1 Introduction
In recent years, conductive inks have received significant interest due to their application and popularity in printed electronics (PE) devices [1]. Printed electronics have a number of attractive attributes, such as lower costs, light weights, optical transparency, compatibility with flexible substrates and large scale production of devices on variety of different flexible substrates [2,3]. PE technology plays a key role in enabling extensive flexible and stretchable electronic devices. Nanomaterials including metal nanowires (NWs), [4,5] metal nanoparticles (NPs) [6,7], 2D nano materials (e.g., graphene [8,9], MXene [10,11]) and carbon (e.g., carbon nanotubes (CNTs) [12,13] are promising suitable candidates for conductive PEs. In recent years, much advancements on PE technologies based on nanomaterials, conductive ink generation, post printing processes such as sintering of printed tracks, and functional device integration have been reported [2]. Conductive patterns have been successfully applied to flexible substrates, paper and polymer, using conductive inks [2,14,15]. Nanomaterial-based conductive inks, made up of nanoscale pieces of conductive material in a liquid medium, are one option for PE devices. These nanomaterial inks are liquid at room temperature allowing them to be easily handled, processed, and deposited. Nanomaterial inks are commonly used in PE technology, using conductive nanomaterials such as silver (Ag) NPs [16], Ag NWs [17], copper (Cu) NPs [18], and CNTs [19, 20]. In recent years, the global conductive inks market has grown significantly due to their application in flexible PEs. Most commonly used functional conductive ink in the flexible PEs is Ag. Even in the oxide state, the Ag material conducts well and therefore the technological risk is low for this materials usage. If the conductivity of the ink is high, only less amount of ink is required for PE device production.
Nanoparticle production techniques are categorized as physical and chemical methods. Chemical reduction by inorganic and organic reducing agents or additives is the commonly used chemical method for NP production. Chemical technique use reducing agents to generate ions and atoms from the chosen precursor whereas, physical method normally uses laser impulse energy to reduce chosen bulk material into ions and atoms. The most important physical techniques are laser ablation and evaporation condensation. Pulsed Laser Ablation in Liquid (PLAL), sometimes given the name Laser Ablation Synthesis in Solution (LASIS), is a physical method of producing nanomaterials in solution. In this method, a pulsed laser is focused on the surface of a solid target in a liquid medium. Since the use of pulsed laser for generating metal nano colloidal solutions in different solvents by ablating metal target surfaces that immerse in the chosen solvent in 1993 by Henglein [21] and Cotton [22], the PLAL method has been extensively used to produce polymer [23], carbon [24,25], semiconductor (e.g., ZnO [26], Si [27], and SiC [28]) and metal (e.g., Au [29], Cu [30], and Ag [31]) NPs in liquid. In this technique laser energy absorbed by the target generates a plasma, which expands and condenses in the liquid environment, forming nanoscale pieces of material as the cavitation bubble expands and collapses. This method can produce ligand-free NPs, and generally doesn't require chemical agents beyond the solvent medium, making the method ecofriendly. The morphology of the NPs produced can be controlled by altering the process parameters such as solvent type, laser power, wavelength, and repetition rate. Using this PLAL technique, nanomaterials can be produced with high purity under normal pressure and temperature conditions. The NP size, their stability in liquid, and NP productivity can easily be controlled in this simple technique by changing the experimental parameters such as laser energy, laser wavelength, repetition rate, solvent etc. [32]. The physicochemical characteristics of nano colloidal solutions strongly affects the rate of NP formation, their particle size and shape and their polydispersity [33]. In the past decades, PLAL method has been widely studied for generating nanomaterials in colloidal solutions as this leads to the production of cost effective PE conductive inks.
Most of the nano colloidal generation via PLAL is static, i.e., the laser ablation of the surface of the chosen target performs within a container of liquid [24,25]. Although this static technique is simple, main limitation of this method is its low productivity which inhibit its acceptance in manufacturing industry [34]. In PE application the NP productivity plays a key role as the quality and conductivity of the printed track depends on the concentration and sizes of NPs in the colloids. Therefore, the present paper focuses on the laser ablation of Ag and Cu in deionized (DI) water in a dynamic recirculation production mode aiming to produce nano colloids with high efficiency for application such as inkjet printing. Here in this technique, the laser ablation occurs on the surface of the target material that immersed in a dynamic liquid. The influence of the process duration on the particle size distribution and production rate was investigated for both Ag and Cu materials. The average particle size distribution for the produced Ag and Cu NP colloids are in the range suitable for inkjet printing applications.
2 Experiment
Nano-colloids were generated using a picosecond, Nd:YAG laser (WEDGE HF 1064, BrightSolutions, Italy) by irradiating laser energy on the Ag and Cu target surfaces. The laser beam power was 1.44 W. The target materials used were Ag and Cu (99.99+% purity, sourced from Goodfellow Cambridge Ltd). The laser beam was rastered using a 2D scanning galvanometer (Raylase SS-12) at variable linear speeds moving in an Archimedean spiral pattern across the surface of the chosen target. A recirculation production mode set up with 100 ml/min liquid flow rate was used for nano colloids production. A flow-cell which is made using a Stratasys Connex1 3D printer with dual material print with VeroWhitePlus (RGD835) photopolymer (65 shore D) was used for keeping substrates and to produce nano-colloids, in DI water, in the recirculation NP production mode (Fig. 1).
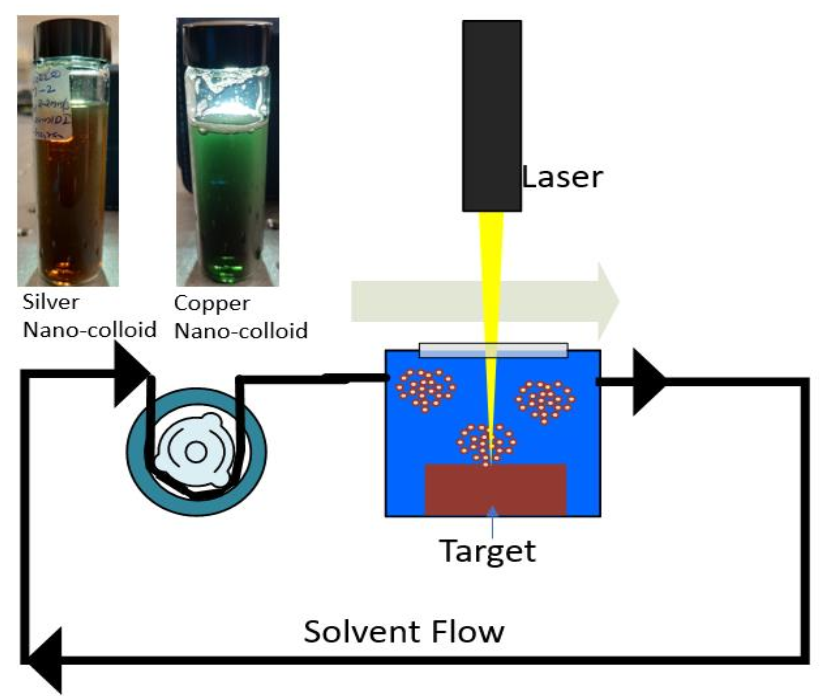 Fig. 1 Schematic of the recirculation production mode for the generation of NP colloids with the images of generated Ag and Cu NP colloids.
Fig. 1 Schematic of the recirculation production mode for the generation of NP colloids with the images of generated Ag and Cu NP colloids.
In this production method, 20 ml of DI water recirculate through a closed-loop piping system and the target material that immersed in a flow cell, for a particular set time. The produced nano colloids were characterized using Dynamic Light Scattering, DLS (NANO-flex® 180° DLS Size, Microtrac Ltd.), and UV-Vis spectroscopy (Libra S22 UV-Vis Spectrophotometer (Biochrom Inc., USA). The NPs productivity in DI water is estimated by evaluating the loss of Ag or Cu target mass before and after the laser ablation.
3 Results and Discussions
3.1 Production of Silver (Ag) Nano colloids
Ag NP solutions were produced by laser ablation of a solid target in DI water by varying target ablation time. Figure 2 (a) shows the UV-Vis absorption spectra of the obtained colloidal solutions. All absorption spectra show the peaks in the ~ 400 nm wavelength region. The location of this peak indicates metallic Ag NPs have been produced [35].The optical properties of Ag NPs are dependent highly on the NP diameter. Nano colloids consist of smaller particle size distribution absorb the incident light and exhibits absorption peaks around 400 nm wavelength region. However, the colloids consist of larger particle size distribution creates light scattering and form broad absorption peak that shift towards the longer wavelength region, is known as, red shifting. The optical properties of Ag NPs change when NPs aggregates. When this happens, the UV-Vis spectra shows shift of the surface plasmon resonance to lower energies leading the scattering and absorption peaks to red shift. The characterization method can be used as reliable and simple technique for recording the NP stability in solution. The intensity of the original peak will decrease with the depletion of the stable NPs and as a result either broad or secondary peaks formation occurs at longer wavelength region. UV/Visible spectroscopy can be used to characterize NP stability in a colloid over time.
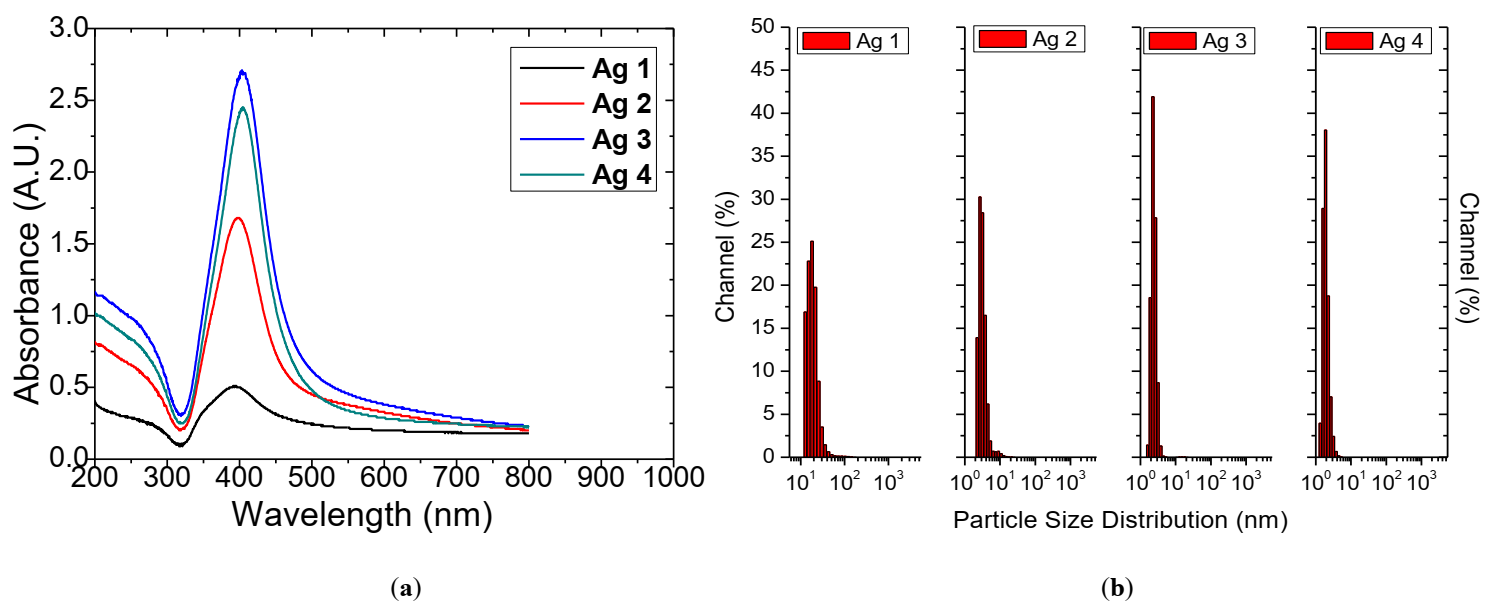 Fig. 2 (a) UV-Vis absorbance vs. wavelength (b) particle size distribution from DLS technique for the Ag NP colloids produced in the dynamic flow PLAL system with 100 ml/min liquid flowrate.
Fig. 2 (a) UV-Vis absorbance vs. wavelength (b) particle size distribution from DLS technique for the Ag NP colloids produced in the dynamic flow PLAL system with 100 ml/min liquid flowrate.
NP size distribution in colloidal solution can be determined by evaluating the random changes in the light intensity that scattered from the colloidal solution. For analyzing NPs after the production DLS technique is commonly used. In suspension, the smaller NPs undergo thermal random motion, which is called Brownian motion. This Brownian movement is modeled by the Stokes-Einstein Eq. As,
where, Dh = hydrodynamic diameter of NPs, η =dynamic viscosity, Dt = translational diffusion coefficient, kB = Boltzman’s constant and T= thermodynamic temperature. Here, temperature is an important parameter consideration due to the presence of the term viscosity as the viscosity is a stiff function of temperature. Most importantly, it explains the particle size analyses from the DLS is the hydrodynamic size.
The DLS NP size distribution plots of the generated Ag NP colloids are shown in Fig. 2 (b). The colloidal solutions exhibit singular peak distribution with the particle size diameter between 2.04 nm to 19.3 nm.
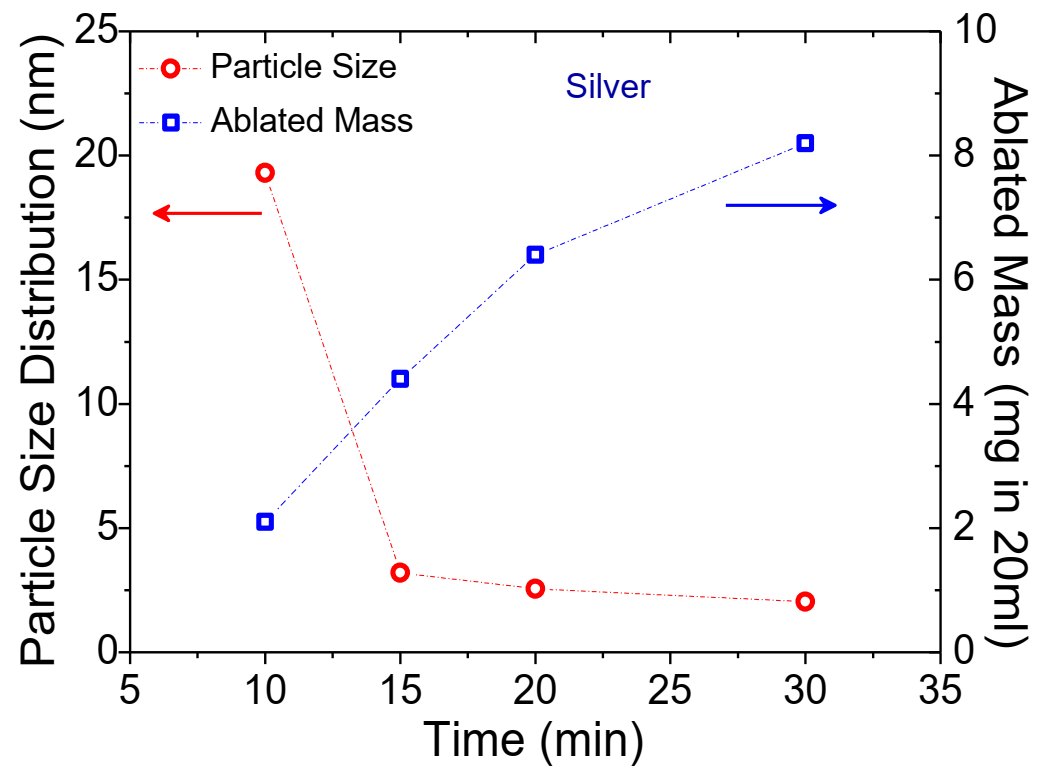 Fig. 3 Average particle size distribution and ablated mass for the Ag NP colloids produced in the dynamic flow PLAL system.
Fig. 3 Average particle size distribution and ablated mass for the Ag NP colloids produced in the dynamic flow PLAL system.
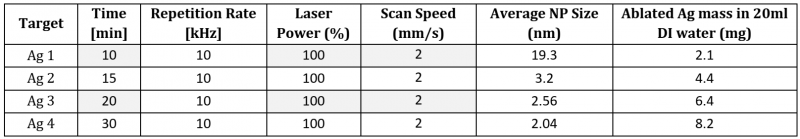
With an increase in the duration of laser ablation (Fig. 3), the yield of ablated Ag mass increases from 2.1 mg (10 min) to 8.2 mg (30 min) in 20 ml DI water. However, average particle size distribution shows a decreasing trend from 19. 3 nm (10 min) to 2.04 nm (30 min). Table 1 list the details of the fixed and varied parameters used for Ag PLAL with the outcomes of ablated mass and the average particle size distribution.
Table 1. Details of PLAL parameters, NP average size distribution, and ablated mass of Cu NPs in 20 ml DI water.
3.2 Production of Copper (Cu) Nano colloids
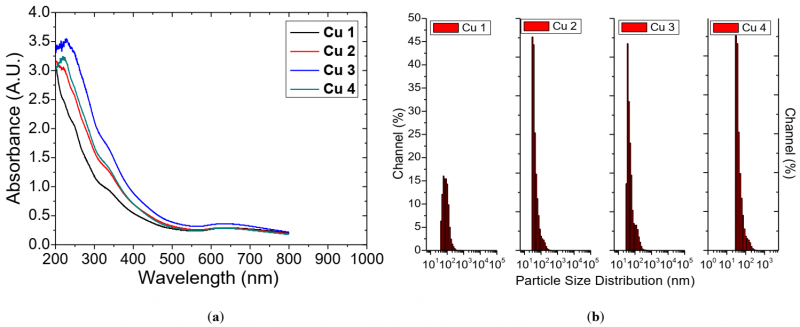
The colloidal solutions of Cu NPs have been generated in DI water (see Fig. 1). The formation of Cu NPs in DI water directly detected due to the colour change of the solvent. The obtained colloidal solutions exhibit green color which indicate the formation oxidized Cu. The UV-VIS absorbance spectra of the obtained Cu NP solutions are shown in Fig. 4 (a). The absorption peaks are observed due to the surface plasmonic resonance of the Cu2O and CuO NPs. The absorption peaks observed between 200 nm -300 nm is due to the formation of CuO NPs and the peaks between 300 nm-400 nm corresponds to the Cu2O Nps.
Fig. 4 (a) UV-Vis absorbance vs. wavelength (b) particle size distribution from DLS technique for the Cu NP colloids produced in the dynamic flow PLAL system with 100 ml/min liquid flowrate.
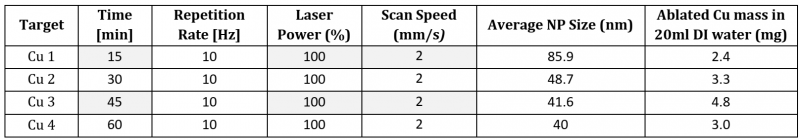
The DLS NP size distribution plots of the generated Cu NP colloids are shown in Fig. 4 (b). The colloidal solutions exhibit singular peak distribution with the particle size diameter between 40 nm to 90 nm. With an increase in the duration of laser ablation (Fig. 5), the yield of ablated Cu mass increases from 2.4 mg (15 min) to 4.8 mg (45 min) in 20 ml DI water, but on further increase in the duration of the laser ablation to 60 min shows a reduction in the ablated mass efficiency, i.e., 3.0 mg in 20 ml DI water. However, average Cu particle size distribution shows a decreasing trend from 85.9 nm (15 min) to 40 nm (60 min).
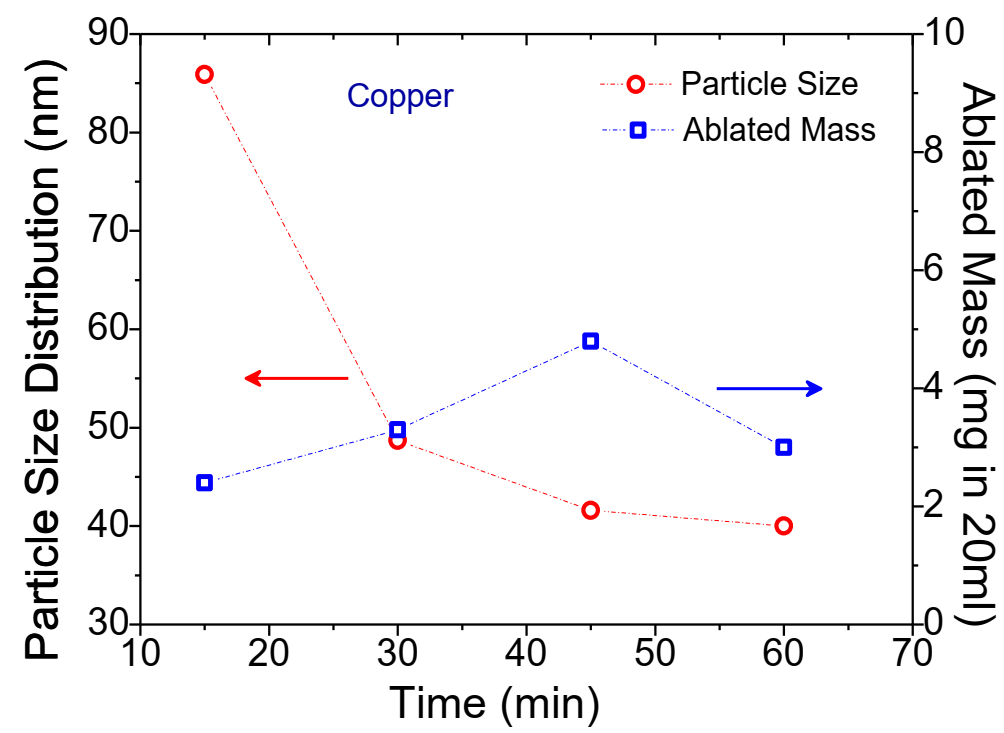 Fig. 5 Average particle size distribution and ablated mass for the Cu NP colloids produced in the dynamic flow PLAL system.
Fig. 5 Average particle size distribution and ablated mass for the Cu NP colloids produced in the dynamic flow PLAL system.
Table 2 list the details of the fixed and varied parameters used for Cu PLAL with the outcomes of ablated mass and the average particle size distribution.
Table 2. Details of PLAL parameters, NP average size distribution, and ablated mass of Cu NPs in 20 ml DI water.
The particle size and production rate of NPs produced in the DI water (20 ml) by laser ablation depends on the laser parameters, such as laser pulse energy and repetition rate, and the duration of the ablation. The highest concentration and NP production rate for Cu target was obtained for a 45-minute duration and for Ag target, it is 30-minute duration.
4 Conclusions
PLAL is an attractive method for producing nanomaterials in solution. Metal NPs in solution are suitable for use as conductive inks which can be used for PEs. The morphology, chemistry, and concentration of the NP colloids produced by PLAL are influenced by the process parameters. As such, the method can be optimized by varying these parameters to achieve the desired properties for a printable, conductive ink.
In this work, colloidal Ag and Cu NPs were successfully prepared by PLAL using a dynamic flow based system. The influence of the process duration on the particle size distribution and production rate was investigated for both materials. It was found that the average particle size decreased with process duration for both materials. This may be due to the re-circulation of the colloid throughout the process, with particles flowing back through the ablation site and being broken up by the laser. The ablated mass produced increased with duration as expected for the Ag NPs, with the rate of increase lowering at higher durations, possibly due to saturation of the colloid. For the copper target experiments, the ablation rate initially increased with duration, however the 60 min duration batch had a lower total mass produced than the 45-minute batch. Nanoparticles may sometimes deposit themselves onto the target after being produced, which could lead to a reduction in total mass [9]. However further reproducibility testing is needed in future work, to determine if this reduction in ablated mass happens consistently.
Acknowledgements
This work is supported in part by a research grant from Science Foundation Ireland (SFI) under Grant Numbers 16/RC/3872, 19/US-C2C/3579, and is co-funded under the European Regional Development Fund.