plants.
37There are two major epidemiological components contributing to the control of bioherbicidal efficacy: a window of temperature and moisture affecting the number of initial infections and the subsequent dispersal and infection of the pathogen within the target weed population. Currently, most research involving bioherbicides does not address the importance of secondary infection. This may be because, conceptually, bioherbicides have been considered as chemical herbicides. The environmental dependency of BCAs limits their control efficacy in variable environments. Consequently, environmental conditions play a fundamental role in guiding the mode of action of bioherbicides. In addition, bioherbicides require several complex and often specific interactions between fungus and water hyacinth. This complexity of interactions is one explanation for the unpredictability and inconsistency often associated with bioherbicides, which has restricted the commercial use of bioherbicides to irrigated systems and to application via aerial spray. The greatest challenge for major promising biocontrol fungi (A. eichhorniae and A. alternata) in water hyacinth is their dependence on ecological factors. A number of challenges need to be overcome in achieving the right formulation of a BCA. The product needs to have good market potential, ease of production and application, adequate product stability and shelf life both during transportation and in storage, and guaranteed propagule viability and efficacy over the long term. Oil formulations may resolve the problem of the dependence of BCAs on environmental moisture.
38Acknowledgements
39This research was financially and technically supported by Belgian Technical Cooperation (BTC) and Phytopathology Unit of Gembloux Agro-Bio Tech.
Bibliographie
Auld B.A., 1993. Vegetable oil suspension emulsions reduce dew dependence of a mycoherbicide. Crop Prot., 12, 477-479.
Auld B.A. & Morin L., 1995. Constraints in the development of bioherbicides. Weed Technol., 9, 638-652.
Babu R.M. et al., 2002. Host range of Alternaria alternata, a potential fungal biocontrol agent for water hyacinth in India. Crop Prot., 21, 1083-1085.
Babu R.M., Sajeena A. & Seetharaman K., 2003. Bioassay of the potentiality of Alternaria alternata (Fr.) Keissler as a bioherbicide to control water hyacinth and other aquatic weeds. Crop Prot., 22, 1005-1013.
Bashan Y., Levanony H. & Or R., 1991. Wind dispersal of Alternaria alternata, a cause of leaf blight of cotton. J. Phytopathol., 133, 225-238.
Bateman R., 2001. IMPECCA: an international, collaborative program to investigate the development of a mycoherbicide for use against water hyacinth in Africa. In: Julien M.H, Hill M.P., Center T.D. & Jianqing D., eds. Biological and integrated control of water hyacinth, Eichhornia crassipes. Proceedings of the 2nd Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, 9-12 October 2000, Beijing, China. ACIAR Proceedings 102. Canberra: ACIAR.
Boyetchko S.M., Pedersen E., Punja Z.K. & Reddy M.S., 1999. Formulations of biopesticides. In: Hall F.R. & Barry J.W., eds. Biopesticides: use and delivery. Methods in Biotechnology, 5. Totowa, N.J., USA: Humana Press, 487-508.
Boyette C.D., Templeton G.E. & Smith Jr. R.J., 1979. Control of winged water primrose (Jussiaea decurrens) and Northern jointvetch (Aeschynomene virginica) with fungal pathogens. Weed Sci., 27, 497-501.
Center T.D. & Dray F.A., 1992. Associations between water hyacinth weevils Neochetina eichhorniae and N. bruchi and phenological stages of Eichhornia crassipes in Southern Florida. Florida Entomol., 75, 196-211.
Charudattan R., 1986. Cercospora rodmannii: a biological control agent for water hyacinth. Aquatics, 8, 21-24.
Charudattan R., 2001. Biological control of weeds by means of plant pathogens: significance for integrated weed management in modern agro-ecology. Biocontrol, 46, 229-260.
Charudattan R., 2005. Ecological, practical and political inputs into selection of weed targets: what makes a good biological control target? Biol. Control, 35, 183-196.
Cilliers C.J., Hill M.P., Ogwang J.A. & Ajuonu O., 2002. Aquatic weeds in Africa and their control. In: Neuenschwander P., Borgemeister C. & Langewald J., eds. Biological control in IPM systems in Africa. Wallingford, UK: CABI.
Cruttwell M.R.E., 2000. Successes in biological control of weeds. In: Spencer N.R., ed. Proceedings of the 10th International Symposium on Biological Control of Weeds, 4-14 July 1999, Montana State University, Bozeman, Montana, USA, 3-14.
Dagno K., 2006. Évaluation des micro-organismes fongiques en tant qu’agents de lutte biologique contre Eichhornia crassipes (Martius) Solms-Laubach dans le bassin du fleuve Niger au Mali. Mémoire : Gembloux Agricultural University-FUSAGx (Belgique).
Dagno K., Lahlali R., Diourté M. & Jijakli M.H., 2011a. Effect of temperature and water activity on spore germination and mycelial growth of three fungal biocontrol agents against water hyacinth (Eichhornia crassipes). J. Appl. Microbiol., 110, 521-528.
Dagno K., Lahlali R., Diourté M. & Jijakli M.H., 2011b. Production and oil-emulsion formulation of Cadophora malorum and Alternaria jacinthicola, two biocontrol agents against water hyacinth (Eichhornia crassipes). Afr. J. Microbiol. Res., 5, 924-929.
de Jong M.D. & de Voogd W.B., 2003. Novel mycoherbicides for biological control of aquatic weeds such as Water Hyacinth and Water Lettuce©: Patent Disclosure. Ann. Plant Prot. Sci., 14, 1.
Egley G.H. & Boyette C.D., 1995. Water-corn-oil emulsion enhances conidia germination and mycoherbicidal activity of Colletotrichum truncatum. Weed Sci., 43, 312-317.
El-Morsy E.M., 2004. Evaluation of microfungi for the biological control of water hyacinth in Egypt. Fungal Diversity, 16, 35-51.
El-Morsy E.M., Dohlob S.M. & Hyde K.D., 2006. Diversity of Alternaria alternata a common destructive pathogen of Eichhornia crassipes in Egypt and its potential use in biological control. Fungal Diversity, 23, 139-158.
El-Sayed W., 2005. Biological control of weeds with pathogens: current status and future trends. Z. Pflanzenkrankh. Pflanzenschutz, 112, 209-221.
Evans H.C. & Reeder R.H., 2001. Fungi associated with Eichhornia crassipes (water hyacinth) in the upper Amazon basin and prospects for their use in biological control. Ascot, UK: CABI Bioscience.
Fayad Y.H., Ibrahim A.A., El-Zoghbyet A.A. & Shalaby F.F., 2001. Ongoing activities in the biological control of water hyacinth in Egypt. In: Julien M.H., Hill M.P., Center T.D. & Jianqing D., eds. Biological and integrated control of water hyacinth, Eichhornia crassipes. ACIAR Proceedings 102. Canberra: ACIAR.
Freeman T.E. & Charudattan R., 1984. Cercospora rodmanii Conway, a potential biocontrol agent for water hyacinth. Florida Agricultural Experimental Station Technical Bulletin No. 842. Gainesville, FL, USA: University of Florida, Institute of Food and Agricultural Sciences.
Gopal B., 1987. Water hyacinth. Amsterdam, The Netherlands: Elsevier.
Hasan S. & Ayres P.G., 1990. The control of weeds through fungi: principles and prospects. New Phytol., 115, 201-222.
Landcard Research, 2008. Inundative control using bioherbicides. TeWhakapau Taru New Zealand BASICS, Nov.
Mullin B.H. et al., 2000. Invasive plant species. Ames, Iowa: Council for Agricultural Science and Technology, Issue Paper No. 13.
O’Connell P.J. & Zoschke A., 1996. Limitations to the development and commercialisation of mycoherbicides by industry. In: Proceedings of the 2nd International Weed Control Congress, 27-28 June 1996, Copenhagen, Denmark, 1189-1195.
Praveena R. & Naseema A., 2004. Fungi occurring on water hyacinth (Eichhornia crassipes (Mart.) Solms) in Kerela. J. Trop. Agric., 42, 21-23.
Senthilkumar N., 2007. Biological control of insect pests and diseases of forestry importance. Curr. Sci., 92, 167.
Shabana Y.M., 1997. Formulation of Alternaria eichhorniae, a mycoherbicide for water hyacinth, in invert emulsions averts dew dependence. J. Plant Dis. Prot., 104, 231-238.
Shabana Y.M., 2005. The use of oil emulsions for improvising the efficacy of Alternaria eichhorniae as a mycoherbicide for water hyacinth (Eichhornia crassipes). Biol. Control, 32, 78-89.
Shabana Y.M., Charudattan R. & Elwakil M.A., 1995. First record of Alternaria eichhorniae and Alternaria alternata on water hyacinth in Egypt. Plant Dis., 79, 319.
TeBeest D.O., 1991. Microbial control of weeds. New York, USA: Chapman and Hall.
US-EPA, 1988. Design manual-constructed wetlands and aquatic systems for municipal wastewater treatment. U.S. Environmental Protection Agency. Report No. EPA/625/1-88/022. Cincinnati, OH, USA: Office of Research and Development.
Walker H.L. & Connick W.J.Jr., 1983. Sodium alginate for production and formulation of mycoherbicides. Weed Sci., 31, 333-338.
Om dit artikel te citeren:
Over : Karim Dagno
Univ. Liege - Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium). E-mail: karimdagno@yahoo.fr – Programme Sorgho. Centre Régional de Recherche Agronomique de Sotuba. BP 262. Bamako (Mali).
Over : Rachid Lahlali
Agriculture and Agri-Food Canada. Saskatoon Research Centre. 107 Science Place. S7N 0X2 Saskatoon, Saskatchewan (Canada).
Over : Mamourou Diourté
Programme Sorgho. Centre Régional de Recherche Agronomique de Sotuba. BP 262. Bamako (Mali).
Over : M. Haïssam Jijakli
Univ. Liege - Gembloux Agro-Bio Tech. Plant Pathology Unit. Passage des Déportés, 2. B-5030 Gembloux (Belgium).






