- Startpagina tijdschrift
- volume 10 (2006)
- numéro 1
- Effectiveness of thiophanate-methyl, trifloxystrobin and vinclozolin on canker caused by Phoma exigua Desm. on ash tree seedlings
Weergave(s): 0 (0 ULiège)
Download(s): 0 (0 ULiège)
Effectiveness of thiophanate-methyl, trifloxystrobin and vinclozolin on canker caused by Phoma exigua Desm. on ash tree seedlings

Nota's van de redactie
Reçu le 29 juin 2005, accepté le 7 octobre 2005
Résumé
Efficacité du thiophanate-méthyl, de la trifloxystrobine et de la vinclozoline vis-à-vis du chancre causé par Phoma exigua Desm. sur semis de frêne. Au cours de ces dernières années, plusieurs cas de chancres provoqués par Phoma exigua sur semis de frêne et menant à une perte totale des parcelles affectées ont été rapportés dans des pépinières belges. Des symptômes semblables ont été observés sur des semis de frêne ailleurs en Europe, notamment en France et en Grande-Bretagne, sans que la pathogénicité n’ait jamais été établie. Des essais d’inoculation et de ré-isolement ont dès lors été entrepris et ont démontré le caractère pathogène de P. exigua sur frêne. Par ailleurs, l’efficacité de trois fongicides (thiophanate-méthyl, trifloxystrobine, vinclozoline) contre le chancre des semis de frêne a été étudiée. Des essais in vitro ont été réalisés pour évaluer la capacité de ces fongicides à inhiber la croissance mycélienne et la germination conidienne de P. exigua. Leur effet sur la réduction des symptômes a été estimé lors d’un essai en plein champ. Les résultats de cette étude ont permis d’obtenir auprès des autorités belges compétentes l’extension d’usage du thiophanate-méthyl pour la lutte contre le chancre des semis de frêne en pépinières forestières.
Abstract
In recent years several cases of cankers caused by Phoma exigua on ash tree seedlings have been reported in Belgian nurseries, leading to a total loss of the affected crops. Similar symptoms have been observed on ash tree seedlings elsewhere in Europe, notably in France and in Great Britain, but the pathogenicity was never established. Inoculation and re-isolation tests were therefore undertaken and demonstrated the phytopathogenic character of P. exigua on ash. Moreover the effectiveness of three fungicides (thiophanate-methyl, trifloxystrobin, vinclozolin) against stem canker of ash tree seedlings was studied. In vitro tests were conducted to evaluate the ability of these fungicides to inhibit mycelium growth and spore germination. The extent to which they reduced the symptoms was estimated in a field trial. The results of this study allowed to get by the Belgian proper authorities the use extension of thiophanate-methyl for the control of canker caused by P. exigua in forest nurseries.
Inhoudstafel
1. Introduction
1Canker of ash tree seedlings (Fraxinus sp.) first appeared in Belgian nurseries at the end of the 1990s. Several cases have been reported since then, in both the northern and southern parts of the country. These cankers have various sizes and shapes but usually appear as cracked tissues. They can also look like small depressions or sometimes surround the whole stem. Developing cankers can coalesce at a later state into large affected areas, causing the plant to break at the slightest shock. Infection is spread rapidly from an affected seedling but the callusing of the cankers is sometimes visible when symptoms are mild.
2In this study, Phoma exigua Desm. var. exigua was isolated from the cankers. The identification was confirmed by the Centraalbureau voor Schimmelcultures of Utrecht, The Netherlands. Similar symptoms have been observed on ash tree seedlings in nurseries elsewhere in Europe, notably in France (Boudier, 1994) and the United Kingdom (Gregory et al., 1989). In addition, Phoma sp. was recently isolated in Belgium from cankers on sprouts of Fraxinus excelsior L. in forest area. It was also associated with stem necrosis on self-sown ash tree seedlings in Polish forests (Przybyl, 2002), although pathogenicity was not confirmed in any of these cases.
3The coelomycete P. exigua, very common in Europe, has a worldwide distribution and may cause various lesions to a large range of host plants as an opportunistic plant parasite. The species P. exigua includes a number of infraspecific taxa, among which is the plurivorous P. exigua var. exigua. Several host-specific P. exigua varieties, e.g. var. populi de Gruyter and P. Scheer on poplar and var. forsythiae (Sacc.) van der Aa et al. on forsythia, have been described (Sutton, 1980; de Gruyter, Scheer, 1998; van der Aa et al., 2000; Boerema et al., 2004). These varieties can be identified by slightly different cultural characteristics. A molecular characterization using AFLP markers has also been described (Abeln et al., 2002).
4According to Boudier (1994), the causal agent is not considered to be a weak parasite: healthy or weakened seedlings can be infested. Humidity favours the establishment of the disease and seedlings less than three years old are the most sensitive.
5Several cases of P. exigua reported in Belgium in recent years led to a total destruction of the affected plots, resulting in substantial economic loss because of the high selling price of ash tree seedlings. Before this study, no fungicide was registered in Belgium for the control of this disease. This lack of registered products is common in horticultural crops whose market share is too limited to interest crop protection companies. This often leads to the use of non-registered fungicides without any knowledge of their effectiveness or the minimal dose needed to provide adequate control. This study therefore sought to evaluate the effectiveness of three fungicides against P. exigua. Two types of tests were carried out: a field trial undertaken to evaluate the effect of three fungicides on cankers caused by P. exigua on ash tree seedlings; several tests performed in vitro to measure the effect of these fungicides on the mycelial growth and the conidial germination of P. exigua.
2. Material and methods
2.1. Fungal isolates
6The strains of P. exigua used in this study (Table 4) were isolated from F. excelsior seedlings from several Belgian nurseries in both the northern and southern parts of the country. Pieces of stems were cut off at the margin of a canker, surface-disinfected in 1% NaOCl for 30 sec, rinsed three times in sterile distilled water and plated aseptically on PDA (Potato Dextrose Agar, Difco). Cultures were incubated at 20°C. Monoconidial strains were generated from all P. exigua isolates and stored on PDA with paraffin oil (VWR International) at 4°C.
2.2. Koch’s postulates
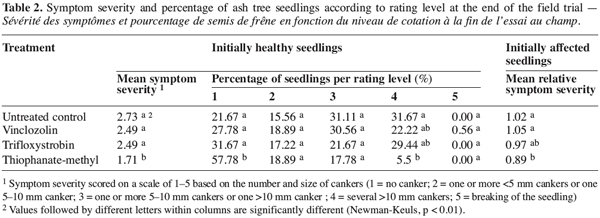
7The pathogenicity was studied under greenhouse conditions using one-year-old ash tree seedlings (F. excelsior). Inoculum consisted of 6 mm-diameter agar plugs from the actively growing front of a two-week-old colony of strain 2310. Twenty-four seedlings were used: 12 were inoculated and 12 served as negative controls.
8The stems were surface-sterilized with 95% ethanol and a fragment of bark was removed at about 10 cm above the root collar. The inoculum was placed on each wound, with mycelium facing the cambium, and the inoculation sites were sealed with parafilm. The inoculum was replaced by a sterile agar plug for the 12 control seedlings. Infection was assessed after 13 days and re-isolations were made on PDA from pieces of infected tissues.
2.3. Field trial
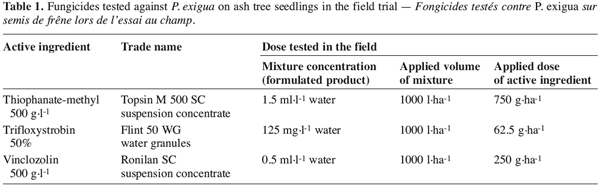
9A trial was conducted in the field on three-year-old ash tree seedlings (F. excelsior) in an experimental plot with a natural infection of P. exigua. The aim of the trial was to compare the effectiveness of thiophanate-methyl (Topsin M500SC), trifloxystrobin (Flint 50WG) and vinclozolin (Ronilan SC) formulations on cankers caused by P. exigua.
10The randomized complete blocks design included six replicates of the three fungicide treatments and of the untreated control. As soon as the first symptoms of the disease appeared naturally, 30 symptom-free and 10 affected seedlings were labelled in each of the 24 plots. Three sprayings of the fungicides were performed under dry weather conditions (11 May, 30 May and 25 June 2001), following the doses recommended by the manufacturers for other woody crops (Table 1).
11Before each spraying, by mid-trial (27 August) and at the end of the trial (24 October), symptom severity of all the labelled seedlings was scored on a scale of 1–5 based on the number and size of cankers (1 = no canker; 2 = one or more < 5 mm cankers or one 5–10 mm canker; 3 = one or more 5–10 mm cankers or one >10 mm canker; 4 = several >10 mm cankers; 5 = breaking of the seedling). The design of the trial and the analysis of results were based on EPPO guidelines PP1/152 (2) for the efficacy evaluation of plant protection products (1999).
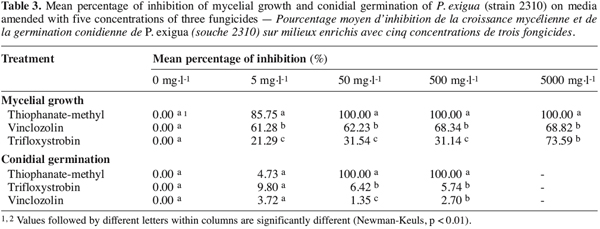
2.4. In vitro tests
12Several experiments were conducted in the laboratory to evaluate the effect of fungicides on the mycelial growth and the conidial germination of P. exigua associated with stem cankers of ash tree seedlings.
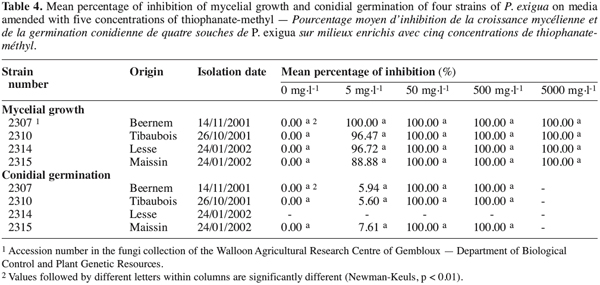
13Mycelial growth
14Comparison of five concentrations of three fungicides. Following the method described by Pionnat (1982; 2001), the growth of strain 2310 was evaluated on PDA media amended with 0, 5, 50, 500 and 5000 mg.l-1 of the three active ingredients previously tested in the field (Table 1). For each fungicide, six replicate plates of each concentration were used.
15Media with fungicides were prepared by adding given quantities of commercial formulations to PDA (50°C) in order to reach the targeted concentrations. Plates were inoculated with one 6 mm-diameter agar plug from the actively growing front of a two-week-old colony of strain 2310. The plates were incubated at 20°C. The growth of P. exigua was measured every seven days with a millimetre-graduated ruler along three diameters previously drawn at the bottom of the plates. The experiment lasted 21 days.
16Comparison of four strains on five concentrations of thiophanate-methyl. A complementary test was undertaken using four strains of P. exigua, including strain 2310 (Table 4). The growth of the four strains was evaluated on PDA media amended with 0, 5, 50, 500 and 5000 mg.l-1 of thiophanate-methyl. For each strain, six replicate plates of each concentration were used.
17As previously, plates were inoculated with one 6 mm-diameter agar plug from the actively growing front of two-week-old colonies of the different strains. The plates were incubated at 20°C. The growth of P. exigua was measured, as described above, every seven days. The experiment lasted 21 days.
18Conidial germination
19Comparison of five concentrations of three fungicides. The conidial germination rate of strain 2310 of P. exigua was also evaluated on PDA media amended with 0, 5, 50, 500 and 5000 mg.l-1 of the three active ingredients previously tested (Table 1). For each fungicide, six replicate plates of each concentration were used.
20A conidia suspension was prepared by flooding a two week old colony of strain 2310 with 5 ml of sterile tap water and diluting the stock spore suspension to 106 spores.ml-1 using a Bürker cell. Media with fungicides were prepared as previously. Plates were inoculated by spreading 100 µl of the spore suspension over the agar surface and incubated at 20°C. The germination was recorded after 48 hours for 50 spores per plate. Conidia were considered to have germinated when the germ tube length equalled the spore diameter.
21Comparison of four strains on five concentrations of thiophanate-methyl. A complementary evaluation of the conidial germination rate was undertaken using strains 2307, 2310, 2314 and 2315 of P. exigua. The germination rate of conidia of the four strains was evaluated on PDA media amended with 0, 5, 50, 500 and 5000 mg.l-1 of thiophanate-methyl. For each strain, six replicate plates of each concentration were used.
22As previously, 100 µl of conidia suspension (106 spores.ml-1) were spread over the agar surface. The plates were incubated at 20°C for 48 hours and the germination rate of P. exigua was assessed for 50 spores per plate as described above.
3. Results
3.1. Koch’s postulates
23After a 13-day inoculation period, each seedling inoculated with P. exigua exhibited typical depressive lesions. P. exigua was successfully re-isolated from all lesions, thereby completing Koch’s postulates. No symptoms were observed on the control seedlings.
3.2. Field trial
24Observations on initially symptom-free seedlings. The results of observations on initially symptom-free seedlings show differences between treatments (Table 2). Mean symptom severity at the end of the trial was significantly lower (Newman-Keuls, p<0.01) on seedlings treated with thiophanate-methyl than on other seedlings. Indeed, mean symptom severity was 1.71 on seedlings treated with thiophanate-methyl (750 g.ha-1, a.i.), 2.73 on untreated seedlings and 2.49 on seedlings treated with trifloxystrobin (62.5 g.ha-1, a.i.) and vinclozolin (250 g.ha-1, a.i.). The means standard error was 0.15.
25At the end of the trial, 58% of the seedlings treated with thiophanate-methyl did not present any symptoms (Table 2). This was significantly higher (Newman-Keuls, p<0.01) than the percentages observed in untreated plots (22%) or in plots treated with trifloxystrobin and vinclozolin (32 and 28%, respectively). The means standard error was 4.8%.
26The percentage of seedlings highly affected by the disease (rating of 4) was significantly lower at the end of the trial for seedlings treated with thiophanate-methyl (6%) than for untreated seedlings (32%) (Newman-Keuls, p < 0.01). The means standard error was 1.9%.
27There was no significant effect of treatment on the percentage of seedlings with mild symptoms (rating of two or three) and the number of seedlings in which the stem had broken (rating of five) was not significant (Newman-Keuls, p < 0.01).
28Observations on initially affected seedlings. Relative symptom severity was calculated for initially affected seedlings by dividing rating at the time of observation by initial rating (Table 2). At the end of the trial, this ratio was significantly lower for seedlings treated with thiophanate-methyl (0.89) than for untreated seedlings (1.02) (Newman-Keuls, p<0.01). The means standard error was 0.05. In addition, a ratio significantly lower than one was observed only for seedlings treated with this fungicide (test of conformity for m=1, p<0.01). This demonstrates a slight curative effect of thiophanate-methyl on cankers caused by P. exigua.
3.3. In vitro tests
29Mycelial growth
30Comparison of five concentrations of three fungicides. The results from the in vitro test with the three fungicides previously tested in the field (Table 3) showed a significantly higher ability of thiophanate-methyl to inhibit mycelial growth of P. exigua for each tested concentration (Newman-Keuls, p<0.01).
31At the end of the experiment, the complete inhibition of P. exigua was observed on media containing at least 50 mg.l-1 of thiophanate–methyl, while growth of the fungus still occurred on media amended with 5000 mg.l-1 of vinclozolin or trifloxystrobin. The percentage of inhibition varied, according to increasing concentrations, from 61 to 69% for vinclozolin and from 21 to 74% for trifloxystrobin. An inhibition of 86% was obtained at 5 mg.l-1 for thiophanate-methyl. The means standard error was 1.5%.
32Comparison of four strains on five concentrations of thiophanate-methyl. The effectiveness of thiophanate-methyl on mycelial growth was confirmed by the in vitro test with four strains of P. exigua (Table 4). The percentage of inhibition on media amended with 5 mg.l-1 of thiophanate-methyl varied from 89 to 100%, depending on the tested strain. The growth of four strains was completely inhibited for higher concentrations (Table 4). The means standard error was 3.1%.
33Conidial germination
34Comparison of five concentrations of three fungicides. After 48 hours, the inhibition of conidial germination was significantly higher on media amended with at least 50 mg.l-1 of thiophanate-methyl (Newman-Keuls, p<0.01) (Table 3). Indeed, conidial germination was completely inhibited on media amended with 50 mg.l-1 of thiophanate-methyl, while total inhibition was never observed for other fungicides. Inhibition varied from 6 to 10% for trifloxystrobin and from 1 to 4% for vinclozolin. No evaluation could be made at 5000 mg.l-1 due to opacity of the amended media. The means standard error was 3.2%.
35Comparison of four strains on five concentrations of thiophanate-methyl. The complete inhibition of conidial germination on media amended with at least 50 mg.l-1 of thiophanate-methyl was confirmed for all strains (Table 4), except for strain 2314, which presented non-viable conidia. As previously, no evaluation could be made at 5000 mg.l-1 due to opacity of the amended media. The means standard error was 1.2%.
4. Discussion
36P. exigua proved to be pathogenic on seedlings of F. excelsior. However, among the P. exigua complex, several host-specific varieties could be differentiated by various techniques. Additional work should be done to establish whether the P. exigua strains isolated from cankers on ash tree seedlings represent a separate variety.
37The various tests undertaken in the field and in vitro showed the great potential of thiophanate-methyl for the control of cankers caused by P. exigua on ash tree seedlings.
38In this study, three preventive sprayings with thiophanate-methyl (750 g.ha-1, a.i.) in the field significantly increased the number of symptom-free seedlings, which were therefore perfectly marketable. In addition, a significantly lower number of highly affected seedlings (rating of four) was observed among seedlings treated with thiophanate-methyl. As seedlings presenting only light damage are commonly marketed, thiophanate-methyl applications therefore influence the number of marketable seedlings. The curative activity of fungicides was evaluated in the field but thiophanate-methyl showed only a slight effect on initially affected seedlings.
39The efficacy of thiophanate-methyl was also demonstrated during in vitro tests performed with five concentrations of three fungicides: the growth and conidial germination of the fungus were totally inhibited on media containing 50 mg.l-1 of thiophanate-methyl. Such complete inhibition did not appear for the two other fungicides tested (trifloxystrobin and vinclozolin), whatever the concentration. This inhibiting effect of thiophanate-methyl was confirmed during complementary tests conducted in vitro on different strains of P. exigua. The different results obtained allowed the use extension of thiophanate-methyl (Topsin M500SC) in Belgium against Phoma sp. in ornamental trees and shrubs. The similarity of results obtained during the field trial and the in vitro tests should also be noted. This underlines the usefulness of in vitro tests as a screening method of fungicides before conducting field trials.
40The literature mentions the high sensitivity of many Phoma species to thiophanate-methyl or, more globally, to benzimidazoles fungicides. Reduced sensitivity or acquired resistance was also reported for most of these species, such as Phoma clematidina (Thüm.) Boerema, Phoma exigua var. foveata (Foister) Boerema, Phoma cucurbitacearum (Fr.:Fr.) Sacc. [teleomorph Didymella bryoniae (Auersw.) Rehm] and Phoma tracheiphila (Petri) L.A. Kantsch. & Gikaschvili (MacCracken, Logan, 1977; Pionnat, 1982; Malathrakis, Vakalounakis, 1983; Decognet et al., 1994; Keinath, Zitter, 1998; van de Graaf et al., 2003; van den Berg, 2004).
41Cultural practices restricting infestation by P. exigua should therefore be preferred for the control of canker of ash tree seedlings, and the systematic application of thiophanate-methyl should not be recommended. This would help lower the risk of reduced sensitivity to this fungicide, while promoting environmentally friendly practices within the framework of sustainable agriculture. Further work should be undertaken to study the resistance to thiophanate-methyl of P. exigua causing canker on ash tree seedlings or to obtain additional registrations of fungicides against this disease in order to diversify their use.
42Acknowledgements
43This research was supported by the Ministry of the Walloon Region (Ministry of Employment – contracts AR258 – conventions 0667 and 0859).
Bibliographie
Abeln ECA., Stax AM., de Gruyter J., van der Aa HA. (2002). Genetic differentiation of Phoma exigua varieties by means of AFLP fingerprints. Mycol. Res. 106 (4), p. 419–427.
Boerema GH., de Gruyter J., Noordeloos ME., Hamers MEC. (2004). Phoma identification manual: differentiation of specific and infra-specific taxa in culture. Oxfordshire, UK: CABI Publishing, 470 p.
Boudier B. (1994). Le chancre des jeunes plants de frênes, une maladie grave certaines années mais des perspectives de lutte. Phytoma 461, p. 35–36.
Decognet V., Cerceau V., Jouan B. (1994). Control of Phoma exigua var. linicola on flax by seed and foliar spray treatments with fungicides. Crop Prot. 13 (2), p. 105–108.
de Gruyter J., Scheer P. (1998). Taxonomy and pathogenicity of Phoma exigua var. nov. populi causing necrotic bark lesions on poplars. J. Phytopathol. 146, p. 411–415.
EPPO (1999). Guidelines for the efficacy evaluation of plant protection products. EPPO Bull. 29, p. 297–317.
Gregory SC., MacAskill GA., Redfern DB., Pratt JE. (1989). Pathology, diagnostic and advisory services — Scotland and northern England. In HMSO. Report on forest research for the year ending March 1989. London, p 42.
Keinath AP., Zitter TA. (1998). Resistance to benomyl and thiophanate-methyl in Didymella bryonae from South Carolina and New York. Plant Dis. 82, p. 479–484.
MacCracken AR., Logan C. (1977). A selective medium for the isolation of Phoma exigua var. foveata from soil. Rec. Agric. Res. 25, p. 71–76.
Malathrakis NE., Vakalounakis DJ. (1983). Resistance to benzimidazole fungicides in the gummy stem blight pathogen Didymella bryoniae on cucurbits. Plant Pathol. 32, p. 395–399.
Pionnat JC. (1982). Le mal sec Phoma tracheiphila (Petri) Kone et Ghik: perspectives sur la lutte chimique et les variétés résistantes. Fruits 37 (4), p. 237–248.
Pionnat JC. (2001). De nouvelles molécules actives contre le mal sec des agrumes, huit sélectionnées appartenant à quatre familles. Phytoma 538, p. 31–33.
Przybyl K. (2002). Fungi associated with necrotic apical parts of Fraxinus excelsior shoots. For. Path. 32, p. 387–394.
Sutton BC. (1980). The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata. Kew, UK: Commonwealth Mycological Institute, 696 p.
van de Graaf P., O’Neill TM., Chartier-Hollis JM., Joseph ME. (2003). Aspects of the biology and control of benzimidazole resistant isolates of Phoma clematidina, cause of leaf spot and wilt in clematis. J. Phytopathol. 151, p. 442–450.
van den Berg G. (2004). Phoma is vaak gelegenheidsparasiet. Boomkwekerij 36, p. 10–11.
van der Aa HA., Boerema GH., de Gruyter J. (2000). Contributions towards a monograph of Phoma (Coelomycetes) VI-1. Section Phyllostictoides: characteristics and nomenclature of its type species Phoma exigua. Persoonia 17 (3), p. 435–456.
Om dit artikel te citeren:
Over : Sophie Schmitz
Département Lutte biologique et Ressources phytogénétiques. Centre wallon de Recherches agronomiques. Rue de Liroux, 4. B-5030 Gembloux (Belgium). E-mail : schmitz@cra.wallonie.be.
Over : Jérôme Zini
Département Lutte biologique et Ressources phytogénétiques. Centre wallon de Recherches agronomiques. Rue de Liroux, 4. B-5030 Gembloux (Belgium).
Over : Michel Etienne
Département Lutte biologique et Ressources phytogénétiques. Centre wallon de Recherches agronomiques. Rue de Liroux, 4. B-5030 Gembloux (Belgium).
Over : Jean-Marc Moreau
Département de Phytopharmacie. Centre wallon de Recherches agronomiques. Rue du Bordia, 11. B-5030 Gembloux (Belgium).
Over : Anne Chandelier
Département de Phytopharmacie. Centre wallon de Recherches agronomiques. Rue du Bordia, 11. B-5030 Gembloux (Belgium).
Over : Marc Cavelier
Département de Phytopharmacie. Centre wallon de Recherches agronomiques. Rue du Bordia, 11. B-5030 Gembloux (Belgium).






